Final report for LNE20-412R
Project Information
Problem, Novel Approach and Justification: The foodborne pathogens Salmonella, Escherichia coli O157:H7 and Listeria monocytogenes cause over 2 million annual illnesses in the U.S, largely due to consumption of contaminated fresh produce and poultry products. Current decontamination strategies using quaternary ammonium compounds or chlorine wash are not completely effective in reducing pathogen load on food products. Additionally, these chemicals pose significant health hazards for farmers that include the risk of cancer. This project aimed to develop novel washing strategies for improved produce and egg decontamination. Our approach combined the antimicrobial efficacy of Generally Recognized as Safe status antimicrobial gas Ozone with novel ultra-fine bubble technology to develop safe and rapid-acting sanitizers for egg and produce washing.
Hypothesis: Ultra-fine ozone (UFO) bubble water would significantly reduce Salmonella, Escherichia coli O157:H7, and Listeria monocytogenes on eggs and fresh produce (cantaloupes, lettuce). The efficacy of UFO bubble water will be more than currently used antimicrobials (e.g. 200 ppm chlorine).
Project Objectives and implementation plan: Our overall goal was to develop a novel washing method using UFO bubble water to improve the microbiological safety of eggs and produce. The first objective investigated the efficacy of UFO bubble water washing in killing Salmonella, Escherichia coli O157:H7 and Listeria monocytogenes on eggs and produce. The second objective implemented an extension/outreach program to disseminate our findings to stakeholders and extension personnel for field adoption of the technology in the Northeast. Our research plan consisted of three stages. In the first stage, UFO bubble water was generated using a dual-machine system consisting of an ozone generator and bubble mixer followed by characterizing the quality and stability of UFO bubbles. In the second stage, the efficacy of UFO bubble water in rapidly killing Salmonella on eggs and Salmonella, Escherichia coli O157:H7 and Listeria monocytogenes on lettuce and cantaloupes was investigated. In the third stage, the effect of organic load on the antimicrobial efficacy of most effective UFO bubble treatment-time combinations was tested. As part of our extension/outreach objective, Dr. Shuresh Ghimire and Dr. Indu Upadhyaya conducted workshops and technology demonstrations for extension personnel (train-the-trainer) in the Northeast to update stakeholders on outcomes of the project and to receive their feedback. They also interacted directly with organic and non-organic vegetable growers, egg-producers in CT and Northeast to understand their perceptions towards ultra-fine bubble technology-based wash solutions.
Results: Washing with UFO bubble water is effective in reducing Salmonella load on eggs and Salmonella, Escherichia coli O157:H7 and Listeria monocytogenes on lettuce and cantaloupes. No deleterious effect on product color was observed when washed with UFO bubble water.
Our goal is to develop a novel washing method using ultra-fine ozone (UFO) bubble water to improve the microbiological safety of eggs and produce in the Northeast. The first objective will investigate the efficacy of UFOB water washing in killing Salmonella, Escherichia coli O157 and Listeria monocytogenes on eggs and produce. The second objective will implement an extension/outreach program to disseminate our findings to stakeholders and extension personnel for field adoption of the technology.
According to the 2010 Census, over 63 million people (21% of US population) live in the Northeast. The region is characterized by a high population density (342 vs. 87 people/square mile-national average), lower rural community (15% vs, 19% nationally), higher per capita income and lower unemployment. Although exact food consumption trends are not available, the data on food availability serves as a proxy for consumption levels. Since 1970, there has been an increase in the availability of fresh fruits and vegetables by ~25%. Northeast produces a higher proportion of fruits and vegetables than the US as a whole. The egg consumption patterns are the highest in the last 7 years with the Northeast region contributing significantly, largely through Pennsylvania. With varied and sloped terrain, the farms in the Northeast are on average smaller and more diverse than the rest of the US. With an increasing demand for locally grown food, improvements in the production system could benefit the farmers and also provide food access in low-income communities. The microbiological safety of food products is of paramount importance for farm profitability since maintaining a safe product leads to respectful recognition in local market and consumer acceptance. Locally grown foods are perceived by consumers and community as safer and wholesome than conventional foods, thereby increasing the responsibility on farmers and processors to ensure product safety and to meet consumer expectations. Therefore, implementing good decontamination strategies for food products is warranted. The CDC recommends farmers to wash and sanitize food products and several FDA recommended antimicrobials are currently used by farmers for poultry carcass and produce washing and decontamination. However, they are minimally effective in reducing pathogen load and foodborne outbreaks continue to occur. Our long-term goal is to help farmers in developing profitable and sustainable poultry and fresh produce production system by providing them with easy to use and effective pathogen decontamination methods for their products.
