Progress report for LNE23-472R
Project Information
Orchardists increasingly plant orchards using dwarf trees with limited root systems. Roots are injured when trees are dug at the nursery and subsequently planted. Rising land costs and the need to return land to production reduces growers’ abilities to rotate orchard land into cover crops for more than a season. This lack of rotation increases the risk of concentrating apple pathogens in the soil. Many growers establish orchards with irrigation and regular fertilization, but these approaches are costly, requiring synthetic or high levels of organic fertilizers, and large quantities of water in dry years.
Many fungi species form associations with apple roots, and there are commercially available formulations that growers can apply to the roots at planting. Previous research found trees inoculated with fungi showed improved survival, growth, and nutrient uptake, and may make trees more resilient to drought. However, few trials have been conducted in commercial northeast orchards.
We will investigate if the addition of commercial mycorrhizal products to newly planted apple orchards, under varying levels of soil phosphorus, improves tree growth, survival, nutrient uptake, and water use efficiency, compared to naturally occurring fungi under various soil, climate, and management conditions found in the northeast. We will assess if fungal colonization, and therefore any subsequent benefits to tree growth, survival, nutrient uptake, and water use efficiency, vary between different apple varieties and rootstocks.
Three research sites will be conducted on commercial orchards in New York, New Hampshire, and New Jersey. We will inoculate 50 trees each with four commercial fungi products, with 50 trees serving as a control. During each of the three years, we will collect data on tree growth, survival, root colonization, root microbial community distributions, nutrient uptake, and water use. Two greenhouse studies will also be conducted. One study will determine the effects mycorrhizae have on drought stress on two varieties and four rootstocks. The second study will look at the effects mycorrhizae and three levels of P fertilization have on nutrient uptake.
A survey conducted in September 2022 showed grower interest in this work. When asked “Would you be interested in using mycorrhizal fungi when planting your new orchards/nurseries?”, 20 responded “Yes”, 1 responded “No”, and 32 responded “Yes, if there was more research documenting clear benefits”. This shows growers are interested in incorporating these materials into their plantings, but more data are needed before they implement these materials.
Growers will play key roles in this project. Field research is taking place at commercial orchards. Growers will apply treatments, maintain sites, and host field meetings. They will play key roles in developing outreach materials. Our advisory committee includes five commercial growers, who will be providing input throughout the course of the project through bi-annual meetings.
While the general benefits of mycorrhizal fungi have previously been documented, more data is needed from field trials on commercial farms in the northeast before growers can be confident in applying these materials on their farms. The knowledge generated from our multi-state on-farm field trials and greenhouse work will provide growers the additional data needed to determine if inoculating trees prior to planting their orchard will provide tangible benefits to their commercial plantings. Positive results may allow them to decrease their fertilizer and irrigation inputs when establishing their future plantings while improving orchard productivity and profitability.
Orchardists increasingly plant orchards using dwarf trees with limited root systems. Roots are injured when trees are dug at the nursery and subsequently planted. Rising land costs and the need to return land to production reduces growers’ abilities to rotate orchard land into cover crops for more than a season. This lack of rotation increases the risk of concentrating apple pathogens in the soil. Many growers establish orchards with irrigation and regular fertilization, but these approaches are costly, requiring synthetic or high levels of organic fertilizers, and large quantities of water in dry years.
Many species of mycorrhizal fungi form symbiotic associations with apple roots. In exchange for carbohydrates, fungi greatly increase the surface area and absorptive capacity of the tree roots. One genus of fungi commonly studied in apple is Glomus, which has been found to form very strong associations with apple roots (Reich, 1988). There are commercially available blends of these Glomus fungi that growers can apply directly to the tree roots when planting the orchard.
Previous studies have found trees inoculated with these fungi showed improved growth and increased nutrient uptake compared to non-inoculated trees (Reich, 1988; Bokszczanin et al. 2021). These studies suggest trees inoculated with fungi may require less nutrient inputs during the establishment phase. High levels of soil phosphorous may also inhibit fungal growth (Van Geel et al, 2015), however the effects of soil phosphorous on the root colonization and subsequent benefits appears inconsistent in the literature, and it is unclear what the effects of pre-plant P incorporation may be on the fungi in northeastern orchards (Miller et al., 1985; Morin et al., 1994). Trees inoculated with these fungi showed improved root development and increased drought tolerance (Huang et al., 2020). This suggests trees inoculated with these materials may be more efficient in their water use, requiring less water during the establishment phase. An unpublished field trial from Pennsylvania found tree survival in a new orchard was improved in trees inoculated with a fungi blend (Racsko, personal communication). These studies suggest mycorrhizal fungi inoculations may improve the survival and productivity of newly established orchards.
Apples are grown on approximately 95,000 acres across the Northeast. While New York, Pennsylvania, and Virginia are large producers, every Northeast state has commercial apple orchards. Growers replace about 5% of their orchards on an annual basis, representing roughly 4,750 acres per year. Growers we discussed this work with are interested in growing healthier trees with fewer inputs, and see these inoculants as an opportunity to improve their tree heath, soil health, and overall farm productivity and sustainability.
Inoculating tree roots with beneficial fungi at orchard planting may allow farmers to establish healthier, more productive trees with fewer fertilizer and irrigation inputs. This would allow them to increase their sustainability and resilience in the long-term. This is particularly critical with climate change and the projected increases in the frequency and severity of droughts across the northeast, as we observed throughout much of the northeast in 2022.
Research
Will the addition of commercial mycorrhizal fungi products to newly planted apple orchards, under varying levels of soil phosphorus, improve tree growth, survival, nutrient uptake, and water use efficiency, compared to naturally occurring fungi under the various soil, climate, and management conditions found in northeast orchards?
Does fungal colonization, and therefore any subsequent benefits to tree growth, survival, nutrient uptake, and water use efficiency, vary between different apple varieties and rootstocks?
Orchard Field Trials
We will establish three orchard field trials to determine if four commercial mycorrhizal products improve root colonization rates, tree survival, tree growth, nutrient uptake, and water uptake under two soil phosphorous levels.
Field sites will be hosted at the following locations, to represent a diversity of site and management conditions from across the northeast.
- Northern Orchards in Peru, NY, Barnsby Pink Lady on M.9 Nic 29 Rootstock, Schroon Fine Sandy Loam, Replant Site
- Apple Hill Farm, Concord, NH, Premier Honeycrisp on G.935 Rootstock, Canterbury Fine Sandy Loam, Replant Site
- Melick's Town Farm in Califon, NJ, Evercrisp on G.935 Rootstock, Washington Loam, Virgin Ground
Treatments: Trees will be treated with one of four mycorrhizal fungi treatments (Mycoapply, MycoBloom, Mykos Gold, BioOrganics) and an uninoculated control). Trees will be treated with one of two levels of phosphorous fertilizer (0 pounds per acre, 200 pounds per acre).
Methods: 3 trees will make up the fungi x P application treatments experimental unit, replicated 5 times down the row in a completely randomized block design for a total of 150 trees per planting. We will apply each mycorrhizal treatment as a root dip or broadcast directly to the roots, as per the manufactures’ recommendation. We will apply P by dissolving triple superphosphate in water, and then applying a gallon of material in a ring around each of the P treatment trees shortly after the trees have settled following planting.
Data Collection:
- Tree growth and survival: at planting, we will measure trunk circumference of each tree at 30cm above the graft union. We will repeat these measurements in October of 2023 through 2025. We will then convert measurements to cumulative annual growth. At each October measurement date, we will rate each tree as alive (0) or dead (1).
