Progress report for LNE20-413R
Project Information
The ultimate goal of this project is to create a sink in local cucurbit pest populations by luring striped cucumber beetle (SCB), Acalymma vittatum F. (Coleoptera: Chrysomelidae) to baited traps, where they are captured and/or killed.
In 2020, we made progress in understanding the season-long attraction of a vittatalactone lure, or the “attract” component of our attract & kill system. In 2021 and 2022, we made progress in developing our experimental bait, or the “kill” component that includes a feeding stimulant (cucurbitacin) and a poison (spinosad). In 2022 and 2023, we worked to develop a synergist for improved chemical control of cucurbit insect pests. Poor pest pressure at field sites meant that we were unable to confirm the efficacy of early intervention on season-long impacts in large-scale experiments. However, we have confirmed that the components of our system effectively attracted and killed or captured all key insect pests in this system, without strong vicinity effects, including striped cucumber beetle (SCB), spotted cucumber beetle (SpCB), Diabrotica undecimpunctata Mannerheim (Coleoptera: Chrysomelidae) and adult & nymph squash bug (SB), Anasa tristis De Geer (Hemiptera: Coreidae), and squash vine borer Melittia cucurbitae Harris (Lepidoptera: Sessiidae).
While the results of our project's research objectives did not yield actionable practices, we shared project results with farmers via various outreach activities and improved knowledge in general principles including but not limited to monitoring for IPM and adopting behavioral control practices for insect management in cucurbits.
The ultimate goal of this project is to develop an effective and adoptable attract-and-kill (A&K) approach for managing striped cucumber beetle (SCB), using attractive odors, selective feeding stimulants, and the judicious use of bee-safe insecticides.
Problem, Novel Approach and Justification:
Striped cucumber beetle (SCB) is a serious pest of cucurbit crops, affecting farmers growing cucumber, melon, squashes, and pumpkin in the northeastern US, representing 56,159 harvested acres, or 18,583 operations, in the NESARE region. While effective chemical controls are available in conventional systems, concern for pollinators visiting these pollinator-dependent crops has driven demand for non-chemical or bee-safe alternatives. Behavioral controls can replace chemical controls, or be integrated with other tools and tactics to achieve control with a dramatic reduction in insecticide use and unwanted non-target effects.
Attract-and-kill (A&K) approaches lure pest insects to a source of poison, which is often combined with an arrestant or feeding stimulant. Here we worked to develop and field test an A&K approach using SCB aggregation pheromone (vittatalactone) to lure beetles to a bait station containing a feeding stimulant (cucurbitacin) laced with a gut poison (spinosad). Our research approach evaluated components of various A&K approaches and relied on a combination of laboratory bioassays, controlled field experiments conducted at research farms, and large-scale data collection at commercial farm sites.
Background & Rationale
While adult SCB will often consume nectar and pollen from a broader range of host plants, members of the Cucurbitaceae family are required for their successful reproduction. Adult beetles overwinter under leaf litter in wild habitats and emerge in the spring to feed and find mates. Eventually they move into cultivated cucurbit crops, often in response to crop flowering. Male striped cucumber beetles emit an aggregation pheromone, vitattalactone, that aids in attracting both male and female conspecifics to hosts as well. Females then lay their eggs at the base of their host plant, and the subsequent larvae burrow down in to the soil to feed on roots until the pupate and emerge in a new generation of adults. In the northeastern United States, SCB will typically complete two generations but longer seasons can allow for a third generation from time to time.
Male striped cucumber beetles emit an aggregation pheromone, vittatalactone (2-methyl-3-(2,4,6,8 tetramethyloctyl) oxetan-3-one), when feeding on a favored host. Chauhan and Paraselli (2017) devised and patented a synthesis that produces eight stereoisomers of vittatalactone with the ring configuration (2R,3R), including the one active isomer. Mixed vittatalactone (eight stereoisomers of (2R,3R)-vittatalactone) has proved highly attractive to both sexes of SCB. This semistereospecific synthesis (i.e. that produce an isomeric mix at three chiral centers, of the five in the molecule) enables less expensive production of the SCB aggregation pheromone in quantities useful for field testing and application.