Cooperators
- (Educator)
- (Educator)
Research
YEAR ONE:
The PI hired a graduate student to work on the project. The PI and graduate student standardized the production of UFO bubbles under various temperature and time combinations. In addition, the effect of ozone in inactivating Listeria monocytogenes on cantaloupe rind and in produce wash water was investigated. Specific details on the experiments are provided below.
Objective 1: Investigating the solubility and stability of ozone in water maintained at different temperatures (25, 4℃).
Rationale: Since ozone is generated on-site, it is critical to study the impact of type of machine, liquid temperature, and bubble time on ozone solubility. Also, since ozone naturally disintegrates to oxygen, understanding the disintegration kinetics of ozone in liquid medium and the various factors that impact the rate of disintegration is important for designing appropriate food safety experiments and develop recommendations for farmers.
Method: The solubility and stability of ozone in water maintained at different temperatures (25, 4℃) was measured at different bubbling time (0, 5, 10, and 15 min). The ozone was generated using an ozone generator (Ivation; ozone generation rate of 600 mg/h) and bubbled in 500 ml of DI water using a micro bubble diffuser. The concentration of ozone was measured post-bubble time at 0, 5, 10, 15, 20, 40 and 60 min using the Vacu-vials kit (SAM, OZONE, CHEMetrics, I-2019). Next, the generation of ozone was investigated using VMUS-4 ozone generator with or without an oxygen concentrator. VMUS-4 produces ozone at the rate of 4 g/h from dry air and 10 g/h from oxygen. This ozone generation rate is significantly higher than Ivation generator due to double corona discharge tubes present in the VMUS-4.
Objective 2: Efficacy of ozone microbubbles in inactivating planktonic cells of Listeria monocytogenes (Scott A strain) in produce wash water.
Rationale: Contaminated wash water can act as a source of cross-contamination between produce and can act as a medium to spread foodborne pathogens. Therefore, it is critical to test the effectiveness of antimicrobial wash treatments in reducing pathogen survival in wash water.
Method: Ozone was bubbled in 500 ml of DI water maintained at 4℃ for 15 min. After bubbling, ozonated DI water was inoculated with L. monocytogenes (Scott A strain) at ~6 log CFU/ml. The wash water was sampled at 1, 5, 10, 15, 30 min and the surviving L. monocytogenes were enumerated by dilution and plating on oxford agar plates followed by incubation at 37℃ for 48 hours. Ozone concentration was simultaneously measured at the aforementioned timepoints. The concentration of ozone at 1 min was ~ 0.74 ppm. The ozone level reduced to ~0.4 ppm by 30 min.
Objective 3: Efficacy of ozone microbubbles in inactivating Listeria monocytogenes on cantaloupe rind plugs.
Rationale: Once the efficacy of ozone microbubbles in inactivating L. monocytogenes was established in wash water, the next step is to test the antimicrobial efficacy of ozone microbubbles against L. monocytogenes present on food product surface.
Method: Ozone was bubbled in 500 ml of DI water maintained at 4℃ for 15 min. Cantaloupe rind plugs (~ 4 cm diameter) were inoculated with LM cocktail (Scott A, AT19115, LM1, LM2 and LM3) at 8 log CFU/plug. The inoculum was allowed to attach for 90 min. Inoculated rind plugs were treated with ozonated water for 1, 5, 10 min at 4℃ and the surviving L. monocytogenes were enumerated by dilution and plating on oxford agar plates followed by incubation at 37℃ for 48 h.
YEAR TWO:
The PI and graduate student custom designed ozone compatible nanobubble generator in collaboration with Aciniti LLC (Japan) that can generate UFO bubbles with size below 200 nm. Once the ozone nanobubble generator was received from Japan, the PI and graduate student standardized the nanobubble production followed by testing the efficacy of UFO bubble water in inactivating Listeria monocytogenes on lettuce. All experiments were repeated at least three times with duplicate samples.
Objective 1: Investigate the stability of UFO bubbles in water.
Experiment 1: Standardization of UFO bubble generation.
Method: UFO bubbles were generated using the oxygen concentrator-ozone generator-nanobubble generator combination at various experiment settings (run time, temperature of water, oxygen pressure, oxygen flow, spin speed of nanobubble generator). Similarly, ozone was produced and dissolved in the water without the nanobubble generator to serve as control.
