Progress report for LNE22-450R
Project Information
Due to a short growing season, late season root crops (e.g., potatoes, sweet potatoes, etc.) are particularly valuable in northeast diversified farming systems. Reduced yield and quality due to infestations by the common insect pests, Colorado potato beetle and wireworm, represents a serious risk for many farmers relying upon the late season revenue provided by these crops. Furthermore, sustainable low-impact options for the control of these pests on both organic and conventional farms are generally limited as most farmers rely heavily upon chemical controls. The high usage of chemical pesticides for the management of CPB and wireworms can significantly increase the risk of pesticide resistance. The development and assessment of novel pest management strategies, as proposed in this proposal, will provide valuable knowledge for the expansion of the currently available IPM toolbox for Northeastern potato growers.
This project will investigate two safe and eco-friendly approaches to reduce wireworm and CPB infestations in root crop plantings. The genesis of this project is the direct outcome of an ongoing participatory action research (PAR) process that currently engages ~30 growers in the Vermont and New York regions. During our recent PAR meetings and indicated via distributed surveys, it became apparent that growers (especially organic growers) are consistently looking to add new and novel techniques to reduce their dependence on the limited number of effective chemical controls (e.g. entrust, neonicotinoids etc.). To best address the growing need for low-impact biorational controls, this project looks to develop and test a benchtop/on-farm formulation of entomopathogenic fungi for the control of both wireworm and CPB. In addition, we will explore the efficacy of a commercially available RNAi product as a potential IPM tactic for conventional growers.
Entomopathogenic fungi Beauveria bassiana and Metarhizium brunneum will be the primary species of fungi that will be cultured for our field trials. These species represent the most effective and easily cultured species of entomopathogenic fungi. For our benchtop formulations we will culture each fungus on commonly available grain substrates (e.g. Millet, barley, etc.). This will allow for the selection of the most cost-effective and efficient substrates for farmers looking to culture their own fungal soil applications.
We will utilize lab experiments, field trials and participatory evaluations to test the following hypotheses: a) grains inoculated with entomopathogenic fungi (I.e. benchtop culturing) will reduce wireworm pressure when applied as a soil application; b) foliar sprays of entomopathogenic fungi and commercially available RNAi will significantly reduce CPB pest pressure in potato; and c) training framers to cultivate their own fungal inoculated grain will significantly reduce the cost of control and expand the IPM toolbox for growers looking to reduce their chemical dependency.
Lab and field trials will be conducted to assess the efficacy of both cultured entomopathogenic fungi and RNAi technologies for the control of wireworms and Colorado potato beetle in root crops. This study will aid in the development of the essential knowledge and skills needed for the effective low-tech culturing of entomopathogenic fungi and subsequent field applications to control these pests. Data generated from RNAi trials will directly inform conventional potato growers of the best practices for utilizing RNAi technologies for CPB control. Both entomopathogenic fungi and RNAi applications will provide growers with innovative strategies to expand their IPM toolbox.
Research
The primary experiments to be conducted:
- Lab trials to assess the most efficacious and cost-effective substrate for both B. bassiana and M. burnneum
- Field trials to test the efficacy of granular formulations of the entomopathogenic fungi, B. bassiana and M. burnneum for the control of CPB and wireworms in potato and sweet potatoes, respectively.
- To develop low-tech protocols for on-farm culturing of B. bassiana and M. burnneum
- Testing the efficacy of commercially available RNAi products for the control CPB on conventional Northeastern diversified farms.
Hypothesis I
Treatments: The lab trials (Table 1) will include formulation of B. bassiana and M. burnneum on different carriers (couscous, millet, and barley). The formulations will be evaluated based on their availability, price, and efficacy, to be considered as a potential root crop pest control.
Mycotized grains application method in furrows has been reported to be as effective as a chemical treatment for the control of soilborne insects. To make mycotized millet, pure inoculums of both fungi will be obtained by culturing the commercial products of B. bassiana (BotaniGard®ES, GHM; BioWorks, Victor, NY, USA) and M. burnneum (Met52®EC and Met52®G, Novozymes Biologicals, Franklinton, NC, USA) on Potato Dextrose Agar (PDA) and Sabouraud Dextrose Agar (SDA) respectively. The fungal formulation will be prepared according to a protocol by Kim et al (2014) by inoculating cocked couscous, millet (Panicum miliaceum L.), and barley (Hordeum vulgare L.). Quality control will be measured by examining conidial germination and condial count. A lab bioassay on wireworm larvae and CPB will be conducted to assess the most effective treatments. The bioassay will include 10 replications of each treatment and control (Table 1.). Approximately 2g of mycotized grains formulation will be added to each treatment. All treatments will be inspected daily to measure mortality rate. The most effective treatments for both fungi will be selected to apply in the field trails (Table 1).
To prepare fungal suspension we will follow the label instruction of the commercial products for B. bassiana and since Met52 is no longer available on the market we will use 108 conidia per milliliter.