Mixed vittatalactone lures were found to attractive to SCB, SpCB, and (surprisingly) squash bug adults and nymphs in preliminary testing during the fall of 2019 (Brzozowski et al. 2022). While replication is necessary to confirm this finding, our cheaper, “new and un-improved’ lure may be an important component for management of multiple key pests of cucurbit crops.
Cooperators
Research
- Understanding vicinity effects associated with bait stations (NH, MD):
- What is the size of the area of arrestment around vittatalactone lures?
- How does the addition of bitter watermelon affect the toxicity of a gut poison?
- How long will our bait last in the field before the poison degrades?
- Does the addition of poison bait mitigate spillover injury near vittatalactone lures?
- Deploying A&K in field (NH, VT, ME):
- Can A&K maintain beetle densities below threshold on nearby plants?
- Can A&K deployed in crop borders achieve the same control as a grower control?
This project attempted to evaluate efficacy of an attract & kill approach for striped cucumber beetle in large-scale field trials, as well as many experiments addressing questions regarding potential components of this system (Fig. 1). Initial project plans included experiments conducted at research farms in NH & MD and commercial farms in NH, VT, and ME. We have also coordinated our efforts with on-going cucumber beetle research outside of our region, and will include pertinent details regarding unpublished results from our collaborations with researchers at South Dakota State University, Virginia Tech University, Cornell University, and Trécé Inc.
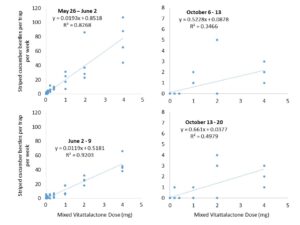
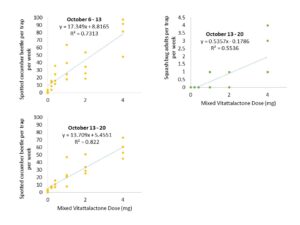
Dose-Response Trials.
Our lure contains a lab-synthesized version of cucumber beetle aggregation pheromone (vitattalactone), which evaporates from our trap to create an attractive odor plume. The potency of our lure could be affected by the concentration of this compound, which is affected by the initial dose applied to our lures. If the dose is too low, the beetles won’t be able to detect it. If the dose is too high, the beetles might not find it attractive.
In order to understand how the dose affects beetle response, we deployed clear sticky traps baited with various concentrations of vitattalactone in farmland with a history of cucumber beetle infestation (Beltsville, MD). We observed how many beetles were trapped weekly. We also changed traps, refreshed lures, and re-randomized treatment locations to replicate the experiment in the spring and fall of 2020.
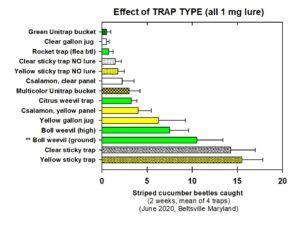
Trap Design Trials.
Our lure is a powerful attractant for striped cucumber beetle, spotted cucumber beetle, and squash bug but we must identify a reliable method of capturing or killing the pest insects lured to aggregation sites in order to avoid vicinity effects.
In order to identify the best trap, we deployed various trap types in farmland with a history of cucumber beetle infestation (Beltsville, MD). Traps were baited with 1 mg vitattalactone, unless they were un-baited as a control. We observed how many beetles were trapped weekly. We also changed traps, refreshed lures, and re-randomized treatment locations to replicate the experiment.
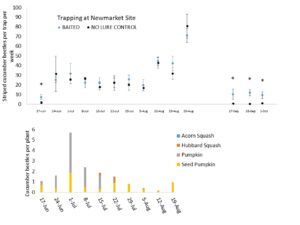
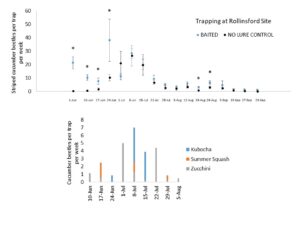
Season-long Lure Trials.
We deployed 8 sticky traps at each of two commercial farm sites in Newmarket & Rollinsford, NH through the 2020 growing season. Half of these traps were baited with 1 mg vittatalactone lures and the other half were unbaited as control. We changed traps, refreshed lures, and re-randomized treatment locations to replicate the experiment through the growing season. We also observed several crops for beetle densities through this same period. At least 20 plants of each crop type were observed each week for the number of striped cucumber beetles, the number of spotted cucumber beetles, the number of squash bug egg masses, nymphs, and adults, signs of injury, and signs of bacterial wilt. We observed the undersides of leaves for at least 30 seconds per plant, if not all leaves. We supplied weekly reports of pest observations to growers, who treated all plants according to their wishes.
