Final report for LNE22-457R
Project Information
Summary
Rapid DNA-based tests were developed for several bacterial pathogens of shellfish to enable the identification of possible sources of mortality events at shellfish hatcheries and field locations. Key design features for these tests included; keeping the assays (and related equipment) as simple, portable and cost-effective as possible, developing user-friendly protocols for aquaculture practitioners at shellfish hatcheries and providing education and training on these protocols to early adopters of the technology.
Problem Addressed
Oysters are particularly susceptible to mortality from naturally-occurring bacterial pathogens at their larval, seed and juvenile stages of development. In shellfish hatcheries, mortality events threaten the productivity and profitability of the operations and remain one of the greatest risks to the aquaculture industry. In particular, the seed supply for shellfish farming is increasingly challenged by hatchery failures (Walker 2017) with myriad potential causes including bacterial pathogens (Jones 2006). A recent study of the causes of ‘production crashes’ at research hatcheries along the East Coast of the US revealed that vibriosis, an infection of larval and juvenile shellfish by one of several species of gram negative, pathogenic bacteria in the genus Vibrio (class Gammaproteobacteria) was a significant cause of mortality (Gray et al. 2022). Additionally, the pathogen responsible for Juvenile Oyster Disease (JOD) – first identified by Boettcher et al. (2005), is known to have caused large-scale crashes in hatchery settings. Determining the cause of mortality events as quickly as possible is critical for recovering from the event and potentially preventing its recurrence.
Solution Pursued
The process of Loop-mediated isothermal amplification (LAMP) has been around for 25 years, when it was introduced as a novel method for detecting the hepatitis B virus (HBV) in less than an hour (Notomi et al. 2000). As its name implies, the LAMP process works at a single (isothermal) incubation temperature (65 °C), which removes the need for the type of expensive thermal-cycling equipment required for Polymerase Chain Reaction (PCR) tests. Furthermore, LAMP offers a variety of ‘readout’ options for interpreting test results, ranging from comparison of changes in fluorescence, turbidity or color of the reaction mixtures in response to targeted DNA amplification (Notomi et al. 2000, Goto et al. 2009, Notomi et al. 2015, Tanner et al. 2015). Although LAMP has been touted as a solution to the problem of detecting pathogenic Vibrio species, many of the existing approaches seem to have not provided end-to-end solutions for the aquaculture industry in the United States – as evidenced by their lack of adoption.
Research Approach
We developed and tested LAMP-based molecular tests to detect five species of Vibrio including V. alginolyticus, V. coralliilyticus, V. harveyi, V. splendidus, V. tubiashii plus the causative agent of Roseovarius oyster disease (ROD), Aliiroseovarius crassostrea. Overall, our research approach included; 1) Surveying the scientific literature and DNA sequence databases for appropriate gene targets that were both species-specific and associated with Vibrio pathogenicity/virulence, 2) Obtaining freeze-dried cell pellets of the targeted bacterial pathogens from several bacterial culture collections including NCMA (Maine, USA), ATCC (Maryland, USA) and DSMZ (Germany) to serve as DNA reference materials and confirm the presence of genetic targets and their exact DNA sequences, 3) Designing species-specific LAMP assays for each of the putative gene targets from each of the 6 bacterial species, 4) Developing a LAMP work-flow and testing the species-specific LAMP assays for reactivity to the targeted species and non-reactivity for all non-target species in our study, 5) Re-designing the LAMP assay if cross-reactivity was detected, 6) Development of species-specific DNA standards (positive controls) using genomic DNA extracts from each of the bacterial species for PCR and molecular cloning 7) Sequencing each of the DNA standards, calculating gene copy numbers per microliter, and testing the sensitivity of LAMP assays, 8) Testing LAMP assays on oyster seed and water samples collected from the vicinity of oyster farms, 9) Comparing results from the hatchery-based, colorimetric detection system (e.g., the self-heating NextMug) with results from the lab-based colorimetric and fluorescence detection system (Bio-Rad PCR and qPCR machines), 10) Training potential end-users on the hatchery-based LAMP protocol. Further details for specific sections are provided below.
Research Conclusions
The LAMP process provided a cost-effective, practical and sensitive approach for developing a hatchery-based molecular toolkit to detect potential pathogens of shellfish. We were able to design, test, and deploy this toolkit at several hatcheries in the Northeastern US, where it will democratize molecular testing at the point of need. As hatcheries and other aquaculture operations become comfortable with this toolkit, we anticipate the need for developing (or deploying – to the extent that they exist) additional LAMP assays for other organisms that pose a threat to shellfish.
Outreach, Impacts, and Potential Impacts on the Aquaculture Community
We presented preliminary research results at the 2024 Northeast Aquaculture Conference and Exposition (NACE) & 43rd Milford Aquaculture Seminar (January 10 – 12, 2024) in Providence, Rhode Island. The oral presentation was entitled ‘A User-friendly, Rapid eDNA Tool to Identify Pathogens Responsible for Diseases in Oyster Aquaculture’ and was presented by Dr. Robin Sleith from Bigelow Laboratory.
A key impact of this project was the development, testing and most importantly, the transfer of LAMP technology to potential end-users in the shellfish industry. We provided materials and copies of our detailed LAMP protocol to five shellfish hatchery operations. As of August 2025, we have conducted three in-person trainings.