Table 1. Treatments, substrates, target organisms, and application types.
|
Treatments |
Target organism |
|
Application |
|
Lab trials |
|||
|
Control (Water) |
Wireworms & CPB |
|
|
|
Couscous (C) |
Wireworms & CPB |
|
|
|
Millet (M) |
Wireworms & CPB |
|
|
|
Barley (B) |
Wireworms & CPB |
|
|
|
Beauveria bassiana GHA couscous (BBC) |
Wireworms & CPB |
|
|
|
Beauveria bassiana GHA millet (BBM) |
Wireworms & CPB |
|
|
|
Beauveria bassiana GHA barley (BBB) |
Wireworms & CPB |
|
|
|
Metarhizium brunneum F52 couscous (MMC) |
Wireworms & CPB |
|
|
|
Metarhizium brunneum F52 millet (MMM) |
Wireworms & CPB |
|
|
|
Metarhizium brunneum F52 barley (MMB) |
Wireworms & CPB |
|
|
|
Field trials |
|||
|
Control (grain) |
Wireworms and CBP |
|
In furrow |
|
Most effective mycotized grain (B. bassiana) |
Wireworms and CBP |
|
In furrow |
|
Most effective mycotized grain (M. burnneum) |
Wireworms and CBP |
|
In furrow |
|
Control (Water) |
CPB |
|
On plants |
|
B. bassiana GHA suspension |
CPB |
|
On plants |
|
M. brunneum F52 suspension |
CPB |
|
On plants |
|
|
|
|
|
Methods:
Experimental design: The experiments will be established in a randomized complete block design on three commercial vegetable farms and two UVM-associated research farms – the UVM Horticultural Research and Education Center in South Burlington, VT and Borderview Research Farm in Alburgh, VT. There will be 15 sweet potato plots and 30 potato plots at each location, including five replicates of control and treatments at each site (Table 1). Same treatments and experimental design will be re-applied in year two.
Experimental unit size: An individual plot size will be 10bdft with a buffer zone of 10bdft between the plots.
Treatment application: To target wireworms, fungal treatments will be applied with in furrow at planting at the rate of 5 g per plot. Fungal suspension will be sprayed on plants to control CPB. All the treatments will be applied three times during the season with three weeks intervals.
Data Collection:
For the field trials (both fungi and RNAi applications), data collection will be conducted by measuring above and below ground larval feeding damage and marketable yield data. Wireworms plant damage estimation will be measured by harvesting 20 sweet potatoes in each plot to collect larval damage (# wireworm mines) and marketable yield (lbs) data. Wireworm density will be measure by establishing bait traps buried in 8-15 cm deep, covered with soil in each plot. Bait traps will be prepared by soaking wheat and barley in water for 24 hours to make the seeds sprout. Wireworms in each trap will be counted separately and identified to the species. To measure CPB plant damage, all potatoes from each plot will be harvested and weighed as total kilograms of potatoes per meter of row. To estimate CBP population, we will collect 10 stems (20–25 cm in length) from random locations within each replicate plot three times during after application of the treatments with four weeks intervals. We will count all live larvae on each stem and categorized as early (first and second) or late (third and fourth) instars.
Hypothesis II
Study Population(s)
Our population will be Northeastern vegetable growers since they are the primary beneficiaries of the project.
Methods
We will provide a low-tech protocol for fungal formulation including 1) preparing a workbench; 2) media preparation: filtering cooked potato through a cheese cloth, add agar, dextrose, and water; 3) sterilization procedures for media and grains: sterilizing the media and grains in 121°C using pressure cooker; 4) grain inoculation: inoculation of sterilized grains by fungi; and 5) maintaining the cultures. We will host workshops in years one and two where we will facilitate a farmer-to-farmer training. Data Collection:
We will design questionnaires and distribute them to the farmers engaged in the training to evaluate the feasibility of the protocol. We will improve the protocol according to trainees’ expressed challenges and suggestions. Trainees’ fungal formulation will be assessed based on contamination and purity of the cultures.
Data Analysis and Presentation of Results:
None needed for this Hypothesis.
-------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Hypothesis III
Treatments:
RNAi product will be purchased from AgroRNA (South Korea). Quality and quantity of the product will be measured using agarose gel electrophoresis and NanoDrop.
Methods:
Experimental design: We will apply RNAi product in complete randomized blocks design at two UVM-associated research farms – the UVM Horticultural Research and Education Center in South Burlington, VT, and Borderview Research Farm in Alburgh, VT. This experiment will be repeated in the second year of the project.
Experimental unit size: An individual plot size will be 10bdft with a buffer zone of 10bdft between the plots.
Treatment application: RNAi product is in liquid form. We will apply AgroRNA following the labeled rate (not available at this time).
Data Collection:
CPB plant damage and population count will be measured as described in hypothesis I.
Data Analysis and Presentation of Results:
We will use the same described in hypothesis I to analyze the data and compare control with the RNAi treated trails.