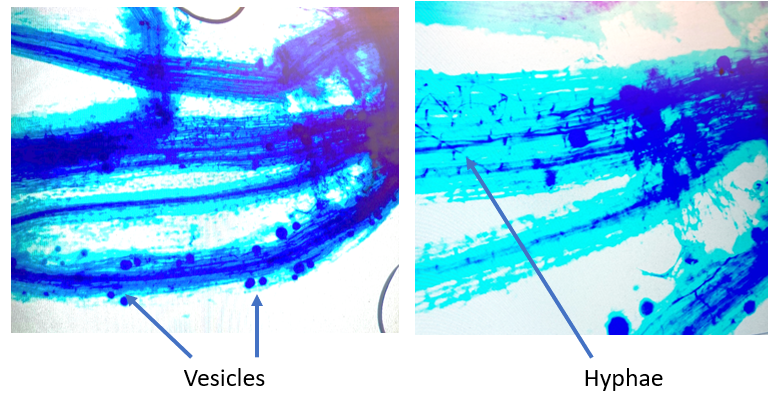
- Root Colonization: in years 1 and 3 of the project, fine (absorptive) roots will be collected from one tree per treatment and preserved in 50% ethanol prior to staining and evaluation. At the laboratory, roots will be stained with trypan blue, stored in acidic glycerol for 72 hours, and installed on microscope slides. To increase throughput, we plan to utilize the newly available AMFinder software (Evangelisti et al., 2021) to identify AMF colonization parameters correlated with increased plant performance (e.g., arbuscules, hyphae, vesicles, and root length colonization). If the AMFinder method fails, we will quantify the percentage of roots colonized by AMF with a compound microscope using the intersection method (McGonigle et al., 1990).
- Soil Community Profiles: To understand how commercial mycorrhizae formulations impact soil microbial community composition, we will collect one soil sample from each field trial prior to planting, and 10 samples (one from each fungi x P treatment) in July of the third year of the trial. Samples will be sent to the Missouri soil health lab for phospholipid fatty acid analysis (PFLA). PLFA analysis is used to quantify total microbial biomass and shifts in microbial community structure by differentiating microbial groups (Gram Positive; Gram Negative; Anaerobe; Actinomycetes; Methanobacter; Fungi; and AMF) to provide estimates of microbial biomass for each group (Frostegard and Baath, 1996).
- Water Use Efficiency: To assess water use efficiency, we will take carbon isotope samples from each treatment replicate around June 15th and October 15th of every year of the study. Three leaves from each treatment replicate will be dried, ground, and submitted to the Cornell Stable Isotope Lab for analysis. Results will give us the concentrations of different carbon isotopes within the plant, which correlates well to seasonal water use efficiency within the plant (Farquhar et al., 1989).
- Nutrient Uptake: In late July of years 2 and 3 of the project, we will collect foliar samples from each treatment replicate for nutrient analysis. Nutrient analysis will be performed by the Cornell Nutrient Analysis Laboratory in Ithaca, NY.
Data Analysis and Presentation of Results: Tree growth, root colonization, water stress, and nutrient uptake data will be analyzed through JMP using the fit model function for each field site. Tree survival data will be analyzed through SAS using the GLIMMIX function. Our analysis will be presented in both tabular form, and through appropriate graphs organized by treatment. All results will be formatted and prepared for both extension style and peer reviewed journal format.
Greenhouse Trials
The benefits of mycorrhizal fungi on young apple trees and the interaction between rootstocks, scions, and abiotic stresses will be detailed through complimentary greenhouse experiments. Grafted, potted trees will be grown in the greenhouse for 3.5-4 months at 24°C with ambient light supplemented using LED lights with a 12-h photoperiod. Pots will be watered to full saturation every 3 days without fertilizer addition. Standard pesticide applications will be applied. Physiological and plant performance traits will be measured throughout as detailed below.
Treatments: Experiment 1 will evaluate the effect of fungi type, soil type, variety, rootstock, and drought treatment on tree growth and physiological performance. Experiment 2 will evaluate the effect of fungi type, phosphorus availability, and rootstock on tree growth and physiological performance.
Methods: Experimental design for experiment 1 is 5 replicate trees x 4 rootstocks (B.9, M.9, G.11, and G.41) x 2 scions (Honeycrisp, Gala) x 4 fungi treatments (no fungi control, 3 commercial products) x 2 water treatments (Control, Drought). Trees will be grown under full watered conditions for 2.5-3 months. Then trees will be exposed to a simulated drought treatment of 14 days with no irrigation. Physiology measures will be collected prior to (every week) and throughout (daily) the drought stress treatment. On day 15, all trees will be fully watered to allow recovery. Growth resumption and recovery metrics will be monitored for an additional 2 weeks before terminating the experiment.
Experimental design for experiment 2 is 5 replicate trees x 1 scion (Honeycrisp) x 4 rootstocks (B.9, M.9, G.11, and G.41) x 4 fungi treatments (no fungi control, 3 commercial products) x 3 P-availability types (None, low: 12 mg/kg, high: 50 mg/kg). Trees will be grown under full watered conditions with no additional fertilizer besides P additions for 2.5-3 months.
Data Collection: Physiological measurements will track growth rate, stomatal conductance, photosynthetic rate, and water potential. Leaf samples will be taken from experiment 1 at the conclusion of the experiment to determine water use efficiency differences through carbon isotope analysis. From experiment 2, leaf ion analysis will be conducted with the idea that fungi inoculations will alter the pattern and concentration of ions. At the end of the study, all potted trees will be destructively sampled to measure root, trunk, branch, and leaf dry matter accumulation. Trunk diameter increment will be calculated by deducting the trunk diameter at the end of the experiment from the initial trunk measurement taken 5 inches above the grafted union. Fine root tissue will be harvested from all experimental trees in both experiments and processed for AMF quantification.
Data Analysis and Presentation of Results: Data will be examined for treatment factors using MANOVA and linear regression methods for single factors and all interaction effects. Analysis will be conducted in the R programming language and results presented as graphics and table formats. All results will be formatted and prepared for both extension style and peer reviewed journal format.
2023 Activities
Field Trials: In 2023, we successfully established our three field research sites at three commercial orchards:
- Northern Orchard in Peru, NY, Barnsby Pink Lady on M.9 Nic 29 Rootstock, Schroon Fine Sandy Loam, Replant Site
- Apple Hill Farm, Concord, NH, Premier Honeycrisp on G.935 Rootstock, Canterbury Fine Sandy Loam, Replant Site
- Riamede Farm in Chester, NJ, Golden Delicious on Bud 9 Rootstock, Parker Gravely Sandy Loam, Replant Site
Prior to planting, soil samples were collected from each field site. Soil samples were analyzed by the University of Missouri Soil Health Assessment Center for Phospholipid Fatty Acid Analysis to estimate the biomass of key microbial functional groups within the soil, and estimated relative percentages of these key groups, including AMF. (See results section for details).
At planting, five mycorrhizal treatments were applied to the trees within the new orchard plantings (1. Symvado, 2. MycoBloom, 3. Mykos Gold, 4. Promate 5. Control). All mycorrhizal treatments were applied at label recommended rates, and were applied according to the manufacturers suggestions for newly planted tree fruit. In NY, trees were planted with a furrow tree planter. Trees were planted into pre dug holes at the NJ and NH field sites.


A few weeks following orchard planting, trees at the NY and NJ field sites also received one of two fertilizer treatments (1. High P and N, 2. Low P and N). Rates were determined based on soil tests taken by the farmers in the previous season. We used polyphosphate and Urea Ammonium Nitrate (UAN) as our fertilizers, as they were both liquid based fertilizers that could quickly make their way to the root zone. Fertilizers were applied by hand, in 1 gallon of water per tree applied directly to the herbicide strip. The NH field site received the fertility treatments of (1. Lime and 2. Control) prior to planting. The rate was determined by the amount of lime required to raise the soil pH of the site to 7.0. We did not adjust P at this site, as the soil test determined that this site was already in the correct range for P.
All treatments were managed similarly, according to each of the growers standard management practices for first year trees. Trees at the NJ field site were irrigated shortly after planting in May. No irrigation occurred at the NY or NH field site.
Shortly after planting, tree circumferences were measured at 30cm above the graft union. The same measurements were done in late October, along with an assessment of tree survival. Growth and survival data were analyzed using the Fit Model feature of JMP Statistical Software. (See results section for details).