We anticipate that our LAMP assays for oyster pathogens will have a positive impact on hatchery operations and will help to democratize the use of genetic techniques outside of traditional research laboratory settings. Specifically, we think that the LAMP approach will help to reduce risks and quickly fill in knowledge gaps when something goes wrong in a shellfish hatchery. Beyond the uses of LAMP for detection of bacterial pathogens, the same equipment can be used to monitor for the presence of harmful algal bloom (HAB) species, fungi and viruses – all of which pose additional threats to aquaculture operations. Our LAMP technology was designed to enhance productivity of American aquaculture and to ensure the safety of our food supply, while supporting rural prosperity. As with the introduction of any new technology, we anticipate that there will be an ‘adoption’ phase, during which time aquaculture professionals may need ongoing training and/or assistance with development of additional LAMP assays. To the extent possible, we are committed to supporting further research, development, and training on molecular approaches for aquaculture applications.
We propose to develop and commercialize loop-mediated isothermal amplification (LAMP) assays for farm-based detection of six virulence marker genes from four Vibrio species associated with shellfish hatchery failures, along with an easy-to-understand Hatchery Instructions Guide. The LAMP method allows for rapid, inexpensive, colorimetric detection of pathogens with minimal sample preparation, giving shellfish hatcheries straightforward diagnostic tools to manage hatchery production. These tools can be used to detect infections and determine management strategies, as well as to check shellfish seed viability prior to shipping to farms from the hatcheries, giving confidence to those purchasing the seed for their farms.
The oyster aquaculture industry depends on hatchery-produced seed. Northeast oyster farms annually purchase their seed from one of 40 Northeast hatcheries, which raise larvae and seed in land-based facilities, typically with 8-12 employees per hatchery. When a hatchery has production failure, farms lose access to seed that would have been their market product in 2-3 years, potentially putting thousands of jobs at risk. The seed supply for shellfish farming is increasingly challenged by hatchery failures (Walker 2017), with myriad potential causes (Jones 2006). Due to difficulty gathering production crash information from commercial hatcheries, recent research compiled production crash case studies from research hatcheries along the East Coast (Gray et al. 2022). Causes of crashes at these research hatcheries included vibriosis, infection of larval and juvenile shellfish by one of several pathogenic Vibrio species that cause mortality (Gray et al. 2022). When there is concern about pathogens, hatcheries currently must send samples to commercial laboratories to identify pathogenic Vibrios through PCR-based tests, waiting 2-4 weeks for results, at which point, the opportunity to control the pathogen has passed. These tests cost upwards of $200/sample, depending on how many genes are targeted, which can be cost-prohibitive for a small business losing product and sales.
This project developed rapid, inexpensive loop-mediated isothermal amplication (LAMP) assays that can be performed onsite with basic equipment, allowing hatcheries to diagnose the presence of pathogenic Vibrio spp. within an hour, and immediately take proactive measures to control the pathogen spread. This project developed LAMP assays to detect six virulence marker genes of four pathogenic Vibrio species known to cause production crashes at shellfish hatcheries (Gharaibeh et al. 2009, Ushijima et al. 2020, Xu et al. 2017, Yang et al. 2021). A survey to Northeast hatcheries elicited anonymous feedback such as ‘This work will certainly help shellfish hatcheries’ and ‘It will be a terrific asset for our industry.’ This technology represents a novel tool for shellfish hatchery pathogen management, and will likely be adopted across hatcheries to create more dependable production. That dependability will increase production efficiency, sustainability, and profitability for hatcheries adopting the approach, and a dependable seed source will increase efficiency and profitability for hundreds of oyster farms purchasing seed from these hatcheries.
Cooperators
- (Researcher)
- (Researcher)
- (Researcher)
Research
Pathogenicity varies between strains of identical species of Vibrio bacteria. Pathogenic strains of Vibrio are known to cause significant losses in oyster aquaculture. Timely and accurate detection of pathogenic strains is therefore necessary to detect and isolate infected seed and feed. This proposal seeks to address a need in the aquaculture community by developing a sensitive and rapid test for pathogenic Vibrio. We hypothesize that loop-mediated isothermal amplification (LAMP) methods will be ideal for detecting pathogenic Vibrio in aquaculture settings across three substrates: water, algae, and oyster seed.
We developed and tested LAMP-based molecular tests to detect five species of Vibrio including V. alginolyticus, V. coralliilyticus, V. harveyi, V. splendidus, V. tubiashii plus the causative agent of Roseovarius oyster disease (ROD), Aliiroseovarius crassostrea. Although there were existing LAMP assays in the scientific literature for some of these Vibrio species, we chose to develop new assays, given the availability of new DNA sequences in public databases and the availability of new bioinformatic software to aid with LAMP designs and optimization (LAMP Designer v. 1.16, PREMIER Biosoft). Overall, our research approach included; 1) Surveying the scientific literature and DNA sequence databases for appropriate gene targets that were both species-specific and associated with Vibrio pathogenicity/virulence, 2) Obtaining freeze-dried cell pellets of the targeted bacterial pathogens from several bacterial culture collections including NCMA (Maine, USA), ATCC (Maryland, USA) and DSMZ (Germany) to serve as DNA reference materials and confirm the presence of genetic targets and their exact DNA sequences, 3) Designing species-specific LAMP assays for each of the putative gene targets from each of the 6 bacterial species, 4) Developing a LAMP work-flow and testing the species-specific LAMP assays for reactivity to the targeted species and non-reactivity for all non-target species in our study, 5) Re-designing the LAMP assay if cross-reactivity was detected, 6) Development of species-specific DNA standards (positive controls) using genomic DNA extracts from each of the bacterial species for PCR and molecular cloning 7) Sequencing each of the DNA standards, calculating gene copy numbers per microliter, and testing the sensitivity of LAMP assays
- Literature Search and Reference Sequences: Relevant literature was searched with SCOPUS and similar online tools using the following keywords and combinations thereof including: Vibrio, alginolyticus, coralliilyticus, harveyi, splendidus, tubiashii, Aliiroseovarius, Roseovarius, crassostreae, virulence, pathogen, aquaculture, clg, collagenase, vcpA, metalloprotease, vhh, hemolysin, toxR, transcription factor, vtpA, ITS, 16S, 23S, LAMP, isothermal, PCR, and qPCR. The GenBank sequence database (https://www.ncbi.nlm.nih.gov/genbank/) was searched using all of the pathogen terms and specific gene identifiers, noted above, to find reference DNA sequences for consideration when designing the LAMP assays.