Field Season 2023:
Hypothesis I: Benchtop granular formulations of B. bassiana and M. burnneum will significantly attract and infect wireworms and CPB larva and result in reduced pest pressure
Lab trail:
Throughout April, May, and June, an extensive array of traps baited with sprouted wheat enclosed in nylon stockings were deployed at the Intervale Community Center, Bear Root Farm, and Burnt Rock Farm to assess wireworm activity. Subsequently, all captured wireworms underwent morphological identification and were relocated to an insect cage within a greenhouse setting. In January 2024, a bioassay is planned to be carried out on the wireworm population in the colony, utilizing both Beauveria bassiana and Metarhizium burnneum.
Field Trial:
- Wireworm traps
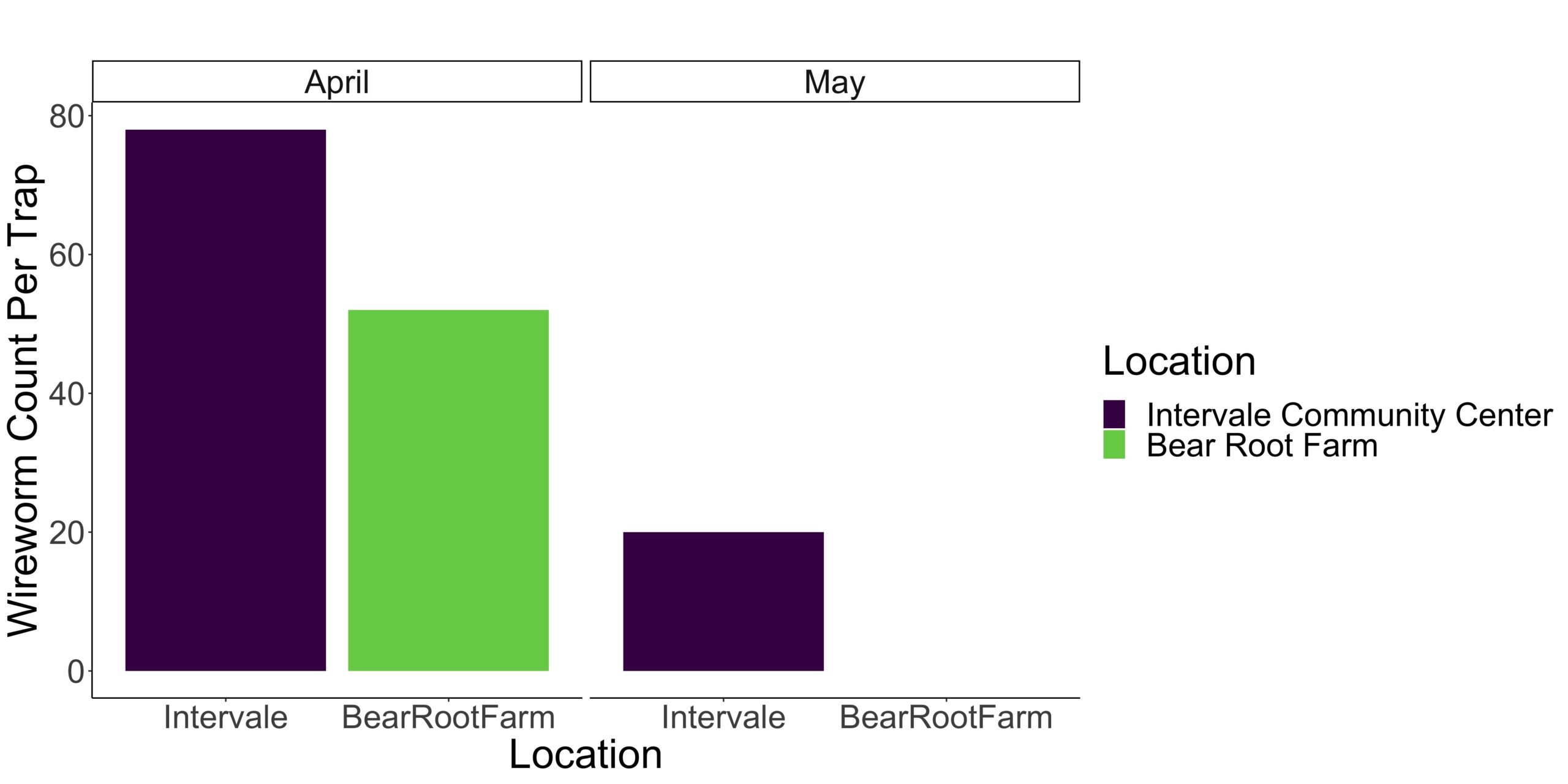
Prior to and during the experiments conducted in April, May, and June, wireworm traps were deployed at the Intervale Community Center, Bear Root Farm, and Burnt Rock Farm. The results from April and May at the Intervale Community Center and Bear Root Farm are displayed in Figure 1. Conversely, no significant wireworm pressure was observed at Burnt Rock Farm. Furthermore, by June, the wireworm population had diminished to such an extent that a significant number were no longer detected in the traps, and as a result, the data for this month is not presented. Morphological identification of wireworms was performed for each field separately to determine species, as depicted in Figure 2A and B. The composition of the wireworm population varied between the fields, with Bear Root Farm exhibiting a greater variety of species. Notably, Limonius infuscatus was the most abundant species at the Intervale (Figure 2A), while Athous sp. predominated at Bear Root Farm (Figure 2B). It is worth noting that identifying the specific species of Athous requires DNA barcoding.
locations.