In July and October, we collected two leaves from each tree to conduct carbon isotope analysis to estimate tree water use efficiency between treatments. Leaves are currently in line to be analyzed by the Cornell Stable Isotope Lab in Ithaca, NY.
In August, root samples were collected from each treatment using a soil corer near the base of each tree, and were stored in ethanol to be stained for future colonization assessments. Colonization estimates are currently ongoing by the Londo lab in Geneva NY, and we expect to have full 2023 results back by June 2024.

Greenhouse Trials:
In year 1, our experimental objective was to test the potential interaction effects of AMF inoculum on apple growth and development. Specifically, our task was to evaluate the impact of different rootstock-scion combinations, different AMF treatments, and different stress treatments with the goal of identifying optimized combinations for stress adaptation. Initially, we proposed to study stomatal function and photosynthetic response, biomass changes, and root colonization metrics as evidence of differential effects. The experiment design thus included 5 biological replicate trees x 3 stress treatments (Control, Drought, Low Phosphorus) x 4 AMF treatments (None, MycoApply, MykoBloom, MykosGold) x 2 scions (Honeycrisp, Aztec Fuji) x 4 rootstocks (B.9, M.9, G.41, G.935) for a total of 480 trees.
Several factors have impacted the initial experiment. One was the late start of the grant in the tree availability season. By the time funds were available, tree selection was minimal. As a result, the trees ordered for the experiment were much larger than anticipated, arriving not as 2-3 foot starts, but as 6ft finished and feathered trees. The first modification to the study was to increase the pot size to accommodate the large trees, and changing from a standard greenhouse to a large polyhouse where the trees could be kept on the floor. While this change facilitated working with the large
trees, it also introduced a lower light level due to the use of shade netting to help keep climate control in the polyhouse.

The experiment was initiated on May 15th where all pots were inoculated with AMF treatments and randomized within the polyhouse and all trunk diameters were recorded with calipers, 6 inches above the graft union. Licor measurements of stomatal conductance and photosynthetic efficiency were taken on June 5th and 6th . Drought and Low phosphorus treatments were initiated July 7th (Low P) and July 10th (Drought). Carbon isotope samples were collected from all trees from control and drought treatments on July 10th . Wilting was observed in drought treatments on July 19th and water volumes were adjusted to compensate for the increasing temperatures in the polyhouse. Carbon isotope samples were collected again on August 8th . Drought treatments were concluded on August 25th (7 weeks of treatment) and trunk diameters were remeasured. The experiment was terminated on September 18th as pest pressure had reached a significant level in the polyhouse. Root tissue was sampled from all trees for AMF quantification on October 2nd . On October 11th , “healthy” trees were transplanted to the field in a randomized design to track tree performance under field conditions in subsequent years. Due to loss of some replicates of rootstock-scion combinations, the field trees represented a balanced design of 400 trees, rather than the full 480 trees.

Data analysis is underway for stomatal conductance and photosynthetic efficiency metrics.
Leaf samples for carbon isotope were collected to examine water use differences between the AMF treatments in control and drought treatments. Those samples have been dried, powdered, and are waiting for analysis at the Cornell COIL facility. The data analysis queue is currently quite long due to repair and personnel issues at COIL. Root staining and analysis of AMF metrics are ongoing. Staining duration is being optimized, initial attempts have identified some instances of AMF vesicles and hyphae.
Our approach going forward is going to change slightly from the original design. The results of the large tree polyhouse experiment were inconclusive. We believe this is in part due to the reduced light and climate control in the polyhouse. We clearly observed plant stress in drought and low Phosphorus treatments, but these observations were not born out in any of the measured metrics. Our plan is to rerun this experiment in year 2 by purchasing smaller, more manageable sized trees and conducting the experiment in greenhouses. In year 2 we are planning to do rooted rootstocks and will verify that AMF colonization has occurred. Then we plan to bud graft scion wood onto the rootstocks for year 3 assessments of scion performance.
2024 Activities
Field Trials:
In 2024, we continued to manage and monitor our three field trials. In May, the New York and New Jersey field sites received their second high and low fertilizer treatments. The high fertility treatments received 53 pounds of total N and K per acre, and was applied as a granular 26-0-26 product applied in a ring around each tree. The low fertility treatment received no fertilizer applications. The New Hampshire field site is comparing high vs. low pH, so this site received the growers standard fertility program in 2024.
In mid-June and October, we collected two recently expanded leaves from each tree to conduct our second year water use efficiency evaluations. These samples were submitted to the Cornell Stable Isotope Lab for analysis.
In Late July, we collected additional leaf samples from each treatment to conduct leaf nutrient analyses. At least 50 leaves from the mid section of the current season's growth were collected from each treatment, and were sent to Dairy One in Ithaca NY for nutrient analysis.
Trees in the NY field trial on August 15 2024. Weed pressure is severe at this site, with perennial grasses dominating the herbicide strip.
In October, we collected our 2024 tree survival and growth data. Trunks were measured again at 30cm above the graft unions, and we recorded if trees were alive or dead.
Data analysis was conducted in November and December.
Greenhouse Trials:
In 2024, our experimental objective was to test the potential interaction effects of AMF inoculum on apple growth and development. Because the results of the 2023 study did not indicate major effects of AMF or stress treatments, we revised our study design to focus on three rootstock cultivars without the complication of variable grafted scions. We examined stomatal function, photosynthetic response, and shoot growth from own-rooted B.9, G.11, and G.935 rootstocks grown with the four AMF treatments (None, Symvado, MykoBloom, MykosGold), and under the same three stress treatments as 2023 (Control, Drought, Low Phosphorus).
The experiment was initiated on April 18th. All pots were inoculated with AMF treatments and randomized within the polyhouse, and all trunk diameters were recorded with calipers at 6 inches above the soil line. Irrigation treatments were initiated on May 3rd and plants with multiple leaders were pruned back to two. Low phosphorus treatments were initiated on June 10th with the first fertigation treatment and drought treatments initiated on June 12th.
Greenhouse trees in the polyhouse.
Licor measurements of stomatal conductance and photosynthetic efficiency were taken on June 24th. Trunk diameter was measured on all trees at the conclusion of the study on September 26th.
Data analysis is underway for stomatal conductance and photosynthetic efficiency metrics.
Root staining and analysis of AMF metrics are ongoing.
2023
Field Studies
Tree Survival
First year tree survival was assessed in October 2023. Trees were rated either alive (1) or dead (0). All trees survived in NY. In NH, one tree was lost in the Symvado x no lime treatment. In NJ, one tree each was lost in the Mykos Gold x low fert treatment, 1 tree was lost in the Mykos Gold x high fert treatment, and 1 tree was lost in the Symvado x low fert treatment. Neither mycorrhizal nor fertilizer treatment had a significant impact on tree survival at any of our sites in 2023.
Tree Growth
Tree growth was measured in May and October 2023, and the cumulative tree growth between the different mycorrhizal and fertility treatments are presented in Table 1. Treatment differences were assessed using the Fit model feature on JMP Statistical Software. Dead trees were removed from the growth analysis.
Table 1. 2023 cumulative tree growth by fungi and fertility treatment at our NY, NJ, and NH field sites. Treatments with differing letters significantly differ (p=.05) according to Tukey's HSD test.
| 2023 Field Trial Trunk Circumference Growth (cm) | ||||
| NY | NJ | NH | ||
| Fungi | Symvado | 0.6 B | 0.9 | 1.4 |
| Mycobloom | 0.7 AB | 0.9 | 1.5 | |
| Mykos Gold | 0.6 B | 1.0 | 1.5 | |
| Promate | 0.9 A | 1.0 | 1.5 | |
| Control | 0.7 AB | 1.0 | 1.4 | |
| P-value | 0.00382 | 0.6282 | 0.87525 | |
| Fertility | Low | 0.73 | 0.9 | 1.5 |
| High | 0.67 | 1.0 | 1.4 | |
| P-Value | 0.1382 | 0.2368 | 0.7481 | |
| Fungi x Fertility | P-Value | 0.6361 | 0.0998 | 0.4124 |
Soil PLFA Analysis
Pre-plant soil Phospholipid fatty acid analysis provided us estimates of the initial amounts and ratio of mycorrhizal fungi present at each of our three field sites prior to the incorporation of commercial fungi products. Please note that this test is only a rough estimate, as it does not test for exact numbers.