Experiment 2: Degradation kinetics of ozone in UFO bubbles in water.
Method: UFO bubbles were generated using an oxygen concentrator-ozone generator-nanobubble generator combination. The run time was 15 min, oxygen pressure of 15 psi, oxygen flow of 3 liters per min, temperature of water was 25oC and spin speed of nanobubble generator was 27 Hz. For control, similar set up was used, except for the nanobubble generator. Dissolved ozone was measured immediately after generation (time zero) and at regular intervals till 24 h.
Objective 2: Investigate the efficacy of UFO bubble water in inactivating Listeria monocytogenes on lettuce.
Method: Circular lettuce samples were prepared using a sterile, steel corer. Five strains of L. monocytogenes (Scott A, AT19115, LM1, LM2 and LM3) were used for this experiment. For inoculum preparation, the individual 10 ml overnight culture was centrifuged at 7000 rpm for 15 minutes at 25℃. The bacterial pellet was washed three times and resuspended in 10 ml of sterile 1X PBS. Equal portion of the washed cultures were mixed and diluted appropriately to yield a final inoculum concentration of 6 log CFU/ml. The average inoculum on lettuce was ~5 log CFU/sample. Cocktail of L. monocytogenes was spot inoculated (200 µl volume with 20 spots of 10 µl each; ~5 log CFU/sample) on the prepared fresh produce samples followed by incubation for 2 h at 25℃ in the biosafety cabinet, to facilitate bacterial attachment. For control, sterile DI water was used for washing. UFO bubbles were generated by using Ozone generator (Oxidation technologies, USA) connected to nanobubble generator (Aciniti, Japan) by running the machine for 30 minutes with 15 Psi of oxygen pressure. The dissolved ozone was measured post-bubble using the Vacu-vials kit (SAM, OZONE, CHEMetrics, I-2019). For treatment, glass containers were filled with 500 ml of DI water (control) or 500 ml of UFO bubble water (treatment). The inoculated lettuce was dipped in the treatment container and treated for 1, 3 or 5 minutes at 25℃. After treatment, the samples were transferred to Whirl-PakTM bag (Nasco, Fisher Scientific) containing 10 ml of Dey-Engley neutralizing broth. The samples were stomached for 1 min at 300 rpm followed by dilution and plating on Oxford agar plates. The plates were incubated at 37℃ for 24-48 hours, followed by bacterial enumeration.
YEAR THREE:
In year 3, the PI and graduate student presented the work conducted in year 1, 2 at the New England Vegetable and Fruit Conference and Trade Show (https://newenglandvfc.org/media/pdf/N/E/V/NEVFC_2022_Proceedings_DRAFTFINAL.pdf). In addition, a demonstration of the working of ozone-nanobubble generator to farmers was performed at the UConn Extension’s 2023 Vegetable and Small Fruit Grower’s Conference. In addition, the effect of nanobubbles on dissolved ozone level in water was tested (objective 1). Further, the PI procured NanoSight 300 machine that provides data on bubble size, and concentration. In addition, experiments investigating the potential of UFO bubble water in inactivating Salmonella spp. on eggs and Escherichia coli O157:H7 on lettuce, cantaloupes were completed. Specific details are provided below.
Objective 1: Effect of UFO bubbles on ozone solubility in water.
Method: UFO bubbles were generated using the oxygen concentrator-ozone generator-nanobubble generator combination (Oxidation Tech-Aciniti) at various experiment settings (run time, temperature of water, oxygen pressure, oxygen flow, spin speed of nanobubble generator). Similarly, ozone was produced and dissolved in the water without the nanobubble generator to serve as control.
Objective 2: Technology Demonstration at the UConn Extension’s 2023 Vegetable and Small Fruit Grower’s Conference.
Method: The design and working of the Oxygen Concentrator-Ozone generator (Oxidation technologies, USA) connected to nanobubble generator (Aciniti, Japan) was demonstrated at the UConn Extension’s 2023 Vegetable and Small Fruit Grower’s Conference using a recorded video. The graduate student described the science of nanobubble generation, mechanism of the machine and results.
Objective 3: Investigating the potential of UFO bubble water in inactivating Salmonella spp. on eggs and Salmonella, Escherichia coli O157:H7 and Listeria monocytogenes on lettuce and cantaloupes.
Objective 3a: To reduce Salmonella on eggs using UFO bubbles applied as a wash treatment and study effect on egg color.