B)

- Fungal treatments
A field trial was carried out across four different farms, each with five treatments: B. bassiana, M. burnneum, B. bassiana with rolled oats, M. burnneum with rolled oats, and a control group. Each treatment was replicated five times at each farm. Experimental plots, each 10 feet long, were set up in a randomized complete block design, with four treatments and five replicate blocks. Approximately 20 potato plants were grown in each plot, totaling 100 potato plants per treatment. To minimize plot interactions, buffers were placed between plots within the same row. Each treatment was applied twice with a three-week interval. The initial applications for all treatments were carried out in the third week of June by incorporating them into the furrows in each plot. The second round of applications for all treatments took place three weeks later, using the same method as the first round.
In October 2023, the potatoes were harvested and assessed. Twenty moderately sized potato tubers were extracted from the center of each plot, cleaned, and then examined for damage incidence and severity on each tuber, including the number of galleries.
Field Trial Site Details and Treatment Application Schedule
- Intervale Community Center: The first treatment application was conducted on June 20th, 2023. Our field, along with others at the Intervale Community Center, was flooded prior to the second application, leading to the discontinuation of the experiment.
2 & 3. Burnt Rock (Hinesburg) and UVM Horticultural Research Center: Due to low levels of wireworm pressure at Burnt Rock Farm and UVM Horticultural Research and Education Center (despite a notable number of adult click beetles at the UVM Horticultural Research Center), no data analysis is reported for these farms. We are considering adding two new sites in the upcoming season at farms with a history of higher wireworm pressure.
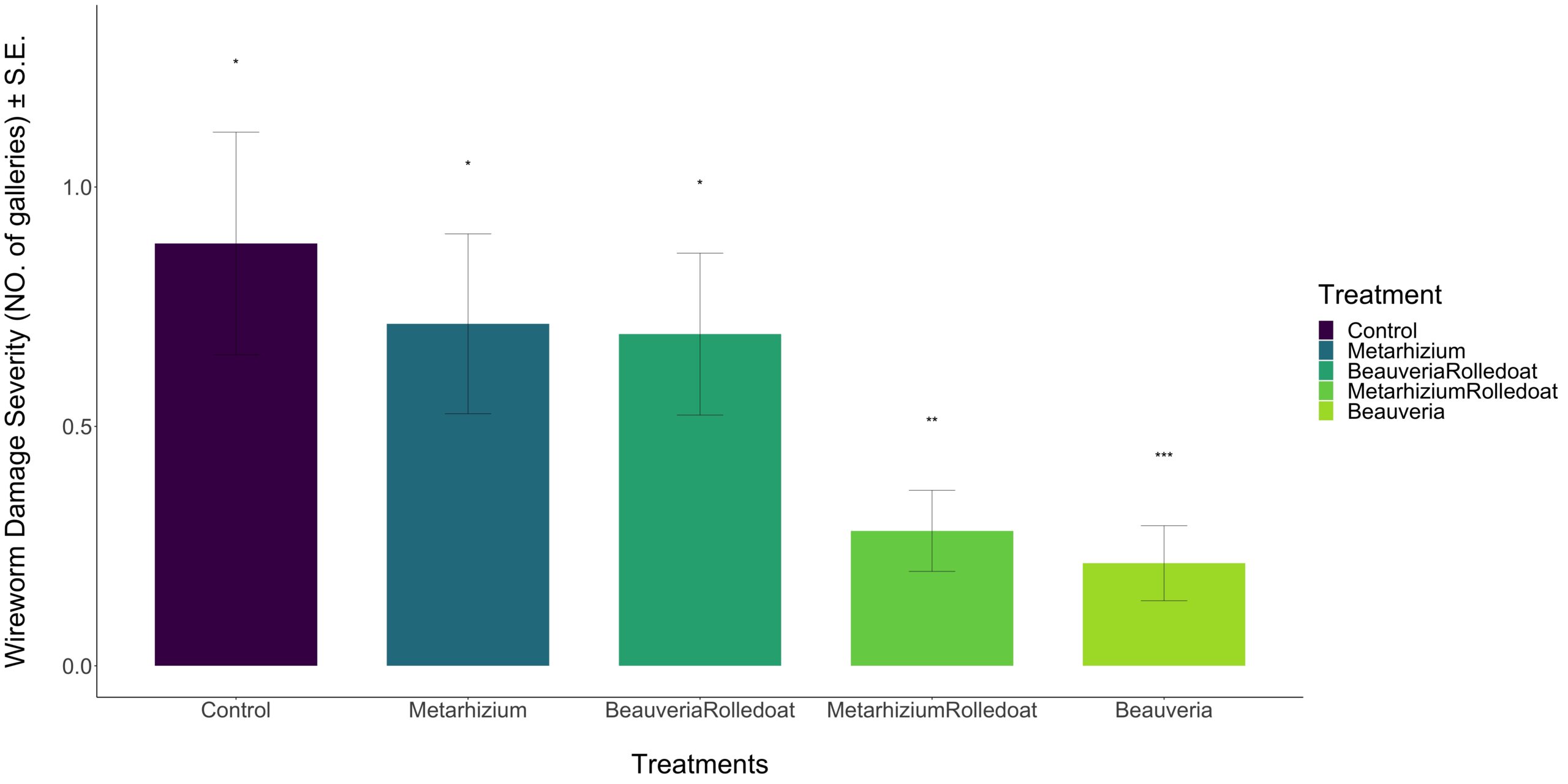
- Bear Root Farm: The first application was performed on June 21st, 2023, and the second on July 14th, 2023. Potatoes were harvested and assessed for wireworm damage on October 3rd, 2023 (refer to Figure 3).