Early-Season Intervention Trials.
In 2020, at three farms (VT, NH, ME), we deployed vitattalactone-baited boll weevil traps in the perimeter of one side of a plot of winter squash to see if early season trapping would remove enough pest insects from the system to result in different pest densities on plants through the season. Traps were 10 m apart and the “treatment” end of each field was at least 50 meters from the “control” end. Weekly visits were made to each site to scout each side of each field for striped cucumber beetles, spotted cucumber beetles, and squash bug egg masses, nymphs, and adults. For each field, we observed the number of beetles on 20 plants on the perimeter and 20 plants in the interior of each plot. The undersides of the leaves were examined on each plant for at least 30 seconds. We supplied weekly reports of pest observations to growers, who treated both plots according to their wishes, and growers supplied us with their spray records at the end of the season.
In 2021, we repeated a similar experiment at five farms (VT, NH, ME), where we deployed vitattalactone-baited stations treated with a feeding stimulant and toxicant in the perimeter of one side of a plot of winter squash; traps were 10 m apart and the “treatment” end of each field was at least 50 meters from the “control” end. Bait stations (Fig. 2) were deployed within a week of planting/transplanting and maintained for 4-5 week, in order to poison insects as they migrated into crop space from overwintering sites. This invasion period typically occurs in June or July and is typically well timed with flower initiation in the crop. We reapplied the feeding stimulant (bitter watermelon juice) and toxicant (spinosad/Entrust) every week and replaced lures (1 mg vittatalactone) every other week .
In 2022, we repeated a similar experiment at four farms (VT, NH), where we deployed 4 different traps/bait stations in the perimeter of a winter squash crop and collected additional field data to compare potential spillover effects of our trap designs. Bait stations (Fig. 2) were deployed within a week of planting/transplanting and maintained for 4-5 week, in order to poison insects as they migrated into crop space from overwintering sites. This invasion period typically occurs in June or July and is typically well timed with flower initiation in the crop. We reapplied the feeding stimulant (bitter watermelon juice) and toxicant (spinosad/Entrust) every week and replaced lures (1 mg vittatalactone) every other week .




We made weekly visits to each site to count the number of pest insects captured or killed by bait stations, and to scout treatment and control plots for striped cucumber beetles, spotted cucumber beetles, and squash bug egg masses, nymphs, and adults. For each plot, we observed the number of beetles on 20 plants, by examining the undersides of all leaves on each plant, or for at least 30 seconds. We supplied weekly reports of pest observations to growers, who treated both plots according to their wishes, and supplied us with their spray records at the end of the season.

Bait Station Efficiency.
In 2022 & 2023, we conduced bioassays to compare the efficiency of trapping methods used in previously described field components. Here we sought to evaluate what proportion of SCB attracted to a zone of aggregation would interact with the bait station or trap in a way that would lead to their death or capture. Insects were collected from insecticide-free host plants July-August and cohorts of 10-20 SCB adults (50:50 male:female) were introduced to screen cages (12x12x12 cm) containing a water wick and one of our bait stations. After 24 h, we observed the number of living and dead or captured beetles in each cage. We replicated this experiment on three dates for a total n = 6.
Roller Bait Station Experiment.
The experimental bait station included in 2021 field experiments includes several components. In order to understand the importance of each component, we attempted to “deconstruct” a bait station with three bait components, all accompanied by an organic-approved insecticide restricted to the bait itself. We evaluated how many pest insects were captured/poisoned by bait stations with or without vittatalactone lure (standard 1mg), and sweet watermelon juice with or without added bitter component (cucurbitacin-E-glycoside; 0.05% v/v).

Cucurbitacin Content of Melon Juice.
In order to make the bait we used in field trials, we crushed and juiced bitter Hawkesbury watermelons grown in UNH greenhouses from Jan-June 2021 or at USDA-ARS research farm in Beltsville Maryland summer 2020. Juice was frozen at -20°C until use, when it was thawed at room temperature.