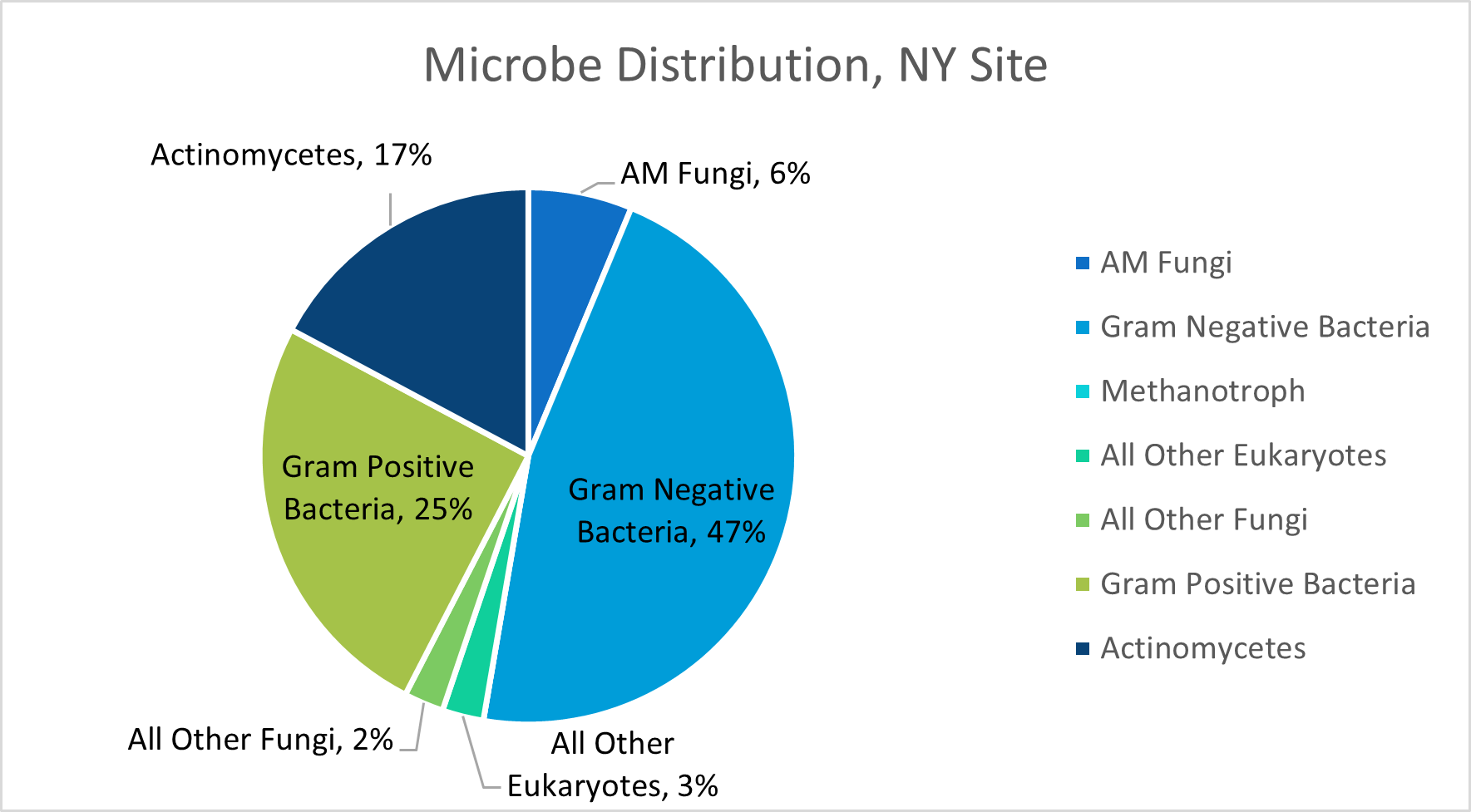
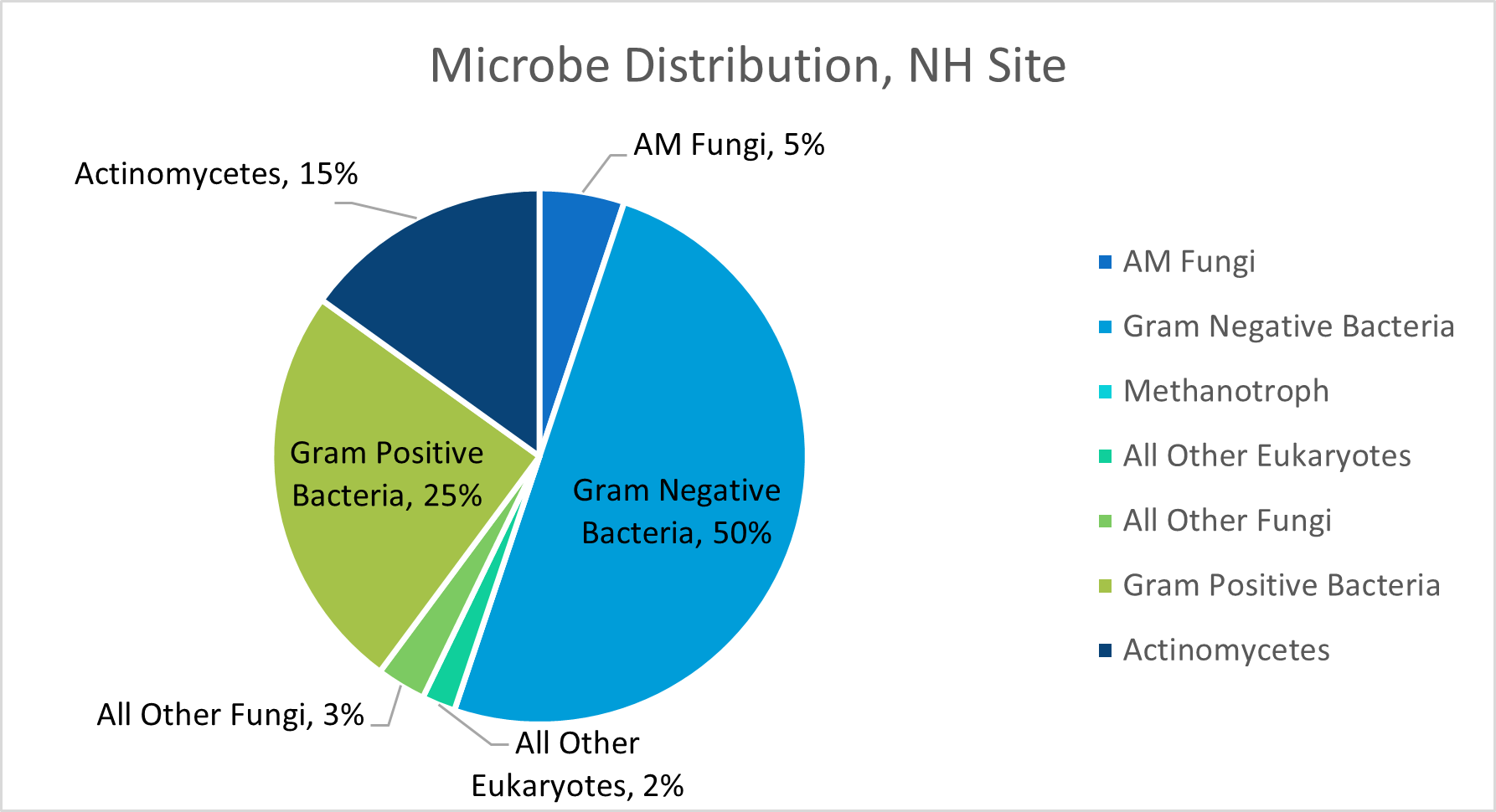
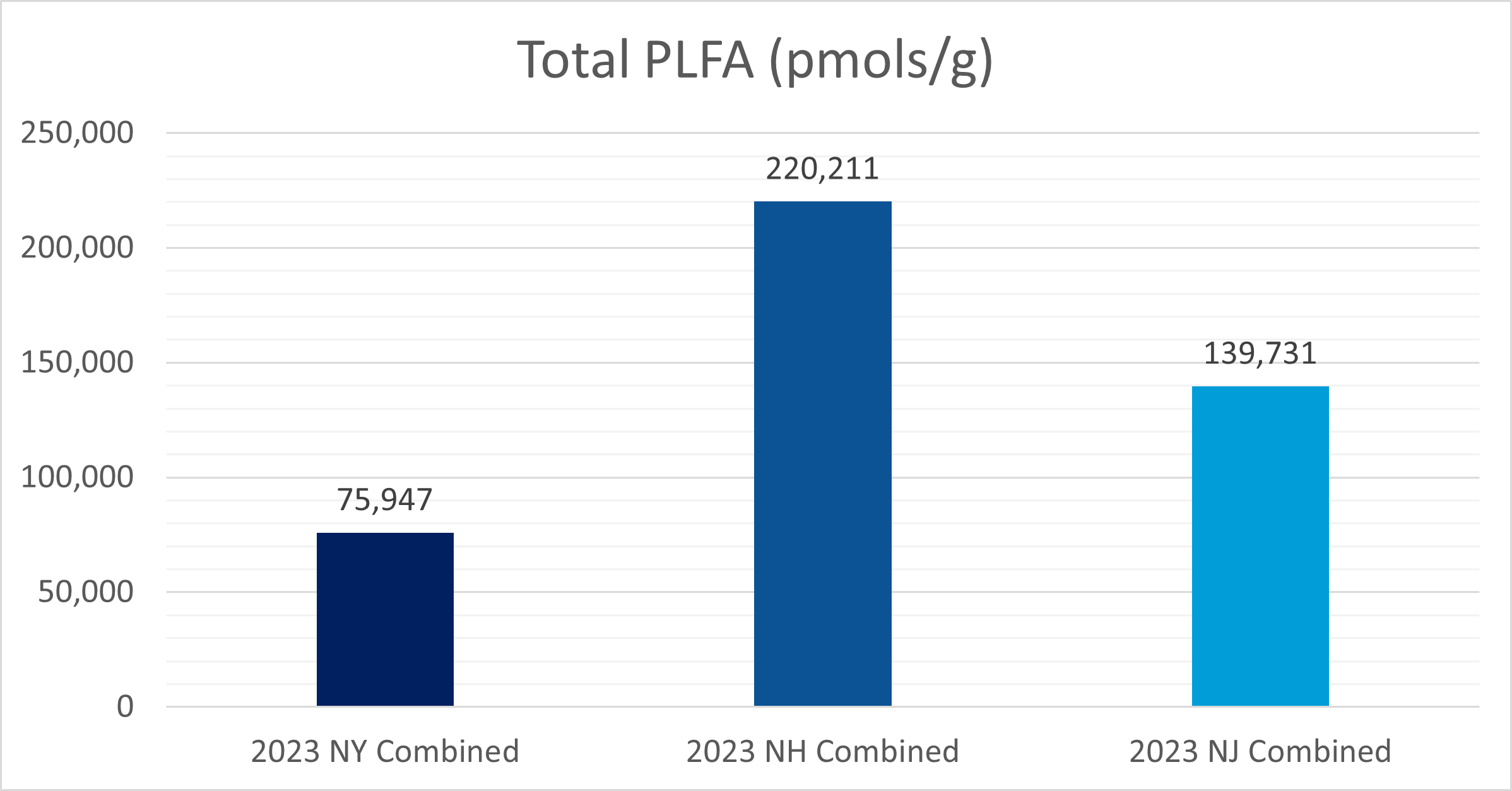
In NY, NH, and NJ, total phospholipid fatty acid content (pmols/g) was analyzed from soil samples from each field site. The NY, NH, and NJ field sites contained approximately 75947, 220211, and 139731 pmols of PLFA per gram of soil, respectively.
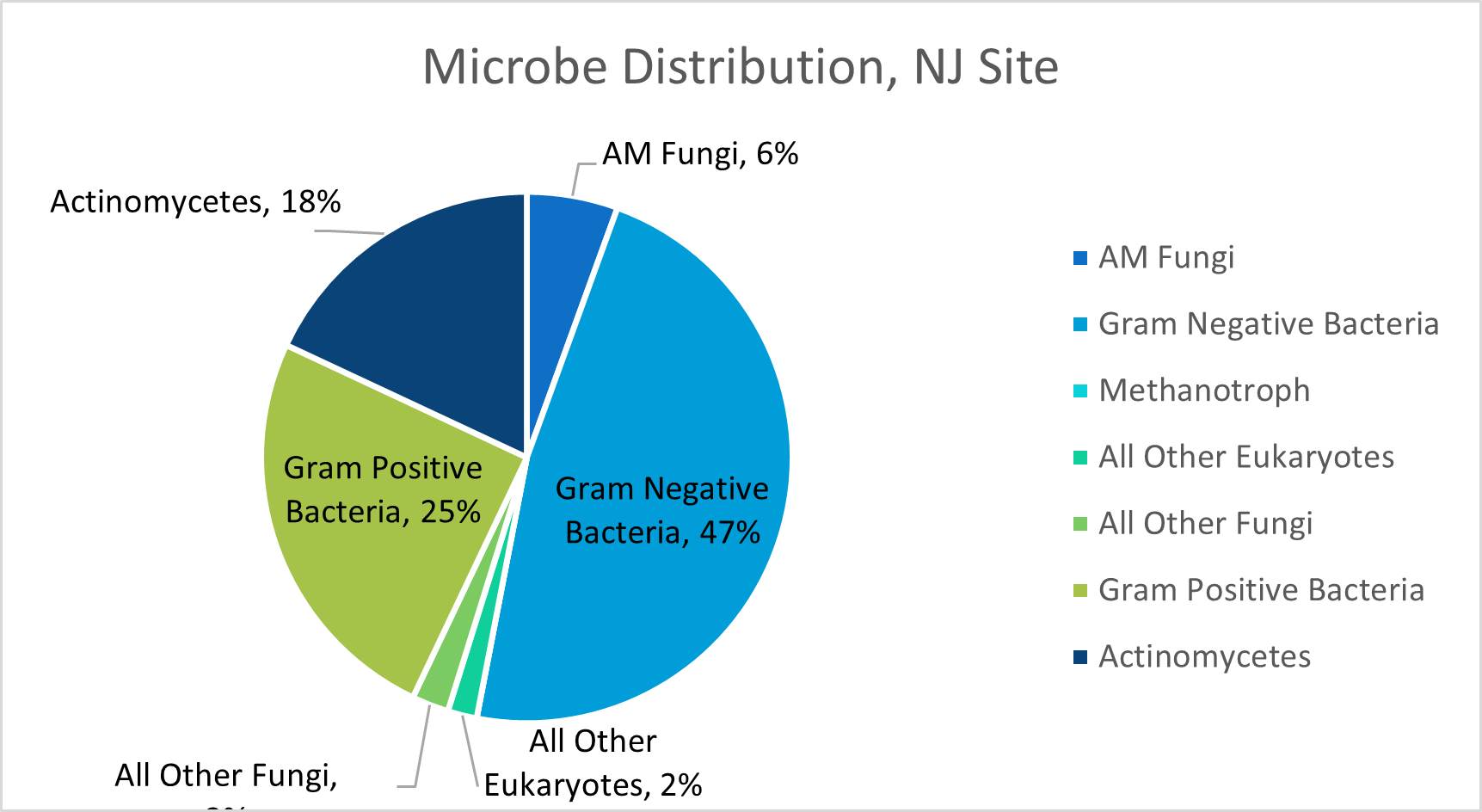
The estimated percentage of AM Fungi associated with the PLFA extracted from the soil samples analyzed for our NY, NH, and NJ field sites were 6, 5, and 6% respectively. These estimates were not from exact counts, but rather from the analysis of different fatty acids present within each soil sample. Other microbial functional groups evaluated in this test included gram negative bacteria, methanotrophs, eukaryotes, non-AM fungi, gram positive bacteria, and actinomycetes. We are curious to see how our commercial fungi blends will impact these percentages over the next two years, so this data simply serves as our "baseline" prior to orchard planting.