Results:
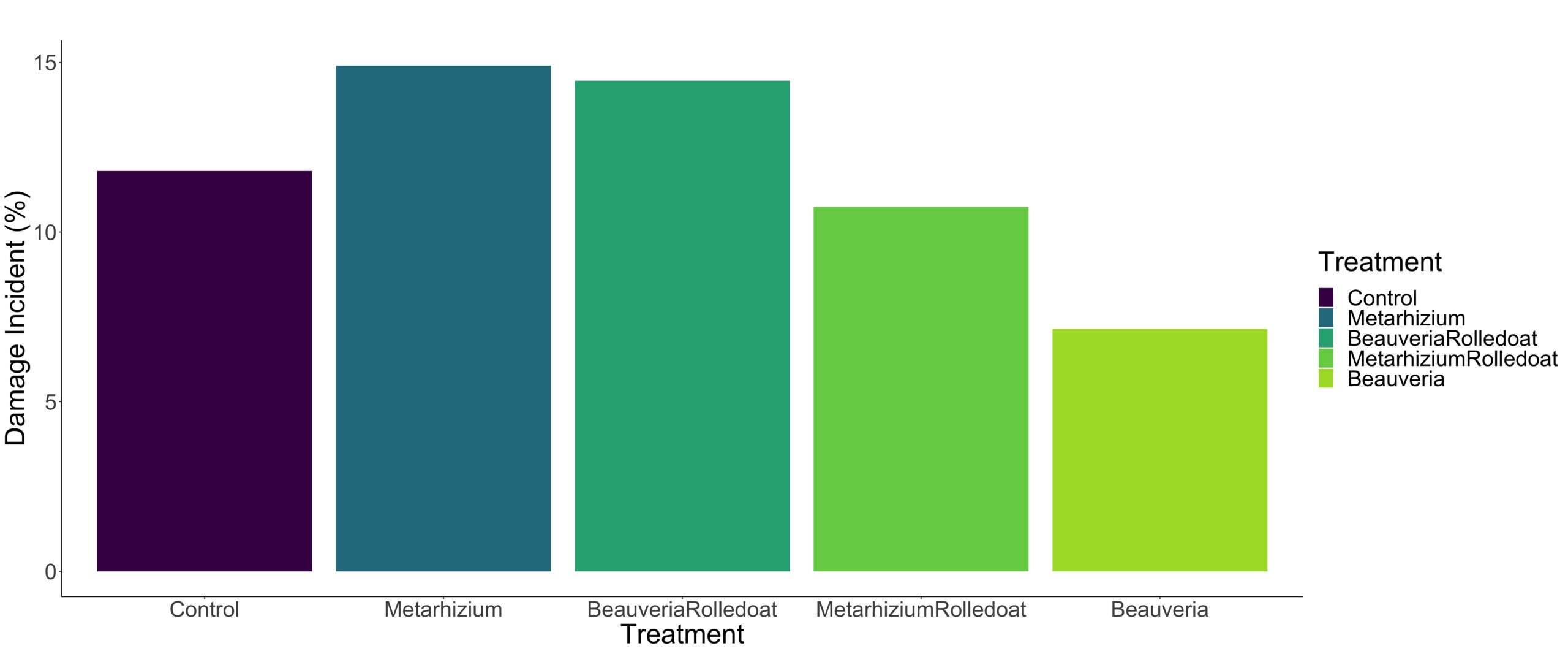
Our data exhibited a non-normal distribution pattern, as indicated by the Shapiro-Wilk normality test (p-value < 0.05). Therefore, a non-binomial generalized model was utilized to accommodate outliers and the non-normal distribution for analyzing potential differences among treatments. Table 1 presents the results of the non-binomial regression analysis for the treatments at Bear Root Farm. The number of galleries in the B. bassiana treatment was found to be significantly different from the control, while the other treatments did not exhibit any significant differences from the control (Table 1). Following B. bassiana, the Metarhizium + rolled oat treatment demonstrated notably low wireworm damage compared to the other treatments. Figures 4A and 4B depict the severity and incidence percentage of wireworm damage under different treatments. It is evident that B. bassiana and Metarhizium + rolled oat significantly reduced wireworm damage.