In addition to producing the raw bitter melon juice for our experiments, we have partnered with Trécé Inc. and researchers at South Dakota State University and Virginia Tech University to develop an insecticide synergist containing water-soluble cucurbitacin. This feeding stimulant (tradename CideTrak L) is derived from the fruit of bitter Hawkesbury watermelon. However, currently available genetic stocks are highly variable in terms of their cucurbitacin content. We conducted a preliminary experiment that determined a wide range in cucurbitacin content in the fruit juice we were relying on for our field experiments (0-1.5 mg/L). While some melons accumulated very high concentrations, we could not detect our compound of interest in ~25% of the lines we tested. For all subsequent field experiments in 2021, we used only the fruit juice of those high producing lines.
Furthermore, we conducted experiments to evaluate the role of parentage and fruit maturity on the cucurbitacin content of fruit grown from seeds of "high accumulators" and "low accumulators". Cucurbitacin synthesis occurs in the leaf tissue but, in order to avoid autotoxicity, the plant will divert these compounds and accumulate them in some other storage structure. For bitter Hawkesbury watermelon, cucurbitacins are accumulated in the fruit. We therefore wanted to investigate the potential that plants from high and low lines were given the appropriate about of time to accumulate cucurbitacins in their fruit by harvesting at various levels of maturity, i.e. early (31±3 d after pollination), at maturity (45±3 d after pollination), past maturity (59±3 d), and well past maturity (73±3 d). We replicated this experiment with 3 high lines and 3 low lines, 9 plants per line, in greenhouse experiments where plants were self-pollenated by hand, and in field experiments relying on open pollination. Watermelon slices (1 cm thick) were cut from the center of each melon, frozen at -20C until shipped to SDU, where cucurbitacin analysis was carried out by Fathi Halewiesh & Trevor Ostlund.
Bait Efficacy in Foliar Applications.
While our project originally aimed to develop A&K bait stations, there is a potential benefit in deploying bait sprays (foliar application of insecticide mixed with cucurbitacin). Efficacy trials were conducted to evaluate the potential benefit of including bitter watermelon juice or a commercial cucurbitacin product (CideTrak) in cucumber (Painter, VA & Durham, NH), summer squash (Painter, VA), and melons (Whitethorne, VA). Treatments were randomly assigned to 20’ plots in an RCB design with 4 replicates. Experiments conducted in Virginia are reproduced with permission from Kuhar et al. (2022; https://www.pubs.ext.vt.edu/content/pubs_ext_vt_edu/en/author/k/kuhar-thomas_p.resource.html). In VA, all foliar treatments were applied with a 3-nozzle boom equipped with D3 spray tips and powered by a CO₂ backpack sprayer at 40psi delivering 30 gpa. In NH, treatments were applied using a hand-pump applicator with one nozzle, delivered using approximately 100 gpa.
In 2022, we conducted a similar experiment in Durham, NH in order to evaluate the potential benefit of adding a feeding stimulant to foliar applications. In this experiment, we compared the number of striped cucumber beetles, spotted cucumber beetles, and squash bug egg masses, nymphs, and adults in plots treated with foliar applications of Entrust, Assail, or Lambda Cy versus the same rates plus the high rate of CideTrak L applied as a dribble next to the plants (presumably lower risk to non-target insects). We repeated this experiment in zucchini, cucumber, and melon crops. We also destructively sampled our zucchini experiment in order to assess squash vine borer damage.

Bioassays.
In 2022 & 2023, we conducted leaf dip bioassays in order to evaluate feeding stimulation from bitter melon juices and excised leaf bioassays to confirm toxicity of our experimental baits. Leaves collected from greenhouse-grown cucumber and bean plants were dipped in bitter melon juice extracts of variable cucurbitacin content. Cucumber leaves were collected from the previously described 2022 Bait Efficacy Trial to evaluate efficacy of foliar treatments over time, 1 DAT & 4DAT. Insects were collected from insecticide-free host plants July-August and 1-10 SCB adults (50:50 male:female), isolate with water for 24 h prior to bioassays, then confined for 24 h with treated leaf tissue in a Petri dish (90 mm diameter). We assessed mortality and leaf area consumed. Dead SCB did not move when prodded with a probe.
Dose-Response Trials.
We concluded the following (Figs. 4 & 5):
- An initial dose of 1 mg vitattalactone is appropriate for further testing.
- We found a positive linear dose-response for striped cucumber beetle, spotted cucumber beetle, and squash bug, with no signs of repellency in high concentrations.