- Reference cultures: In most cases, reference cultures were selected based on literature reports that a particular Vibrio culture would test positive for a particular LAMP target that indicated virulence of the specific pathogen. For Aliiroseovarius crassostreae, putative virulence genes have been identified (Kessner et al., 2016) but their function in the etiology of ROD do not appear to have been confirmed. We therefore focused on developing a LAMP assay for the ITS region of the rRNA operon. It should be noted that the type species for A. crassostreae was first isolated from the Damariscotta River, ME, in 1997 (Boettcher et al. 1999). The primary research institutions involved in this study are located along this same river system. Detailed information on each of the reference cultures is presented in Table 1.
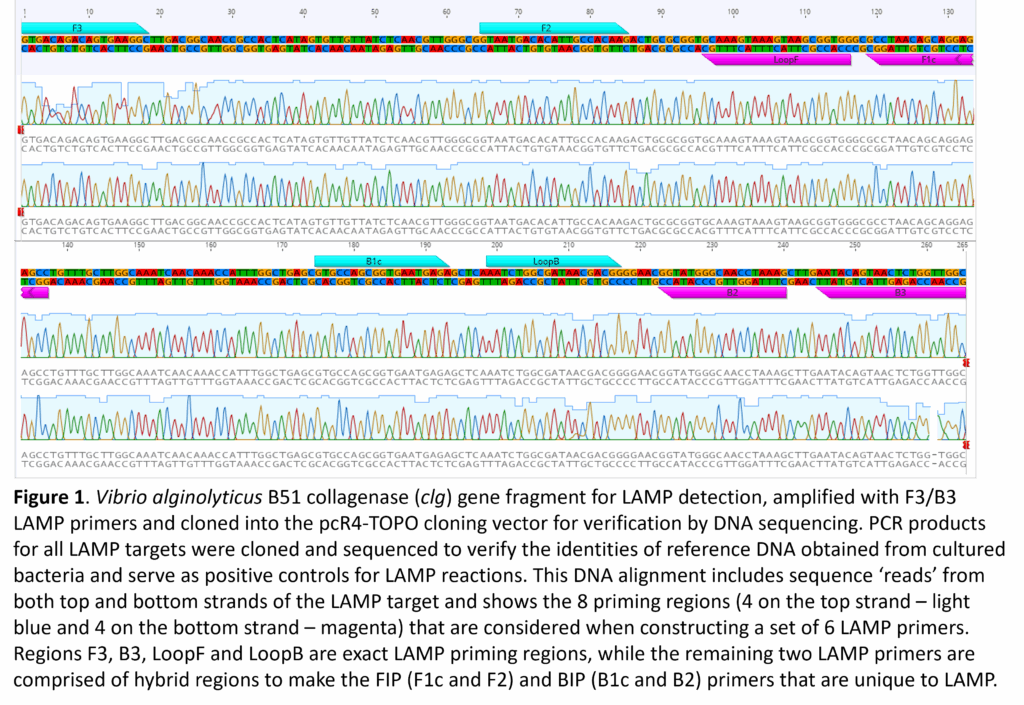
- Design of LAMP assays: DNA sequence alignments for each of the targeted species were imported into sequence visualization software (Geneious v 9.1.8, Dotmatics) to search for suitable regions for LAMP assay design. Consensus DNA sequences were imported into LAMP Designer v. 1.16, (PREMIER Biosoft) for one-step assay design and evaluation of six LAMP primers for each of the six pathogens. A key advantage of LAMP Designer is its ability to rank LAMP assays as ‘Poor’, ‘Good’, or ‘Best’, which saves time and resources when testing the efficacy of a particular set of primers. LAMP primers were confirmed for each of the pathogens using DNA sequences that were generated from the reference cultures (Fig. 1). LAMP Designer finds a total of eight regions that are used to generate the six primers needed for LAMP. It should be noted that the LAMP Forward Inner Primer (FIP) and Backward Inner Primer (BIP) are hybrid primers composed of the non-adjacent F1c and F2 priming regions (FIP) and the B1c and B2 priming regions (BIP). All primers were ordered from Integrated DNA Technologies (Coralville, IA) or from Eurofins Genomics (Louisville, KY), selecting the ‘salt-free’ option for purification.