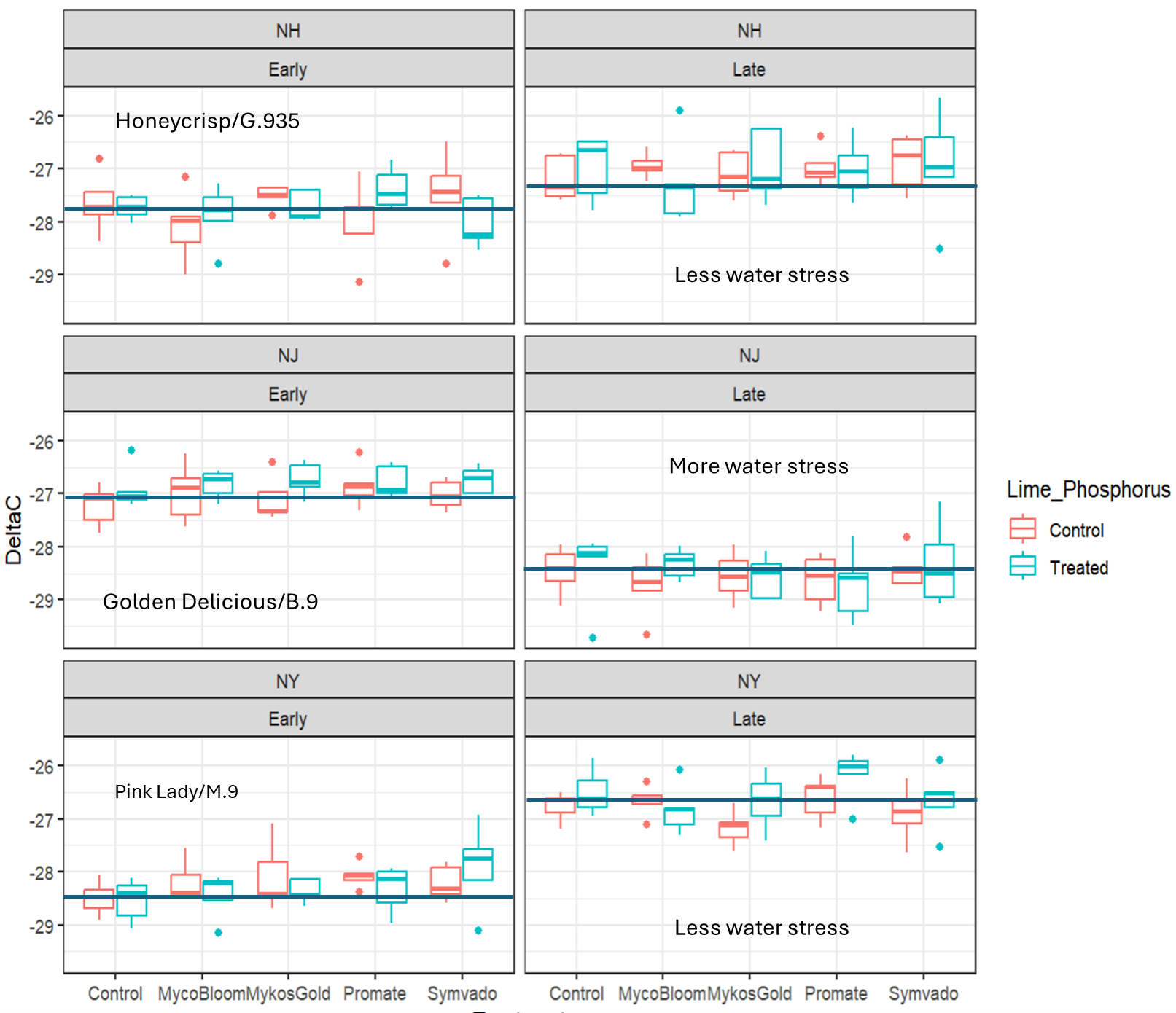
Water Use Efficiency
In 2023, we examined the C12/C13 carbon isotope ratio of field collected leaf tissue to assess if AMF treatments meaningfully impacted plant water use. If AMF inoculation reduced water stress through colonization and expansion of accessible water in the soil, then we would expect carbon isotopes to be less negative in value. Results of the study did not show that any AMF product shifted carbon isotope ratios across all scion/rootstock x location combinations. For trees in New Hampshire (Honeycrisp/G.935) there were clear differences of early and late season water use, with indications of less water stress in late season. For Golden Delicious/B.9 in New Jersey, carbon isotope results suggested water stress was elevated later in the season. Finally, for Pink Lady/M.9 in New York, results suggested that water stress was greater later in the season. Applications of lime (NH) or phosphorus (NJ and NY) were also tested in these contrasts, and no consistent benefit to water use efficiency was noted for any AMF product. Taken together, there was no consistent benefit of using AMF products for altering water use efficiency in the field trees.
Water use efficiency ratings from carbon isotope sampling. No significant reductions in water stress were observed in any of the fungi treatments relative to the control.
AMF Root Colonization
Root colonization was extremely limited in all of the treatment samples we collected from field sites in 2023. So few were recovered that statistical analysis could not be completed. This suggests there was very little root colonization in any of our samples four to five months following treatment, or that our sampling procedure did not yield usable samples.
Greenhouse Studies
Stomatal Conductance and Photosynthetic Efficiency
Initial interpretation does not suggest that AMF treatments had any meaningful impacts on these performance metrics, with the large caveat that the movement of the experiment to the polyhouse resulted in a much lower light level than is optimal. As a result, physiological measurements were very close to the baseline. Differences could be seen between scions in these traits, and both stomatal conductance and photosynthesis was lower in drought and low phosphorus treatments.
Trunk Diameter
Trunk diameter measures across the experiment were similarly underwhelming. No significant effect of AMF treatment, or stress treatment, were detected. Significant effects of scion and rootstock were detected, with Aztec Fuji demonstrating increased growth on G.935 relative to Honeycrisp, and the opposite response observed on M.9.
Root Colonization
Our 2023 greenhouse samples did not have strong indication of AMF colonization. So few were recovered that statistical analysis could not be completed.
2024
Field Studies
Tree Survival
Second year tree survival was assessed in October-December 2024, depending on the trial site. Trees were rated either alive (1) or dead (0). All trees survived in NY. In NH, one tree was lost in the Symvado x no lime treatment. In NJ, one tree each was lost in the Mykos Gold x low fert treatment, 1 tree was lost in the Mykos Gold x high fert treatment, 1 tree was lost in the Symvado x low fert treatment, and one tree was last in the Mycobloom x high fert treatment for four trees total now. Neither mycorrhizal nor fertilizer treatment had a significant impact on tree survival at any of our sites in 2024.
Tree Growth
Tree growth was measured in May 2023 and Fall 2024, and the cumulative tree growth between the different mycorrhizal and fertility treatments are presented in Table 2. Treatment differences were assessed using the Fit model feature on JMP Statistical Software. Dead trees were removed from the growth analysis. Fungi treatment has not had any impact on tree growth at any of the field sites to date. In NJ, trees receiving the high fertilizer treatment have grown significantly more than those receiving the reduced fertilizer program. We are not seeing a fertility treatment effect in New York, nor are we seeing a treatment effect of adjusting soil pH in NH. We saw no significant treatment interactions between fungi and fertilizer treatments at any site.