- This dose-response is strongest in the early season, before host plants are present.
Trap Design Trials.
We concluded the following (Fig. 6):
- We found that sticky traps, boll weevil traps, and yellow gallon jug traps captured more striped cucumber beetles than unbaited traps, and captured more beetles than other baited designs.
- Given concerns regarding non-target trapping of beneficial hymenopterans, we opted not to use yellow sticky cards or yellow gallon jug traps in further testing, and continued our testing with clear sticky cards and boll weevil traps.
Season-long Lure Trials.
We concluded the following (Fig. 7 & 8):
- When there are not actively growing crops, significantly more beetles were captured by baited traps than un-baited controls.
- Stimuli from crop plants likely outcompete our man-made attractants at this trap density.
Early-Season Intervention Trials.
The efficacy of our A&K approached was found to be inconclusive, despite successfully killing/capturing large numbers of pest insects at all farm sites (Figs 9&10), with no indication of problematic vicinity effects (Figs 11&12).
Bait Station Efficiency Assays.
We found no significant difference in the number of beetles that would theoretically be killed or captured by either of our bait stations or the standard clear sticky trap, while boll weevil traps are significantly less efficient according to an ANOVA blocked by experiment (F = 6.5, p = 0.0036; Fig. 13).
Roller Bait Station Experiment
We evaluated how many pest insects were captured/poisoned by bait stations with or without vittatalactone lure (standard 1mg), and sweet watermelon juice with or without added bitter component (cucurbitacin-E-glycoside; 0.05% v/v).
- Both SCB and SpCB were captured by the traps.
- Striped CB captures were most strongly influenced by presence of their own pheromone, which resulted in a 14-fold increase in captures. Melon juice and cucurbitacin also increased captures, but only 4-fold each (Table 1).
- In contrast, spotted CB responded most strongly to the presence of cucurbitacin, but also to vittatalactone and melon juice (Table 2).
The roller bait traps rely on a combination of all three factors: pheromone lure (presumably for long-distance volatile attraction), melon juice (presumably augmenting the volatiles, and possibly also encouraging bait consumption), and cucurbitacin (a known feeding stimulant and arrestant with no volatility). Both species rapidly succumbed to the 0.4% Entrust, which is a fraction of the field rate for a foliar spray application. This material is registered in all cucurbits but does not have either cucumber beetle on the label. However, when ingested, as in this bait, it is very effective.
This preliminary trial shows not surprisingly that vittatalactone is the most important factor in capture of striped cucumber beetles, since it is their aggregation pheromone. Since 2020 we have known that SpCb and SB are attracted to vittatalactone (Brzozowski et al. 2022), presumably eavesdropping on the signal from the other species, so the 6-fold attraction of SCB to this pheromone is not surprising in that context. Spotted cucumber beetles are even more influenced as to trap captures by the presence of the cucurbitacin, at an almost 13-fold rate.
Table 1: Proportions of striped cucumber beetle captured by roller bait traps, October 2021, Beltsville, MD. Total captured n=60.
|
striped cuke btl |
PLUS |
MINUS |
MULTIPLE |
uncorrected P< |
proportion |
lower95 conf.int. |
upper95 conf.int. |
|
PHERO |
56 |
4 |
14.0 |
0.0001 |
0.933 |
0.838 |
0.982 |
|
MELON |
48 |
12 |
4.0 |
0.0001 |
0.800 |
0.677 |
0.892 |
|
CUX |
48 |
12 |
4.0 |
0.0001 |
0.800 |
0.677 |
0.892 |
|
TOTAL = 60 |
Table 2: Proportions of spotted cucumber beetle captured by roller bait traps, October 2021, Beltsville, MD. Total captured n=824.
|
spotted cuke btl |
PLUS |
MINUS |
MULTIPLE |
uncorrected P< |
proportion |
lower95 |
upper95 |
|
PHERO |
707 |
117 |
6.0 |
0.0001 |
0.858 |
0.832 |
0.881 |
|
MELON |
491 |
333 |
1.5 |
0.0001 |
0.596 |
0.562 |
0.630 |
|
CUX |
764 |
60 |
12.7 |
0.0001 |
0.927 |
0.907 |
0.944 |
|
TOTAL = 824 |
Cucurbitacin Content of Melon Juice.