- LAMP Work-Flow: The process of testing environmental samples for the presence of a pathogen via LAMP involves numerous choices and detection methods (Fig. 2). A key feature of LAMP is that it is amenable to most sample types – even those containing impurities that might inhibit the Polymerase Chain Reaction (PCR). While a kit-based DNA extraction procedure will likely result in the cleanest DNA for LAMP, crude cell lysis with a detergent-based lysis solution (or simply DNA-free water) in combination with mechanical cell disruption (pestle or bead-beating) can yield enough free DNA for detection. Regarding kit-based DNA extractions, the one required piece of lab equipment that is often cost-prohibitive is a bench-top centrifuge, capable of 14,000 xg. For this reason, we largely focused on preparing samples via the crude cell lysis (CCL) protocol. The CCL method has worked well in the past for extracting marine plankton samples for quantitative PCR (Countway and Caron, 2006). That said, shellfish tissues, with their copious mucopolysaccharides can present challenges for DNA-based assays that rely on CCL methods. Another consideration for DNA extraction is whether a particular extraction method is compatible with colorimetric assays, which rely on changes in the pH of the LAMP reaction as a result of DNA amplification. For this reason, preparation of CCLs using DNA-free water might be preferable to the lysis buffer described in Countway and Caron (2006).

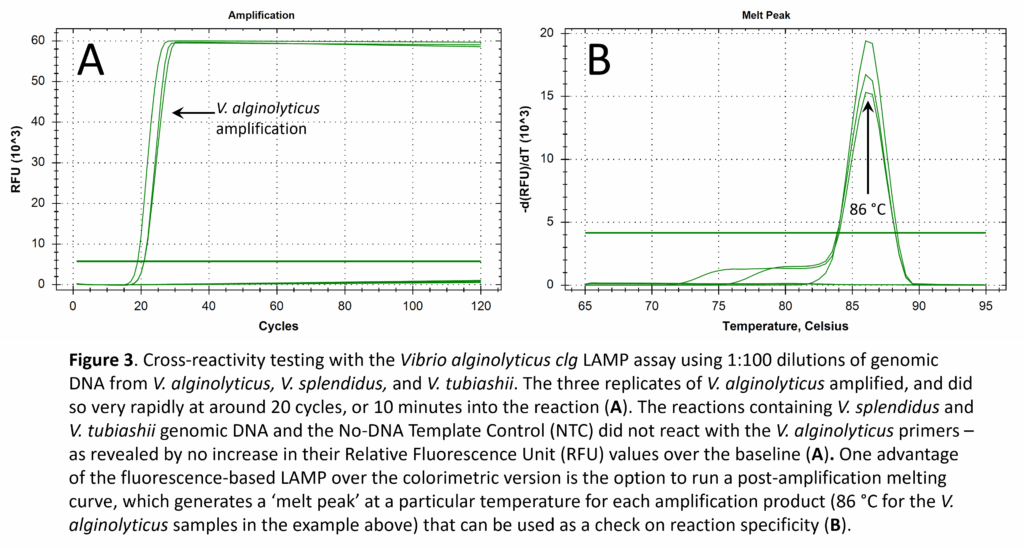
Key features of LAMP include its high specificity and rapid amplification of the gene target. We checked the reactivity of all six of our LAMP assays against genomic DNA from every target and non-target species and did not find any cases of cross-reactivity. For example, the Vibrio alginolyticus LAMP assay, targeting the clg (collagenase) gene, exhibited a positive reaction for V. alginolyticus genomic DNA (as expected), but was negative for V. splendidus and V. tubiashii genomic DNA (Fig. 3). The V. alginolyticus LAMP assay was also negative for V. coralliilyticus, V. harveyi, and A. crassostreae.
- LAMP Primer Re-design: The single-stranded DNA primers that are required for LAMP are relatively inexpensive (roughly $10-$15 each or approximately $60-$90 for a complete set of six), making re-design a viable option if a particular set did not work as expected. Although first iterations of most LAMP designs worked well, there was some degree of trial-and-error at the lab bench and working with LAMP Designer in generating the final set of primers.