Table 2. Cumulative tree growth by fungi and fertility treatment at our NY, NJ, and NH field sites. Treatments with differing letters significantly differ (p=.05) according to Tukey's HSD test.
| May 23-Fall 24 Total Field Trial Trunk Circumference Growth (cm) | ||||
| NY | NJ | NH | ||
| Fungi | Symvado | 1.0 | 3.1 | 3.0 |
| Mycobloom | 1.2 | 2.8 | 3.2 | |
| Mykos Gold | 1.1 | 3.0 | 2.9 | |
| Promate | 1.2 | 3.0 | 3.4 | |
| Control | 1.0 | 3.2 | 3.3 | |
| P-value | 0.3696 | 0.5154 | 0.6822 | |
| Fertility | Low | 1.1 | 2.4 B | 3.2 |
| High | 1.1 | 3.6 A | 3.1 | |
| P-Value | 0.7496 | <.0001 | 0.4370 | |
| Fungi x Fertility | P-Value | 0.8149 | 0.1351 | 0.7782 |
Leaf Nutrient Analysis
Our leaf nutrient analyses looked at leaf N, P, K, Ca, Mg, Mn, Fe, Cu, B, and Zn (Table 3). There were no differences in any foliar nutrient levels between fungi treatments in New York or New Hampshire. In New Jersey, the Promate and Mycobloom treatments had higher leaf Mg levels than the Symvado treatment, but there were no differences within the other nutrients we evaluated.
There were significant differences in leaf nutrient concentrations between the high and low fertility treatments in NY and NJ. In NY, fertilized trees had higher leaf levels of N, Ca, Mg, and Cu. In NJ, fertilized trees had higher levels of N, Ca, Mg, Fe, Cu, and B. Interestingly, P was actually higher in the low fertilizer treatment in NJ. No differences were observed between the lime and control treatment in NH.
When we looked at interactions between fungi and fertilizer treatments, in NY we saw higher concentrations of K in the leaves of the low fertilizer x Mykos Gold and the low fertilizer x Promate treatments compared to the high fertilizer x control treatment. In NH, we saw significantly higher K concentrations in the lime x Symvado treament compared to the lime x Mykos Gold treatment. We saw no differences in treatment interactions in NJ.
Table 3. Leaf nutrient analysis results for our NY, NJ, and NH field sites. Treatments with differing letters significantly differ (p=.05) according to Tukey's HSD test.
| 2024 Leaf Nutrient Analysis | ||||||||||
| NY | ||||||||||
| Fungi | N | P | K | Ca | Mg | Mn | Fe | Cu | B | Zn |
| Symvado | 1.75 | 0.32 | 1.41 | 1.14 | 0.30 | 55 | 35.6 | 5.40 | 31.0 | 21.0 |
| Mycobloom | 1.82 | 0.32 | 1.40 | 1.18 | 0.29 | 48 | 35.7 | 5.39 | 30.9 | 20.3 |
| Mykos Gold | 1.92 | 0.37 | 1.55 | 1.21 | 0.28 | 46 | 38.7 | 5.95 | 31.2 | 22.0 |
| Promate | 1.75 | 0.33 | 1.60 | 1.04 | 0.28 | 56 | 35.4 | 5.06 | 30.6 | 20.0 |
| Control | 1.71 | 0.27 | 1.35 | 1.16 | 0.31 | 55 | 34.0 | 5.47 | 30.8 | 21.8 |
| P-Value | 0.4936 | 0.3346 | 0.0637 | 0.1488 | 0.3078 | 0.0791 | 0.4397 | 0.2274 | 0.9733 | 0.7387 |
| Fertility | N | P | K | Ca | Mg | Mn | Fe | Cu | B | Zn |
| Low | 1.68 B | 0.35 | 1.53 A | 1.04 B | 0.27 B | 51 | 34.7 | 5.07 B | 31.6 A | 20.9 |
| High | 1.90 A | 0.29 | 1.39 B | 1.26 A | 0.32 A | 53 | 37.0 | 5.84 A | 30.2 B | 21.2 |
| P-Value | 0.0078 | 0.0650 | 0.0228 | 0.0001 | <.0001 | 0.6314 | 0.1393 | 0.0024 | 0.0160 | 0.7564 |
| Fertility x Fungi | N | P | K | Ca | Mg | Mn | Fe | Cu | B | Zn |
| Low Symvado | 1.60 | 0.30 | 1.32 AB | 1.01 | 0.28 | 57 | 33.8 | 5.04 | 30.8 | 21.7 |
| Low Mycobloom | 1.79 | 0.33 | 1.38 AB | 1.11 | 0.28 | 46 | 34.1 | 5.18 | 31.4 | 19.3 |
| Low Mykos Gold | 1.84 | 0.46 | 1.77 A | 1.14 | 0.25 | 42 | 40.3 | 5.55 | 32.2 | 19.7 |
| Low Promate | 1.73 | 0.36 | 1.77 A | 0.96 | 0.25 | 56 | 35.5 | 5.03 | 31.6 | 20.5 |
| Low Control | 1.42 | 0.28 | 1.42 AB | 0.95 | 0.26 | 55 | 29.6 | 4.55 | 31.9 | 23.0 |
| High Symvado | 1.89 | 0.33 | 1.5 AB | 1.27 | 0.31 | 53 | 37.3 | 5.76 | 31.2 | 20.3 |
| High Mycobloom | 1.85 | 0.31 | 1.41 AB | 1.25 | 0.31 | 50 | 37.2 | 5.60 | 30.4 | 21.3 |
| High Mykos Gold | 2.00 | 0.27 | 1.32 AB | 1.28 | 0.31 | 50 | 37.1 | 6.35 | 30.2 | 24.4 |
| High Promate | 1.78 | 0.29 | 1.42 AB | 1.12 | 0.31 | 56 | 35.3 | 5.09 | 29.6 | 19.5 |
| High Control | 2.00 | 0.26 | 1.27 B | 1.37 | 0.35 | 54 | 38.3 | 6.39 | 29.7 | 20.7 |
| P-Value | 0.2292 | 0.1412 | 0.0161 | 0.2178 | 0.1982 | 0.7273 | 0.1922 | 0.1856 | 0.5382 | 0.3040 |
| NJ | ||||||||||
| Fungi | N | P | K | Ca | Mg | Mn | Fe | Cu | B | Zn |
| Symvado | 2.29 | 0.3 | 1.5 | 1.46 | 0.22 B | 154 | 65 | 10 | 32 | 27 |
| Mycobloom | 2.14 | 0.27 | 1.30 | 1.49 | 0.27 A | 151 | 62 | 10 | 34 | 25 |
| Mykos Gold | 2.13 | 0.23 | 1.26 | 1.51 | 0.26 AB | 149 | 63 | 10 | 33 | 25 |
| Promate | 2.12 | 0.28 | 1.36 | 1.51 | 0.27 A | 141 | 63 | 11 | 33 | 26 |
| Control | 2.27 | 0.29 | 1.32 | 1.53 | 0.26 AB | 151 | 64 | 10 | 32 | 27 |
| P-Value | 0.2653 | 0.6222 | 0.3330 | 0.8820 | 0.0119 | 0.5760 | 0.9080 | 0.6049 | 0.4594 | 0.7626 |
| Fertility | N | P | K | Ca | Mg | Mn | Fe | Cu | B | Zn |
| Low | 1.8 B | 0.37 A | 1.37 | 1.3 B | 0.24 B | 149 | 54 B | 9 B | 34 A | 27 |
| High | 2.58 A | 0.18 B | 1.32 | 1.7 A | 0.27 A | 150 | 72 A | 11 A | 32 B | 25 |
| P-Value | <.0001 | <.0001 | 0.5120 | <.0001 | 0.0002 | 0.7879 | <.0001 | 0.0018 | 0.0042 | 0.1538 |
| Fertility x Fungi | N | P | K | Ca | Mg | Mn | Fe | Cu | B | Zn |
| Low Symvado | 1.98 | 0.42 | 1.62 | 1.25 | 0.2 | 162 | 56 | 9 | 33 | 30 |
| Low Mycobloom | 1.61 | 0.35 | 1.22 | 1.3 | 0.26 | 151 | 53 | 9 | 34 | 28 |
| Low Mykos Gold | 1.66 | 0.29 | 1.26 | 1.28 | 0.24 | 143 | 55 | 9 | 33 | 26 |
| Low Promate | 1.80 | 0.39 | 1.34 | 1.38 | 0.26 | 137 | 54 | 9 | 34 | 25 |