While fruit maturity did not have an effect, according to a generalized log-linear model assuming a Poisson distribution, we found significant effects of parental line in greenhouse trials where plants were self-pollinated by hand in 2021 (F = 16.2, p = 0.006) and 2022 (F = 17.6, p = 0.0002), as well as in open-pollinated field trials (F = 11.7, p = 0.0018; Fig. 14).
Bait Efficacy in Foliar Applications
Efficacy trials found no added benefit of including bitter watermelon juice or a commercial cucurbitacin product (CideTrak) to foliar applications of standard crop protection materials in cucumber (Painter, VA; Fig. 15), summer squash (Painter, VA; data not shown), and melons (Whitethorne, VA; data not shown).
In 2022, we compared control of foliar applications to the same material combined with CideTrak, but dribbled the same rate/volume per plot next to the plant rather than applied to the crop (zucchini, melon, cucumber; Durham, NH). Pest pressure was too low to draw any conclusions about management of SCB. However, we found that SCB adults were likely more attracted to plots with CideTrak additions, which may have made infestations worse than controls (Fig. 16). When we destructively sampled zucchini plots to observe squash vine borer infestations, we found that all of our treatments had fewer squash vine borer larvae than control plots (Fig. 17). This was a surprising and encouraging finding, and to our knowledge, the first record of this feeding stimulant having activity on this species.
Excised Leaf Bioassays.
While there were numerical differences in mortality between bioassays conducted with treated plant tissue 1DAT & 4DAT, there was significantly higher mortality in all treatments compared to controls 1DAT and 4DAT Fig 18).We found no difference in the amount of leaf tissue consumed by beetles after 24 h.
Most of the products evaluated in this project are not commercially available, therefore we are not concluding this project with any grower recommendations. This work does confirm several aspects of cucurbit IPM that should be held in mind when growers are attempting any behavioral control approach, including trap cropping, mass trapping, and attract & kill:
- Behavior of all pests in this complex can be manipulated while insects are moving between overwintering sites and their host plants in the spring and fall. Insects cannot be drawn away from a crop once they have established.
- Flowering cucurbit crops tend to be more attractive than attract & kill components.
- Pest aggregations must be managed in some manner to avoid unwanted spillover effects. Chemical pesticides may be necessary when populations are high. Cultural control tactics are appropriate to suppress populations over many years.
Education & Outreach Activities and Participation Summary
Educational activities:
Participation Summary:
Consultations included one-on-one education or recommendations made by the team, or via Extension specialists in our network, regarding topics on general cucurbit IPM or using behavioral controls to manage cucumber beetles.
Journal articles are in various stages of the submission process, including:
- Pasteur, Khrimian, Guzman, Haber, Weber. Dose and trap type affect response of striped and spotted cucumber beetles to synthetic vittatalactone. In prep for Environmental Entomology.
- Weber, Ostlund, Halaweish, Wallingford, Haber. Effect of cucurbitacin B glycoside, vittatalactone, and watermelon juice on bait stations for capture of cucumber beetles. In prep for Journal of Economic Entomology.
- Weber, Wallingford, Pasteur, Khrimian, Guzman, Haber, Boyle, Shee, Kuhar, Kaplan. Season-long attraction of synthetic vittatalactone to striped cucumber beetle (Acalymma vittatum), spotted cucumber beetle (Diabrotica undecimpuntata howardi) and squash bug (Anasa tristis). In prep for Environmental Entomology.
- Wallingford, Hernandez, Goyette, Weber. Influence of parentage and growing practices on cucurbitacin content of bitter Hawkesbury watermelon, feedstock for an insecticide synergist. In prep for Agrochemicals.
Webinars, talks, presentations is an estimate of the number of annual "research updates" from project activities that were included along with regular grower meetings/webinars, including but not limited to:
- Webinar on fruit & vegetable IPM hosted by New England Vegetables & Berry Growers (nevbga.com/winter-growers-meetings/). February 2022.
- In-person "Vegetable IPM Season Kickoff" Workshop, featuring talks on cucurbit breeding and IPM. UNH's Merrimack County Extension Office. April 2022.
- In-person workshop hosted by Maine Organic
Farmers & Gardeners Association Farmer to Farmer Conference. Belfast, ME. November 2022.