B)
Hypothesis II: Farmers will independently produce their granular formulation of fungi using a low-tech protocol
At the NOFA-VT winter conference 2023, we hosted a workshop titled "D.I.Y Beneficial Fungi: Grow Your Own Fungal Insecticide at Home" (Figure 5). The abstract for the workshop was as following:
"The Vermont Entomology and Participatory Action Research Team (VEPART) and Soil Ecology lab at the University of Vermont offered a workshop designed to empower growers to cultivate their own beneficial fungi cultures for controlling insect pests in agricultural settings. The workshop covered topics such as the use of beneficial fungi for pest control, basic cultivation techniques using readily available equipment (most of which can be found in your kitchen), and effective application methods. Participants had the opportunity for hands-on experience in growing beneficial fungi. We also evaluated the practicality of the methods taught for different types and scales of production systems and reflected on our successful field applications of the beneficial fungi."
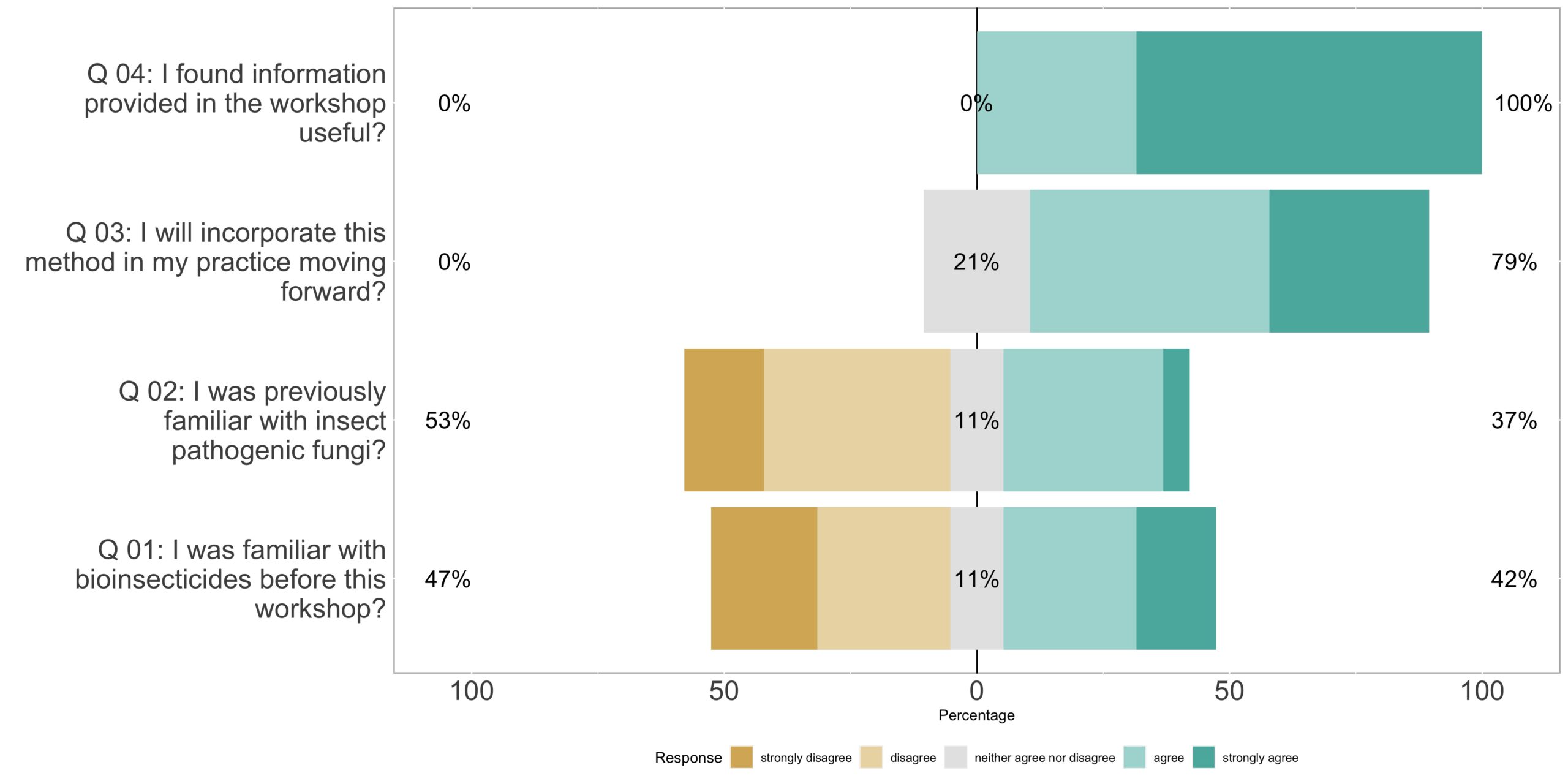
We provided a handout to participants including a detailed explanation and visual representation of each step involved in growing entomopathogenic fungi (Figure 6). The workshop attracted 28 participants, with 19 of them completing our survey. Feedback from the participants indicated a high level of satisfaction with the information presented during the workshop, and they expressed a strong interest in implementing the method in their own practices (Figure 7).