Methods:
Bacterial strains and culture conditions: Five S. Enteritidis isolates pre-induced for resistance to 50 μg/mL of nalidixic acid (NA) were used. Each strain was cultured (37°C-24 h) separately in tryptic soy broth containing NA. Equal volumes of the cultures were combined and centrifuged (3,600×g for 15 min). The bacterial pellet was washed twice, resuspended in phosphate-buffered saline (PBS, pH 7.0), and used as the inoculum.
Inoculation of eggs: Fresh eggs procured from the university farm were washed in sterile deionized water, and air-dried for 30 min. The eggs were spot-inoculated with 200 μl of a 5-serotype cocktail of S. Enteritidis (~ 7 log CFU/ml) and air-dried for 1 h. The final pathogen load was ~ 6 log CFU/egg. Based on the USDA recommendation on the minimum temperature for the water used for egg washing, the efficacy of UFO bubbles as an antimicrobial wash for killing S. Enteritidis on shell eggs was investigated at 32°C. Five eggs were used per treatment/time point (n=15) and the study was repeated three times.
UFO bubble wash and microbiological analysis: We used the high-pressure air-water shearing method for generating UFO bubbles. The UFO bubbles were generated in deionized water maintained at 4°C by using a micro/nanobubble generator (operation time ~ 10 min). Batches of 15 eggs each were placed in a 2L sterile plastic bucket containing 1000 ml of sterile deionized water (control) or water with UFO bubbles and washed in a shaker water bath at 32°C for 5, 10, 15 or 30 min. Water containing 200 ppm of chlorine and peracetic acid will be included as industrial controls. The concentration of UFO bubbles will be standardized based on the minimum bactericidal concentration of ozone. Our preliminary data indicate that a dissolved ozone concentration of 0.5 ppm kills ~5 log S. Enteritidis population in wash water in 5 min.
After treatment, each egg was separately transferred to a sterile bag containing 30 mL of neutralizing broth and rubbed by hand for 1 min. S. Enteritidis was enumerated by plating the neutralizing broth on XLD+NA plates incubated at 37°C for 48 h. A batch of treated and control eggs were refrigerated (to mimic post-harvest storage) in egg cartons for 3 weeks, and Salmonella populations on eggs were determined on days 7, 14 and 21 of storage.
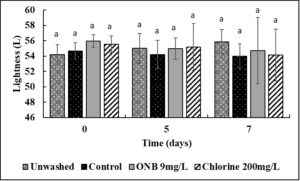
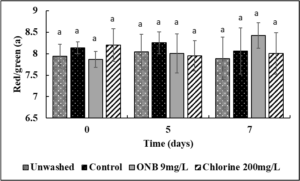
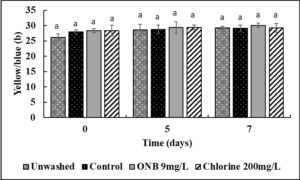
Egg color analysis: Un-inoculated shelled eggs were washed with UFO bubble water followed by egg color analysis using a chroma meter (MiniScan XE Plus) following the guidelines from International Commission on Illumination (CIE) for L* (lightness), a* (green red spectrum), b* (blue/yellow spectrum) values. The chroma meter was calibrated against a white ceramic surface before measurements were recorded on 0, 5, and 7 days of refrigerated storage.
Objective 3b: To reduce Salmonella, Escherichia coli O157:H7 and Listeria monocytogenes on lettuce and cantaloupes using UFO bubble wash.
Methods:
Bacterial strains and culture conditions: The strains of Salmonella are similar as mentioned previously. Five strains of E. coli O157:H7 and L. monocytogenes (pre-induced for resistance against nalidixic acid) were used. The E. coli O157:H7 and L. monocytogenes count were confirmed by plating on MacConkey-Sorbitol agar and modified Oxford agar both containing 50 μg/mL of Nalidixic Acid, with incubation at 37°C for 24 h.
Inoculation of fresh produce: Fresh lettuce and cantaloupes were procured from a local farm. Two hundred microliters of a 5- strain mixture of S. Enteritidis, E. coli O157:H7 or L. monocytogenes in PBS (8 log CFU/ml) were used to spot inoculate the sample separately. The inoculum was spotted (~ 50 spots, 20 μl each) evenly on the surface of individual sample and air-dried for 30 min at 23°C.