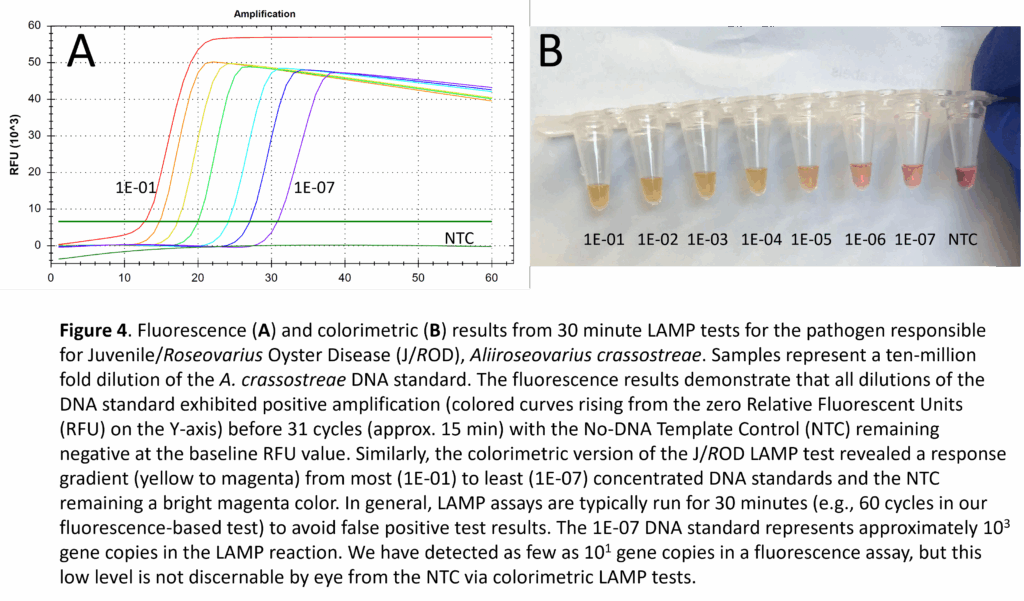
- Preparation of LAMP DNA Standards: Although our LAMP assays are primarily intended for use in determining the presence/absence of a pathogen, the use of DNA standards can serve as semi-quantitative positive controls – similar to quantitative PCR. Vibrio virulence genes were amplified by PCR using the F3/B3 LAMP primers while the Aliiroseovarius crassostrea ITS region was amplified with universal primers for the 16S/23S rRNA gene. PCR products were ligated into the pCR4-TOPO cloning vector and transformed into chemically competent TOP10 E. coli cells (Invitrogen) following the manufacturer’s protocol. Plasmid DNA was recovered from 3 bacterial clones for each LAMP target and sequenced to verify the exact base-pair composition. Glycerol stocks were prepared for each bacterial clone, ensuring an unlimited supply of plasmid DNA for future LAMP applications. The advantage of using cloned plasmid DNA for Vibrio or J/ROD positive controls (rather than genomic DNA from cultures) is the ability to add a known number of gene copies to a LAMP reaction. For example, a 10-million-fold dilution series was prepared for the J/ROD plasmid DNA and tested in both fluorescence (Fig. 4A) and colorimetric (Fig. 4B) modes. The 1E-07 dilution represented approximately 103 gene copies per reaction and was easily identified as a positive result when compared to the NTC reaction for each of the respective detection modes. Further testing was conducted in fluorescence mode, with amplification detected for dilutions containing 102, 101, and 100 gene copies per reaction (not shown). In general, the colorimetric testing mode has a visually-identifiable (by eye) testing limit of 102-103 copies per reaction. It is conceivable that a machine-based spectrophotometric reading of the colorimetric results could lower this limit to 101-102 copies per reaction.
- Sequencing and Testing LAMP DNA Standards: LAMP DNA standards for each of the pathogens were sequenced to confirm their identities and to determine the exact nucleotide (A, C, G, T) composition of the LAMP targets. Based on the nucleotide composition of the LAMP targets plus the published nucleotide composition of the pCR4-TOPO cloning vector – into which the LAMP targets were inserted, the molecular weight (MW) of each standard was calculated. Knowing the MW and the DNA concentration of each standard, gene copy per microliter was calculated for the undiluted standards. As an example, the undiluted DNA standard for Aliiroseovarius crassostreae (J/ROD) had 1.17 x 1010 gene copies per microliter, with the 1:10,000,000 dilution (1E-07) having just 1.17 x 103 gene copies per microliter. Three microliters of DNA standard were used for LAMP in testing the J/ROD dilution series, in which the target DNA copy number per reaction ranged from 3.5 billion (1E-01 dilution) to 3.5 thousand (1E-07 dilution) copies of the gene target (Figs. 4A, 4B).
We periodically tested water samples from different stages of treatment at Mook Sea Farm (MSF), but did not detect any of the targeted Vibrio species in the hatchery. The non-detects for Vibrio at MSF were a primary reason that we added the JOD pathogen, Aliiroseovarius crassostreae, to the toolkit, as this species was detected in association with juvenile oysters outside of the hatchery. Our water testing inside MSF included sequencing bacterial isolates from TSA and mTCBS plates to make sure that we weren’t missing an obvious pathogen. The TSA plates yielded DNA sequences from the non-pathogenic, gram-negative Alphaproteobacteria, Donghicola eburneus, while a single colony from the mTCBS (which selects for Vibrio, if present) yielded a sequence from Pseudomonas marincola, a non-pathogenic gram-negative member of the Gammaproteobacteria. Testing of water samples from the dock at MSF using the newly-designed Vibrio LAMP assays revealed several positive results for V. splendidus, indicating its presence in Maine waters. Testing of juvenile oysters at a second hatchery in Maine indicated a low levels of Vibrio alginolyticus, however the presence of this potential pathogen wasn’t associated with any known shellfish mortality issues. The most recent testing of the new LAMP methods occurred at a hatchery in Massachusetts, where we tested two sources of treated water, the algal culture Tisochrysis lutea (T-Iso) and two juvenile shellfish samples. Testing was restricted to the assay for Vibrio tubiashii, which had previously been identified as an issue at this hatchery. Four rounds of testing confirmed the presence of V. tubiashii in one of the two shellfish samples via fluorescence and colorimetric-based assays.
This project developed six rapid and relatively inexpensive Loop Mediated Isothermal Amplification (LAMP) assays that can be performed at the point of need (e.g., aquaculture hatcheries, field locations). The LAMP assays require only basic lab equipment to test for several species of Vibrio as well as the causative agent of Juvenile Oyster Disease (JOD) – now known as Roseovarius Oyster Disease (ROD, Malloy et al. 2007), renamed Aliiroseovarius crassostrea by Park et al. (2015). These tests allow hatcheries to detect the presence of one of several pathogenic Vibrio spp. or JOD in about an hour, enabling them to take proactive measures to control the spread of the pathogen and protect their assets. Furthermore, the assays can be used to evaluate cleaning processes to remove pathogens, to check algal food sources for potential Vibrio contamination and to test oyster seed for pathogen presence, prior to its distribution. Adoption of LAMP technology will provide an early warning system for the presence of potential shellfish pathogens in hatcheries and could lead to development of improved mitigation methods to eliminate these pathogens quickly, thereby reducing shellfish hatchery production failures, increasing sustainability, resiliency, and profitability. After investing in the basic lab equipment (see the associated LAMP SOP for suggested equipment) we estimated that the direct cost of running a single sample, plus one positive and one negative control was approximately $10.