Other Educational Activities includes all other outreach efforts where NESARE funding was acknowledged, including but not limited to scientific presentations and outputs directed to the general public:
- Symposium on "Novel tools and opportunities for cucurbit IPM in North America". International IPM Symposium. Denver, CO. March 2022.
- Wallingford, A. 2022. Attract-and-Kill strategies for sustainable striped cucumber beetle management. Northeast IPM Center Online Conference (www.northeastipm.org/index.cfm/about-us/publications/ipm-online-conferences/2022-northeast-integrated-pest-management-research-update-conference/). March 2022.
- Wallingford, A., S. Lewins, V. Izzo, and D. Weber. 2023. Regulatory barriers to adoption of behavioral controls. In Member symposium: Current Issues in Agricultural Pest Management. Eastern Branch Annual Meeting of the Entomological Society of America. Providence, RI. March 2023.
- NHAES MacFarlane Greenhouse Open House (colsa.unh.edu/new-hampshire-agricultural-experiment-station/news-events/macfarlane-research-greenhouses-open-house). April 2022, 2023.
- Instagram @Wallingbug @UNHExtension @NHAES
- Lab website: wallingfordlab.weebly.com/
Number of farmers/ranchers who participated in education and outreach activities is an estimate that includes farmers who participated in the project, as well as farmers who we interacted with during "research updates" at regular grower meetings/webinars.
Number of agricultural educator or service providers who participated in outreach activities is an estimate of Extension professionals that we worked with directly and those who attended scientific presentations.
Learning Outcomes
While the results of our project's research objectives did not yield actionable practices, we shared project results with farmers via various avenues through out the project. Among cucurbit growers participating in the project, growers attending regular UNHCE meetings, growers within our county specialists' networks, we observed changes in knowledge and behavior regarding the following basic principles:
Monitoring for IPM. We provided weekly reports to our collaborators following each visit, which provided them with pest monitoring data they used to make IPM decisions. These reports typically resulted in no action, delayed pesticide application or eliminated pesticide application. In some instances, we identified early incidence of cucurbit downy mildew, which resulted in timely pesticide application. We often highlight regional monitoring networks for tracking disease incidence at grower talks, including the website that hosts cucurbit downy mildew incidence, and assume some level of gain in awareness among our farmer community.
Behavioral controls for managing cucumber beetle attacking cucurbits. While informal updates on our research did not yield actionable practices, we did present "refresher" content about using trap cropping to control key insect pests. Data on trap crop adoption was not collected, but at least 3 educators reported recommending behavioral controls to their clients, and we commonly received feedback on an increased awareness of host preference in the field. Cucurbit growers reported seeing more pest pressure on preferred crop species and opted to focus on those crops for pest management efforts (physical & chemical controls) instead of whole fields, due to connections made in our research updates.
Project Outcomes
The following manuscripts are in preparation for publication in peer reviewed journals:
Pasteur, Khrimian, Guzman, Haber, Weber. Dose and trap type affect response of striped and spotted cucumber beetles to synthetic vittatalactone. In prep for Environmental Entomology.
Weber, Ostlund, Halaweish, Wallingford, Haber. Effect of cucurbitacin B glycoside, vittatalactone, and watermelon juice on bait stations for capture of cucumber beetles. In prep for Journal of Economic Entomology.
Weber, Wallingford, Pasteur, Khrimian, Guzman, Haber, Boyle, Shee, Kuhar, Kaplan. Season-long attraction of synthetic vittatalactone to striped cucumber beetle (Acalymma vittatum), spotted cucumber beetle (Diabrotica undecimpuntata howardi) and squash bug (Anasa tristis). In prep for Environmental Entomology.
Wallingford, Hernandez, Goyette, Weber. Influence of parentage and growing practices on cucurbitacin content of bitter Hawkesbury watermelon, feedstock for an insecticide synergist. In prep for Agrochemicals.
The current paradigm for cucurbit IPM relies on foliar applications of broad-spectrum insecticides and/or the use of physical barriers (i.e. row cover) and good sanitation practices. This project found encouraging evidence that pest behavior could be manipulated using attractive stimuli without increasing pest risk. However, we have not found convincing evidence that the approaches evaluated here are reliable enough to replace the current standard approach.
Future work will seek to identify approaches that are comparable to the industry standards in terms of labor required for deployment and reliable pest control.