UFO bubble wash treatment and microbiological analysis of fresh produce: Individual lettuce and cantaloupes were submerged in appropriate volume of sterile deionized water (with or without UFO bubbles) maintained at 25°C in a plastic tub for a period of 1,5, or 10 min. The treatments also included 200 ppm chlorine as industry control. After treatment, fresh produce were removed and divided into two groups. One group was stored at refrigeration temperatures and the other at room temperature. Fresh produce were sampled at 0, 1, 3, 5 and 7 days of storage to determine surviving Salmonella, E. coli O157:H7 and L. monocytogenes counts. Five samples (n = 5) were used per treatment/time point and the study was repeated twice. For enumerating the surviving pathogen populations on produce samples at each time point, the samples were immersed in appropriate volume of neutralizing broth and rinsed followed by serial dilutions and plating of the neutralizing broth on XLD, SMA or Oxford agar plates. The plates were incubated aerobically at 37oC for pathogen enumeration. In addition, 1 mL portion of neutralizing broth was enriched in Cysteine Selenite broth (for Salmonella), E. coli O157 enrichment broth, and Listeria enrichment broth followed by plating on selective media as described above. Determination of the effect of treatments on produce surface color was analyzed as described above.
Effect of organic load on the antimicrobial efficacy of UFO bubble water: The effect of organic load (chicken droppings, soil) on the antimicrobial efficacy of UFO bubble wash water on eggs and produce was investigated. Briefly, 3% w/v chicken dropping, and 5% w/v soil load was prepared in distilled water. Eggs and produce were washed in UFO bubble wash water containing 3% chicken droppings and 5 % soil and the reduction in pathogen load was tested as described above.
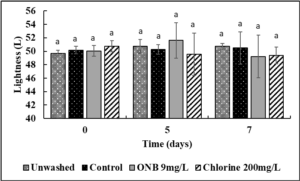
Cantaloupe and lettuce color analysis: Un-inoculated cantaloupes and lettuce were washed with UFO bubble water followed by color analysis using a chroma meter (MiniScan XE Plus) following the guidelines from International Commission on Illumination (CIE) for L* (lightness), a* (green red spectrum), b* (blue/yellow spectrum) values. The chroma meter was calibrated against a white ceramic surface before measurements were recorded on 0, 5, and 7 days of refrigerated storage.
YEAR ONE:
Results and Discussion (objective 1): The temperature of the liquid had a significant effect on Ozone solubility. For example, approximately 1 ppm of dissolved ozone was observed in the water maintained at 4℃ after 15 min of bubbling time. However, when the water was maintained at 25℃, the dissolved ozone level obtained after 15 min of bubbling time was ~ 0.5 ppm (~50% less solubility). The ozone disintegration time was also higher in water maintained at 25℃ than at 4℃. After 60 min post-bubble time, the ozone level reduced by ~90% in water maintained at 25℃ whereas the ozone level reduced by ~30% in water maintained at 4℃ suggesting that ozone has a higher stability at lower temperature. Use of oxygen concentrator facilitated in the production of ~5 ppm of ozone in 1500 ml of DI water maintained at 25℃ in 5 min. Without the oxygen concentrator, a maximum ozone concentration of ~1 ppm was observed in 5 min. These results suggest that in order to generate a higher ozone concentration in water, an ozone generator with a higher ozone output (g/h) is required. Moreover, an oxygen concentrator facilitates in generating higher level of dissolved ozone in solution.
Results and discussion (objective 2): Ozone micro bubbles (dissolved ozone concentration ~ 0.74 ppm) inactivated L. monocytogenes to below detection limits (~5 log CFU/ml reduction) as early as 1 min of treatment time indicating that dissolved ozone is very effective in inactivating L. monocytogenes in wash water.
Result and Discussion (objective 3): A reduction of ~ 1 log CFU/ml of L. monocytogenes was observed as early as 1 min of treatment time. Additional treatment times (5, 10 min) did not result in increased antimicrobial efficacy indicating that the maximum inactivation occurred in the first min of interaction between dissolved ozone and L. monocytogenes present on cantaloupe rind.
YEAR TWO:
Results and discussion (objective 1, experiment 1): We observed that a dissolved ozone level of ~ 3-4 ppm was obtained after 15 min of run time in both control (without nanobubble generator) and treatment (with nanobubble generator). When the water temperature was lowered to 4oC, a higher dissolved ozone level of ~ 9 ppm was obtained.
Results and discussion (objective 1, experiment 2): The figure (Fig. 1) below show the data obtained for experiment 2. We observed that a dissolved ozone level of ~ 3.5-3.8 ppm was obtained after 15 min of run time in both control (without nanobubble generator) and treatment (with nanobubble generator). After 1 h of storage at room temperature, the dissolved ozone level in ozonated nanobubble water (Blue line) was significantly higher as compared to normal ozone water (orange line) indicating that ozone nanobubbles might be facilitating higher dissolved ozone levels in the water.