Oyster Seed and Water Tests: Four oyster seed samples that we tested previously for J/ROD via qPCR were used to evaluate our LAMP assays. QPCR testing indicated that three of the four seed samples were positive for J/ROD, including samples 10s, 20/25s, and 10/12/14, with sample 18s testing negative. LAMP testing indicated positive results (yellow) for both undiluted and 1:10 dilutions of samples 10s and 20/25s after 30 minutes, while the 10/12/14 and 18s samples tested negative (pink) over that same time interval (Fig. 5). The previous qPCR testing suggested that samples 10s and 20/25s had the highest concentrations of J/ROD, which aligns with the rapid detection for these same two samples via LAMP. It is possible that the LAMP test for sample 10/12/14 would have turned positive with a longer incubation.
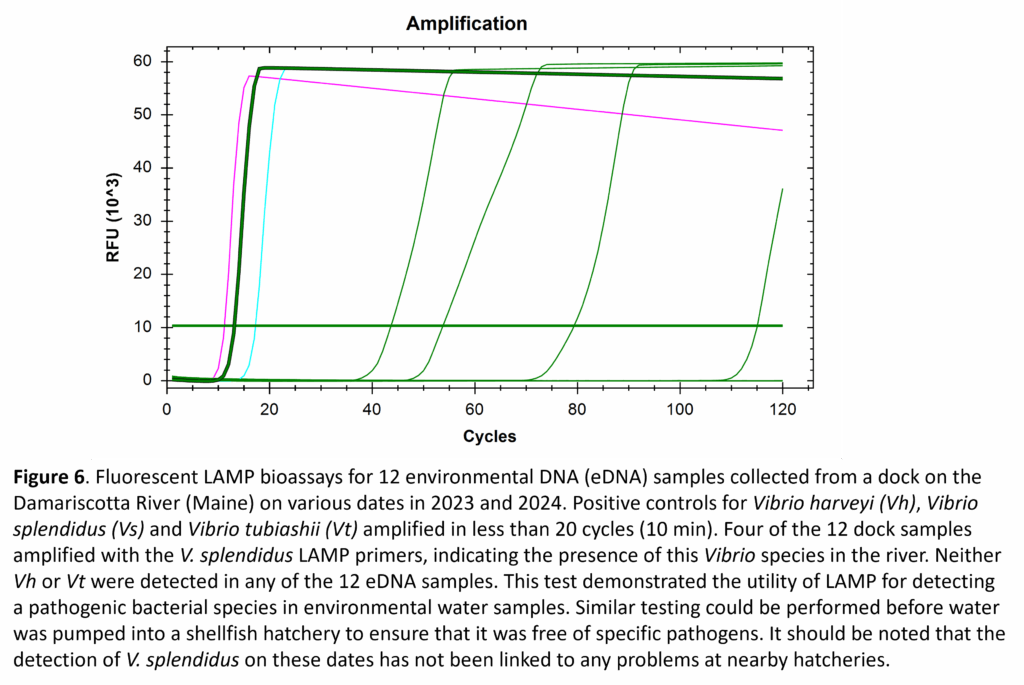
Although testing oyster seed is the most-likely use for our LAMP assays, testing filtered water samples for potential pathogens was also evaluated. Samples from a dock on the Damariscotta River (ME) were collected weekly in 2023 and 2024. Twelve of these samples were selected for pathogen testing via LAMP assays for V. harveyi, V. splendidus, and V. tubiashii. Of the 36 total LAMP tests, only four resulted in positive detections of a pathogen, with all four indicating the presence of V. splendidus (Fig. 6). Given the retrospective nature of this testing, it was not possible to link the detection of V. splendidus in the environment to any problems in nearby hatcheries.
Comparison of Hatchery- and Lab-based LAMP: The hatchery-based LAMP solution was developed to provide a first line of defense against potential oyster pathogens, to democratize DNA testing capabilities, and to enable aquaculture professionals to get the answers they need as quickly and affordably as possible. We have tested the LAMP assays on the hatchery-based (self-heating mug) and lab-based (PCR machine) amplification platforms and found them to give largely comparable results (Figs. 4, 5). The one caveat to our comparability assessment is the fact that fluorescence detection by a real-time qPCR machine is more sensitive than colorimetric detection via the human eye. We encourage users of the hatchery-focused LAMP technology to archive samples that test positive for pathogens (or any samples that yield ambiguous results) for confirmation of the results by a secondary method such as fluorescence LAMP, qPCR or DNA sequencing.