Hypothesis III: RNAi products will significantly reduce pest pressure associated with CPB infestations in conventionally managed potato crops.
At the UVM Horticultural Research Center, a field consisting of 60 plots, each measuring 10 ft with 15 ft buckwheat buffer in between to prevent spraying drift, was prepared. Throughout the week, the number of CPB was diligently recorded to assess population levels ahead of RNAi and EPF applications. However, the presence of a high abundance of predatory insects has prevented the realization of significant CPB pressure required for the application of our treatments.
To tackle this challenge, we've launched a greenhouse bioassay at the UVM Greenhouse, focusing on CPB larvae and employing the same treatments. This ongoing experiment is aimed at generating results that will be presented next year. Currently, we are testing six treatments, including a control, B. bassiana, M. burnneum , RNAi, B. bassiana + RNAi, and M. burnneum + RNAi, with each treatment consisting of 9 replications. Due to the controlled environment of the assay, we intend to explore various approaches, such as targeting different larval stages and pupae within the soil. Our objective is to determine the most effective method for applying fungi in preparation for the upcoming field season.

2022 Field Season Report
Lab trials were postponed to the winter of 2022-2023 as we were unable to capture a sufficient number of field caught wireworm larvae. After several attempts using suggested bait/trap protocols, we decided to postpone the lab experiments for the winter season and substitute mealworms (an easily reared and similar taxa) for the bioassays.
RNAi trials will also begin in the 2023 season, as the two current commercial sources of the product were unable to supply us with the sufficient product to satisfy our experimental needs. Furthermore, we wanted to calibrate the dosage for the below ground applications (i.e. entomopathogenic fungi trials) before setting up a full above/below ground trial.
Hypothesis 1a: grains inoculated with entomopathogenic fungi (I.e. benchtop culturing) will reduce wireworm pressure when applied as a soil application
For the 2022 field season we conducted our first field trial at the Intervale Community Farm located in Burlington, VT. This trial looked to test the efficacy of entomopathogenic fungi as a a control for wireworm larvae in sweet potato plantings.
Sweet potato slips were planted in 40’’ wide beds with 4 rows per bed and 1 foot spacing within rows. Experimental plots (10 bedft long) were established in a randomized complete block design with four treatments and five replicate blocks. Each plot contained ~20 sweet potato plants for a total of 100 sweet potato plants per treatment. We also included buffers between plots within the same row to reduce interactions among plots.
Figure 1. Treatments application
The treatments included an untreated control (no grain), sterilized millet grains (grain control), mycotized millet grains (2g/plant) and the organic grower chemical control Seduce (44 lbs/acre). Seduce is a spinosad based chemical bait used by some organic root crop growers. The first applications of all treatment were made on June 16 by adding them through the opening of the plastic around each plant incorporated into the soil. Second applications of all treatments, August 1st, and last application on September 14th in the same manner as above (Figure 1).
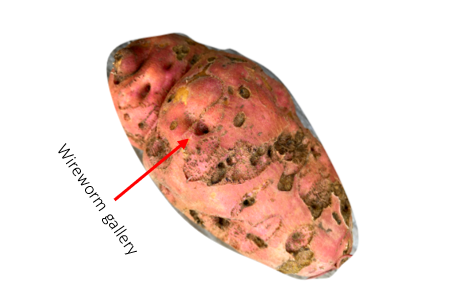
Sweet potatoes were harvested and scored in October 2022. At harvest, 20 moderately sized sweet potato tubers were dug from the center of each plot, washed, and then evaluated for damage incidence and severity on each tuber (number of galleries) (Figure 2). Mean damage incidence and mean damage severity were designated as dependent variables, and differences among treatments were determined via a generalized linear model.