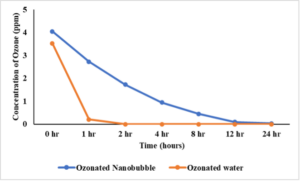
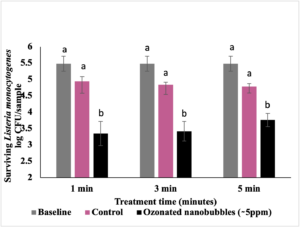
Results and discussion (objective 2): The effect of ozone nanobubble water in inactivating L. monocytogenes on lettuce at 25oC is presented in Fig. 2. In case of control (washing lettuce with water), no reducing in pathogen load was observed as compared to baseline (lettuce inoculated with pathogen but not subjected to any wash treatment). Washing with water containing ozone nanobubbles for 1 min reduced pathogen load by ~ 2 log CFU/sample as compared to control (P<0.05). Increasing the wash time from 1 to 5 minutes did not further enhance the antimicrobial efficacy of ozone nanobubbles. Results suggest that ozone nanobubbles washing is effective in reducing L. monocytogenes on lettuce.
YEAR THREE:
Result and discussion (objective 1): The solubility of ozone when passed through the nanobubble generator increased 5 folds as compared to control. For example, when ozone was bubbled in 10 L of water for 15 min at 25oC without the nanobubble generator (control), the dissolved ozone level was ~ 1 ppm. However, when ozone was bubbled in 10L of water for 15 min at 25oC through the nanobubble generator, the dissolved ozone level observed was ~ 5 ppm (P<0.05). This increase in dissolved ozone level is due the increased capture of the gas in nanobubbles.
Result and discussion (objective 2): Significant interest in the farming community was observed for the nanobubble technology. Major follow up questions included cost of procuring the machine, operation cost, effectiveness etc.
Result and discussion (objective 3):
3a. Inactivation of Salmonella on eggs using UFO bubble wash treatment and effect on egg color (Fig. 3).
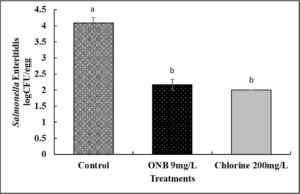
Inactivation of Salmonella on eggs using UFO bubble wash treatment.
In case of control, after washing with 1 min with just water, ~ 4 log CFU Salmonella was recovered per egg. Washing of eggs inoculated with Salmonella for 1 min with UFO bubble water or chlorine treatment reduced survival of the pathogen by ~2 log CFU as early as 1 min of treatment time (Fig. 3 ; P<0.05). Increasing the treatment time to 5, 10 or 30 min did not further increase antimicrobial efficacy of UFO bubble water or chlorine treatment (data not shown).
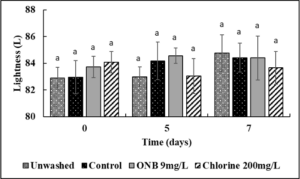
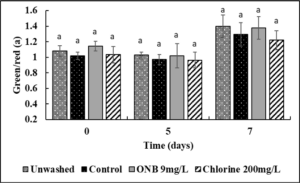
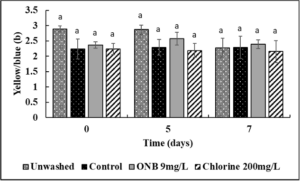
Effect of UFO bubbles and chlorine wash on egg color (Fig. 4,5,6).
No change in the Lightness (Fig.4), green/red spectrum (Fig. 5) and yellow/blue spectrum (Fig. 6) was observed when eggs were washed with UFO bubble or chlorine wash for 1 min (P>0.05).
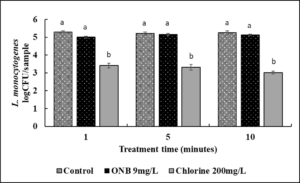
3b. Inactivation of Salmonella, Escherichia coli O157:H7 and Listeria monocytogenes on lettuce and cantaloupes using UFO bubble wash.
Inactivation of L. monocytogenes on cantaloupes and lettuce using UFO bubble wash treatments (Fig. 7,8).