Training Potential End-Users: LAMP technology was designed and tested in collaboration with end-users at shellfish hatcheries to develop a practical toolkit for marine pathogen detection (Fig. 7). We have trained personnel at four hatcheries in the Northeastern region of the US, and plan to conduct a virtual training event with hatchery personnel in Virginia in the fall of 2025 with funding support from Bigelow Laboratory. We have already heard of additional interest in the technology from shellfish growers in Maine and expect to continue our training efforts through a fee-based course at Bigelow Laboratory, or with potential future grant support. A new internally-funded project at Bigelow Laboratory will develop portable LAMP technology for a pathogen of a commercially-fished crab species in Alaska, providing additional opportunities to train stakeholders in novel molecular approaches. Participants in this Northeast SARE project (P. Countway and K. Johnston from Bigelow Laboratory) will collaborate with two other Bigelow colleagues on the crab project – bringing their experiences from this project to a new research topic. One Master’s student (R. McCann) from Northeastern University’s Roux Institute received training through this Northeast SARE project as part of his internship at Bigelow Laboratory, in partial fulfillment of his MS degree in biotechnology.
The LAMP Standard Operating Procedure (SOP) is available here: LAMP_SOP_2025.
Challenges and Unexpected Outcomes
Shellfish samples provide unique challenges for DNA extraction due to their high concentrations of mucopolysaccharides that can interfere with DNA amplification. Although mollusc- and/or tissue-based DNA extraction kits can generate highly purified DNA samples, the kits require high-speed centrifuges, which generally aren’t found in aquaculture labs, and/or use of toxic chemicals. Since the goal of this project was to generate low-cost molecular protocols for use in shellfish hatcheries, we focused on low-tech extraction protocols to prepare crude cell lysates with various combinations of homemade cell/tissue lysis buffer, DNA- and nuclease-free water, plastic tissue grinding pestles, cell/tissue-disruption beads, a vortex mixer and a heated dry bath. Sample preparation will vary with sample type and may require some trial and error. DNA standards for the various LAMP assays can be spiked into samples during extraction testing, to check for inhibition of amplification as a result of sample impurities. In many cases, sample impurities and amplification inhibitors can be overcome by dilution of the crude lysate with DNA- and nuclease-free water.
The colorimetric LAMP reagents contain the Phenol Red indicator dye, which presented a challenge due to its sensitive response to changes in pH. Normally, as DNA amplifies in the LAMP reactions, the pH drops, and the reactions turn from magenta to yellow. Occasionally, a No-DNA Template Control (NTC) – containing just water and the reaction mixture, would change from magenta to yellow without any obvious sources of contamination. According to Tech Support at New England Biolabs, this can sometimes happen if CO2 in the head-space of the PCR tube diffuses into the reaction mixture, where it causes a drop in the pH and leads to the color change. The problem can be exacerbated by amplification in a system without a heated lid (like the NextMug), where a heated lid in a traditional PCR machine prevents sample evaporation and mixing with air in the reaction headspace. We were able to overcome this problem in the NextMug by using an overlay of Vapor Lock (Qiagen) on top of the LAMP reagents, which prevented exchange between the air in the reaction headspace and the LAMP mixture. However, it is unclear why this issue only occurred for our V. alginolyticus LAMP assay, and not with any of the other pathogen assays. When the same reaction was run on a PCR machine with a heated lid but no Vapor Lock, the NTC for the V. alginolyticus assay remained magenta. There are other colorimetric indicator options, including a mixture of hydroxynaphthol blue, calcein, and manganese (Pang et al. 2019) that we will explore in the future.
Research Conclusions
The LAMP process provides a cost-effective, practical and sensitive approach for developing a hatchery-based molecular toolkit to detect potential pathogens of shellfish. We were able to design, test, and deploy this toolkit at several hatcheries in the Northeastern US, where it will democratize molecular testing at the point of need. As hatcheries and other aquaculture operations become comfortable with this toolkit, we anticipate the need for developing (or deploying – to the extent that they exist) additional LAMP assays for other organisms that pose a threat to shellfish.
Impacts and Potential Impacts on the Aquaculture Community
We presented preliminary research results at the 2024 Northeast Aquaculture Conference and Exposition (NACE) & 43rd Milford Aquaculture Seminar (January 10 – 12, 2024) in Providence, Rhode Island. The oral presentation was entitled ‘A User-friendly, Rapid eDNA Tool to Identify Pathogens Responsible for Diseases in Oyster Aquaculture’ and was presented by Dr. Robin Sleith from Bigelow Laboratory. The talk was very well attended and generated interest among the attendees. Project collaborators S. Zimmerman, R. Sleith, and P. Countway attended the meeting in person.
We are planning to attend the 2026 NACE conference in January of 2026, in Portland, Maine, to discuss the finalized version of the LAMP toolkit and to follow-up with early adopters of the Vibrio LAMP technology. Bigelow Laboratory scientists Countway and Sleith are committed to advancing the use of molecular tools for aquaculture applications and are planning to seek additional lines of funding to support continued development of this toolkit, and help to support existing users in the aquaculture industry.
A key impact of this project was the development, testing and most importantly, the transfer of LAMP technology to potential end-users in the shellfish industry. We provided self-heating LAMP incubation smart mugs (e.g., NextMug), thermometers and copies of our detailed LAMP protocol to five shellfish hatchery operations including; Mook Sea Farm (Walpole, ME), The University of Maine’s research hatchery at the Darling Marine Center (Walpole, ME), Muscongus Bay Aquaculture Hatchery (Bremen, ME), A. R. C. Hatchery (Dennis, MA), and the hatchery owned by Mobjack Bay Lease Holdings (Foster, VA). As of August 2025, we have conducted three in-person trainings that included personnel from Mook Sea Farm, the Darling Marine Center, and Muscongus Bay Aquaculture hatcheries. A fourth in-person training session is planned at the A.R.C. Hatchery during the last week of August, 2025.