Figure 2. Wireworm damage on a sweet potato
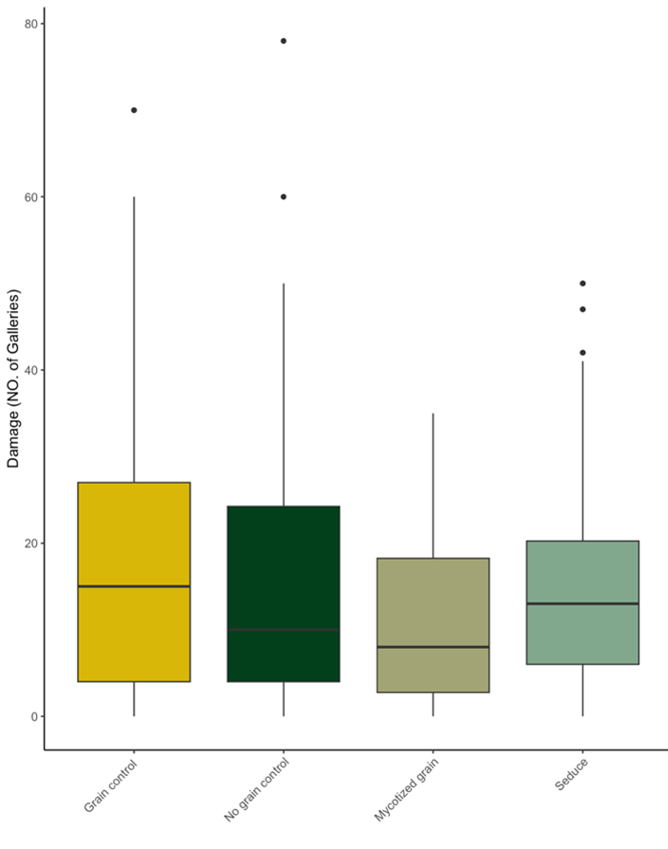
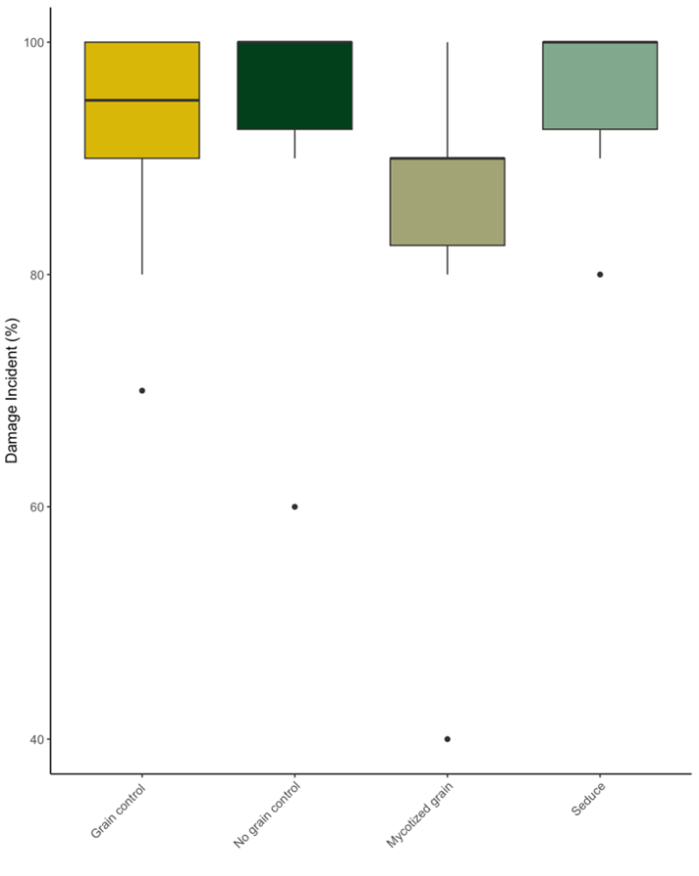
There was no significant difference between the mean incidence of wireworm damage on sweet potatoes with any of the treatments applied (Table 1, Figure 3, Figure 4). However, the severity of wireworm damage (number of galleries) on sweet potatoes was significantly lower in mycotized grains than grain control and seduce. In addition, the number of galleries was lower in mycotized grain treatment damage severity was not significantly different of those in no grain control (Table 1). This could be the result of high variation in the number of galleries.
|
Table 1. Mean incidence and severity (± SE) of wireworm damage on sweet potatoes at the Intervale Center VT, summer 2022 |
||
|
|
Mean Damage Incidence (%) ± SE |
Mean Damage Severity (gallery/tuber ± SE) |
|
Untreated control (no grain) |
94 ± 4 |
0.15 ± 0.015 |
|
Millet grain control (2g per plant) |
92 ± 3.2 |
0.19 ± 0.017 |
|
Mycotized grain (2 g per plant) |
85 ± 5.42 |
0.10 ± 0.009 |
|
Seduce 44lbs/acre |
96 ± 2.21 |
0.15 ± 0.012 |
|
p-value |
0.2303 |
0.0022 |
Figure 3. Mean severity of wire worm damage on 20 sweet potatoes per plot (n=100) grown at the Intervale Community Farm, VT in 2022
Figure 4. Mean incidence of wire worm damage on 20 sweet potatoes per plot (n=100) grown at the Intervale Community Farm, VT in 2022.