Approximately 7 log CFU L. monocytogenes was inoculated on cantaloupe surface. Washing cantaloupe with just water (control) removed loosely attached cells but ~ 6 log CFU L. monocytogenes was still recovered indicating that washing with water is not sufficient in reducing pathogen load (Fig.7). Washing cantaloupe with water containing UFO bubbles did not significantly reduce pathogen load after 1 min of wash time. No significant difference in the efficacy of UFO bubble wash treatment was observed between 1,5 or 10 min suggesting that increasing the wash time did not have any effect on the antimicrobial efficacy of UFO bubble wash treatment. Washing with 200 ppm chlorine (industry standard) reduced L. monocytogenes by ~2 log CFU as early as 1 min. The efficacy of chlorine did not increase further with increase in wash time to 5 or 10 min (P>0.05).
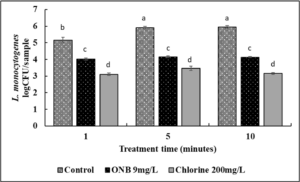
Washing Lettuce with water containing UFO bubbles reduced L. monocytogenes by ~1 log CFU after 1 min of wash time (Fig. 8). After 10 min of wash treatment, a reduction of ~ 2 log CFU L. monocytogenes was observed as compared to control (P>0.05). Washing with chlorine for 1, 5 or 10 min reduced pathogen load by an additional ~ 0.5 to 1 log CFU as compared to UFO bubble wash treatments (P<0.05). The reduced efficacy of both UFO bubbles and chlorine treatment on cantaloupe as compared to lettuce could be attributed to the highly rough surface of cantaloupe which facilitates strong attachment of pathogens. Moreover, the rough surface also reduces the interaction between pathogen and disinfectants that further protects bacteria and pathogens hiding in the netting of the cantaloupe.
Inactivation of E. coli O157:H7 on cantaloupes and lettuce using UFO bubble wash treatments (Fig. 9,10).
Unlike L. monocytogenes, UFO bubble wash treatment reduced the survival of E. coli O157:H7 on cantaloupes (Fig. 9). For example, washing of cantaloupes with UFO bubble water reduced E. coli O157:H7 counts by ~1 log CFU as early as 1 min (P<0.05). No further increase in efficacy of UFO bubble water was observed. Chlorine treatment reduced E. coli O157:H7 by ~ 1.5 log CFU by 1 min of treatment time (P<0.05). No significant difference in the efficacy of chlorine and UFO bubble water treatment was observed at 1 min. However, at 5 and 10 min of treatment time, chlorine was slightly better (~ 0.5 log CFU) in reducing pathogen load than the corresponding UFO bubble water wash treatment.
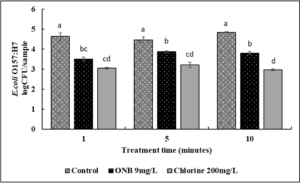
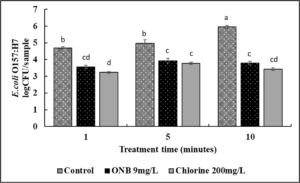
In case of lettuce samples (Fig. 10), by 1 min of wash time, both UFO bubble and chlorine wash treatments reduced E. coli O157:H7 by ~1.5 log CFU as compared to control (P<0.05). No difference in the efficacy of UFO bubbles and chlorine treatments was observed in any of the test timepoints (P>0.05). No further increase in antimicrobial efficacy was observed for either UFO bubbles or chlorine wash treatments.
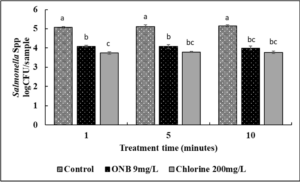
Inactivation of Salmonella spp. on cantaloupes and lettuce using UFO bubble wash treatments (Fig. 11, 12).
The cantaloupes were inoculated with ~ 6 log CFU/sample of Salmonella. Washing cantaloupes with DI water (control) removed loosely attached Salmonella by ~ 1 log CFU. Since DI water does not exert any antimicrobial effect, significant pathogen load was detected in wash water. This could lead to cross contamination of other cantaloupes during washing and is a significant food safety hazard. Washing cantaloupes with water containing UFO bubbles or chlorine for 1 min reduced Salmonella load on the cantaloupes by an additional ~1 log CFU (P<0.05). Increasing the wash time to 5 or 10 min did not increase the efficacy of antimicrobial wash treatments (P>0.05). No surviving Salmonella were detected in the wash water indicating that the presence of UFO bubble treatment in wash water significantly reduces the risk of cross contamination.