Members of the advisory board will be given final tool kits, training, and up to 10 samples from them for LAMP testing validation over the next year at no cost for the purposes of additional testing and introduction of the toolkit to a broader swath of the aquaculture industry.
We anticipate that our LAMP assays for oyster pathogens will have a positive impact on hatchery operations and will help to democratize the use of genetic techniques outside of traditional research laboratory settings. Specifically, we think that the LAMP approach will help to reduce risks and quickly fill in knowledge gaps when something goes wrong in a shellfish hatchery. Beyond the uses of LAMP for detection of bacterial pathogens, the same equipment can be used to monitor for the presence of harmful algal bloom (HAB) species, fungi and viruses – all of which pose additional threats to aquaculture operations. Our LAMP technology was designed to enhance productivity of American aquaculture and to ensure the safety of our food supply, while supporting rural prosperity. As with the introduction of any new technology, we anticipate that there will be an ‘adoption’ phase, during which time aquaculture professionals may need ongoing training and/or assistance with development of additional LAMP assays. To the extent possible, we are committed to supporting further research, development, and training on molecular approaches for aquaculture applications.
Education & outreach activities and participation summary
Participation summary:
August 2022, Project Advisory Committee Meeting. We met virtually with our advisory committee and had a useful discussion around priorities for oyster hatcheries and growers.
November 2022, Peter Countway and Robin Sleith attended the Marine Genomics in the Gulf of Maine Symposium/Networking Event at the University of New Hampshire. This event brought researchers working on diverse genomic projects together to discuss problems relevant to the Gulf of Maine (and beyond). The subject of Vibrio pathogens came up several times and we were able to network with other regional partners studying pathogens.
In January 2024 Countway, Sleith, and Zimmerman attended the 2024 Northeast Aquaculture Conference & Exposition (NACE) and the 43rd Milford Aquaculture Seminar (MAS) in Providence, RI. Sleith presented the work that McCann accomplished in Fall 2023 and we gathered members of the advisory board for a brief check-in on our progress. We received good feedback on our project and have had follow-up conversations with the Bivalve Hatchery Health Consortium as we are planning to collaborate and include LAMP assays in the toolkits that are being distributed to hatcheries.
The finalized LAMP SOP will be communicated to the shellfish community at NACE 2026, which will be attended by our team.
Learning Outcomes
This project provided an opportunity to develop, validate and test low-cost, user-friendly genetic tools in support of shellfish aquaculture. To date, Over 40 individuals from the aquaculture industry have been presented the data from this project. Personnel from four hatcheries (including MSF) have been trained in colorimetric LAMP testing for several Vibrio species and the agent of Juvenile Oyster Disease, Aliiroseovarius crassostreae. In all cases the trainees were able to conduct successful LAMP assays, encountering only minor challenges upon first use of the technology. Although the primary focus of this work was to develop and deploy LAMP assays, an equally important aspect was testing the extraction of environmental samples and juvenile shellfish for genetic testing with basic lab equipment (e.g. a heat-block and vortex mixer). We tested a low-cost/low-tech approach for DNA extraction that was used previously for qPCR (Countway and Caron, 2006). This method involved the use of a DIY lysis buffer (comprised of NaCl, EDTA, SDS and Tris-base), heating, and bead-beating to prepare a Crude Cell Lysate (CCL). We also tested extraction of shellfish using water, instead of lysis the buffer. In general, both methods worked – even with challenging shellfish samples that contain mucopolysaccharides, but may require further optimization. To our knowledge, there are no commercially available kits that are aimed at extraction of DNA/RNA from shellfish or water samples with only basic lab equipment. Further purification of samples with standard lab equipment (e.g., a high-speed centrifuge) and use of organic chemicals improved the speed of Vibrio tubiashii detection but overall didn’t indicate any positive results in samples that had already tested negative via the CCL approach. Overall, the LAMP methods were practical and useful for the initial adopters of the new technology. It is possible that shellfish labs will want to invest in a basic high-speed centrifuge to enhance their capabilities for DNA extractions, particularly from crude cell lysates of shellfish samples, which would benefit from enhanced DNA purification. The project generated an end-to-end protocol for sample processing and testing for six shellfish pathogens, and has already been deployed at four hatcheries. Scientists at Bigelow Lab and their collaborators will be seeking additional sources of funding to support further research, development, technical support and setup of LAMP-based testing capacity.
Project Outcomes
We are seeking new collaborations with any aquaculture group that is interested in the LAMP toolkit and will be seeking additional sources of external funding to support this work. Dr. Peter Countway has received internal funding from a Bigelow Kickstarter grant to develop LAMP technology with his Bigelow colleague, Dr. Maya Groner, who works on diseases of marine animals. Their project builds on the successes with this recent NE-SARE project and focuses on developing LAMP for the parasitic dinoflagellate Hematodinium. This parasite infects snow crabs in the Gulf of Alaska, making them unmarketable – due to the bitter taste which the parasites impart on the crab meat, and ultimately killing the infected crabs. If successful, the internal project will be leveraged by Dr. Groner and colleagues to seek larger, more sustained sources of funding. Dr. Countway has recently established a fee-for-service lab at Bigelow – the Center for Quantitative Molecular Ecology (CeQME) which will be able to offer LAMP-based detections for aquaculture samples using the newly developed Vibrio and JOD assays.