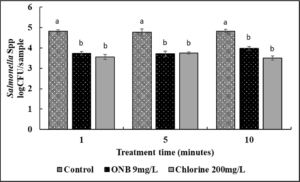
In case of lettuce samples (Fig. 12), a significant reduction in Salmonella load was observed on lettuce by ~ 1 log CFU as early as 1 min of wash time with UFO bubble treatment. No significant difference in the UFO bubbles and chlorine treatment was observed. The efficacy of antimicrobial washing with either UFO bubbles or chlorine treatment did not improve as wash time increased from 1 min to 5 or 10 minutes (P<0.05).
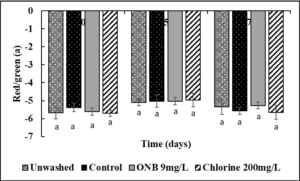
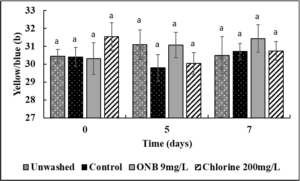
Effect of ONB and chlorine wash on cantaloupe (Fig. 13,14,15) and lettuce color (Fig. 16,17,18).
It is critical to ensure that the wash treatments do not negatively impact produce color. Using a standard protocol, the effect of UFO bubbles and chlorine wash (10 min time point) on cantaloupe and lettuce color was investigated during post-washing storage (0,5,7 days) at room temperature. No change in the lightness (L; Fig. 13), Red/green spectrum (a; Fig. 14) or yellow/blue spectrum (b; Fig. 15) was observed during storage of cantaloupes (P>0.05) indicating that the surface color of cantaloupe is not affected by the wash treatments. Similar results were observed with lettuce (Fig. 16, 17, 18) wherein no change in color of lettuce was observed after washing with UFO bubbles water.
In this project, we investigated the efficacy of UFO bubble water wash in killing the three major foodborne pathogens (Salmonella spp., Listeria monocytogenes and E. coli O157:H7) on eggs, cantaloupes and lettuce. Overall, our results indicate that Ultrafine ozone (UFO) bubble water at ~9 ppm is able to reduce pathogen load on the aforementioned produce without deleteriously affecting product color. Moreover, the UFO bubble water is similar in its efficacy to chlorine (200 ppm). It is noteworthy that although the dose of UFO bubble water is ~20 times less than chlorine, it is able to exert similar antimicrobial effect. Therefore, in our future research, we aim to focus on improving the antimicrobial efficacy of UFO bubble water by (1) increasing the dose of ozone (2) increasing the stability of bubbles in water by changes in pH of solution (3) combining UFO bubble water with other commercially available antimicrobial for potential synergy.
Education & Outreach Activities and Participation Summary
Educational activities:
Participation Summary:
The activities performed as part of the outreach activity in the project are described below.
Presentation:
In year 3, the PI and graduate student presented the work conducted in year 1, 2 at the New England Vegetable and Fruit Conference and Trade Show (https://newenglandvfc.org/media/pdf/N/E/V/NEVFC_2022_Proceedings_DRAFTFINAL.pdf).
Technology Demonstration at the UConn Extension’s 2023 Vegetable and Small Fruit Grower’s Conference:
A demonstration of the working of ozone-nanobubble generator to farmers was performed at the UConn Extension’s 2023 Vegetable and Small Fruit Grower’s Conference. The design and working of the Oxygen Concentrator-Ozone generator (Oxidation technologies, USA) connected to nanobubble generator (Aciniti, Japan) was demonstrated at the UConn Extension’s 2023 Vegetable and Small Fruit Grower’s Conference using a recorded video. The graduate student described the science of nanobubble generation, mechanism of the machine and results.
Newsletters and media mentions:
Drs. Abhinav Upadhyay, Indu Upadhyaya, Shuresh Ghimire developed a newsletter published in Cross Talk (Volume 18, Issue III — October 2022) wherein they described the Application of ultra-fine bubble technology to reduce Listeria monocytogenes contamination of Romaine lettuce.
https://ipm.cahnr.uconn.edu/wp-content/uploads/sites/3216/2022/10/Crop-Talk-October-2022-FV-reduced.pdf
Dr. Abhinav Upadhyay developed a media report wherein he described the ultra-fine bubble technology and its application in poultry food safety.
Learning Outcomes
The key areas in which farmers and educators reported change in knowledge, attitude, skills and/or awareness are as follows:
-Ultrafine ozone bubble technology.
-Survival capability of foodborne pathogens (Salmonella, Listeria monocytogenes, Escherichia coli O157:H7) on eggs, lettuce, and cantaloupes.