| Low C | 1.92 | 0.41 | 1.41 | 1.31 | 0.24 | 150 | 56 | 9 | 33 | 26 |
| High Symvado | 2.60 | 0.18 | 1.37 | 1.68 | 0.28 | 147 | 75 | 10 | 32 | 25 |
| High Mycobloom | 2.66 | 0.19 | 1.38 | 1.68 | 0.28 | 151 | 71 | 10 | 33 | 24 |
| High Mykos Gold | 2.59 | 0.18 | 1.25 | 1.73 | 0.25 | 155 | 71 | 10 | 32 | 23 |
| High Promate | 2.44 | 0.18 | 1.37 | 1.64 | 0.28 | 145 | 72 | 12 | 32 | 26 |
| High C | 2.62 | 0.17 | 1.23 | 1.75 | 0.27 | 153 | 73 | 10 | 31 | 28 |
| P-Value | 0.1749 | 0.5285 | 0.4643 | 0.5384 | 0.5355 | 0.5446 | 0.9939 | 0.5932 | 0.8449 | 0.3406 |
| NH | ||||||||||
| Fungi | N | P | K | Ca | Mg | Mn | Fe | Cu | B | Zn |
| Symvado | 2.21 | 0.18 | 1.62 | 0.76 | 0.17 | 38 | 58 | 6 | 39 | 10 |
| Mycobloom | 2.24 | 0.17 | 1.52 | 0.77 | 0.15 | 36 | 57 | 6 | 37 | 11 |
| Mykos Gold | 2.28 | 0.17 | 1.53 | 0.81 | 0.16 | 38 | 55 | 7 | 38 | 11 |
| Promate | 2.27 | 0.17 | 1.57 | 0.81 | 0.16 | 34 | 54 | 7 | 38 | 11 |
| Control | 2.27 | 0.17 | 1.6 | 0.81 | 0.16 | 38 | 55 | 7 | 39 | 11 |
| P-Value | 0.8430 | 0.6882 | 0.4031 | 0.7701 | 0.5795 | 0.2925 | 0.6855 | 0.5313 | 0.3743 | 0.2976 |
| Fertility | N | P | K | Ca | Mg | Mn | Fe | Cu | B | Zn |
| Low | 2.23 | 0.17 | 1.56 | 0.78 | 0.16 | 36 | 55.0 | 6 | 38 | 11 |
| High | 2.28 | 0.17 | 1.58 | 0.8 | 0.16 | 37 | 57.0 | 7 | 39 | 11 |
| P-Value | 0.3497 | 0.9672 | 0.4826 | 0.5501 | 0.6751 | 0.8265 | 0.4214 | 0.9319 | 0.1543 | 0.9163 |
| Fertility x Fungi | N | P | K | Ca | Mg | Mn | Fe | Cu | B | Zn |
| Low Symvado | 2.13 | 0.18 | 1.48 AB | 0.76 | 0.18 | 40 | 60 | 6 | 37 | 10 |
| Low Mycobloom | 2.20 | 0.16 | 1.53 AB | 0.75 | 0.15 | 35 | 55 | 6 | 37 | 10 |
| Low Mykos Gold | 2.22 | 0.17 | 1.61 AB | 0.77 | 0.16 | 34 | 55 | 7 | 39 | 10 |
| Low Promate | 2.31 | 0.17 | 1.54 AB | 0.83 | 0.16 | 30 | 50 | 7 | 36 | 10 |
| Low Control | 2.29 | 0.17 | 1.63 AB | 0.82 | 0.16 | 43 | 55 | 7 | 39 | 12 |
| High Symvado | 2.28 | 0.17 | 1.75 A | 0.77 | 0.15 | 36 | 56 | 7 | 42 | 10 |
| High Mycobloom | 2.27 | 0.17 | 1.52 AB | 0.8 | 0.15 | 37 | 58 | 6 | 37 | 11 |
| High Mykos Gold | 2.35 | 0.17 | 1.45 B | 0.85 | 0.17 | 42 | 55 | 7 | 37 | 11 |
| High Promate | 2.22 | 0.17 | 1.61 AB | 0.78 | 0.16 | 38 | 58 | 6 | 39 | 12 |
| High Control | 2.25 | 0.18 | 1.58 AB | 0.8 | 0.16 | 33 | 56 | 7 | 38 | 11 |
| P-Value | 0.1132 | 0.3057 | 0.0243 | 0.2713 | 0.2434 | 0.0316 | 0.5977 | 0.3954 | 0.078 | 0.3444 |
These results suggest there was little to no effect of our fungi treatments on tree nutrient acquisition in 2024. It may be that our fungi applications still have not colonized the trees adequately, but at this point we would not recommend replacing fertilizer treatments with fungi applications.
Water Use Efficiency
2024 field samples are still awaiting processing.
Greenhouse Studies
Summary results for the greenhouse experiment do not indicate major impacts of AMF treatments in 2024.
Tree Growth
Initial growth rates were highly variable by individual tree, so growth comparisons were made in a two-week period after cutting back all primary growth to leave 2 buds. New growth from these two buds was initiated, but no significant differences in growth rate were detected in either the AMF or stress treatments. Clear phenotypic evidence of drought stress was observed, but the stress did not severely impact overall growth. Numerically, drought and low phosphorus treatments appeared to impact the growth of B.9 and G.11, but the AMF treatments did not seem to buffer this decrease. The caveat of this observation is that overall growth rates were quite low, so treatment differences would be difficult to detect.
Stomatal Conductance and Photosynthetic Efficiency
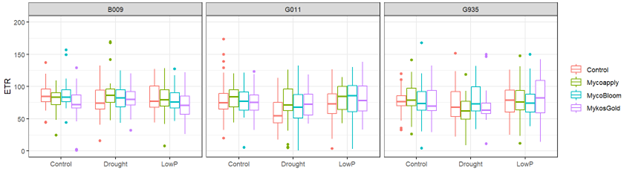
Examination of stomatal conductance and photosynthetic efficiency responses also did not indicate strong treatment effects. When examining the impact of treatment combinations on electron transport, there were some numerical trends in treatment and AMF response. Namely, drought appeared to reduce ETR measurements when there was no AMF included, but was buffered with all three AMF treatments. For B.9, Symvado and MycoBloom appeared to be more effective at this buffering response than Mykos Gold. For G.11, all three AMF products appeared to help buffer the drought depression in ETR measurements. However, no major effect was observed for G.935. For low phosphorus treatments, it appeared that B.9 had the greatest reduction in ETR. All three AMF products appeared to increase ETR for G.11 relative to control. Again, no major effect was observed for G.935.
Electron transport respiration (ERT) data shows drought response in B.9 and G.11 were numerically buffered by fungi treatments. No effects were observed in G.935.
Additional 2024 data analysis is ongoing.
Education & Outreach Activities and Participation Summary
Educational activities:
Participation Summary:
The following events were held in 2024:
We held a Soil Health Webinar on February 26th. At this meeting, Mike Basedow gave an overview of the project and our initial results. In addition, we also had presentations on soil health testing, and research project looking at improving soil health with organic matter additions. This meeting was attended by 60 growers.
A 15 minute talk on our project was given at the Tree Fruit Research Plot Tour at the Snyder Research and Extension Farm in Pittstown NJ on May 17th. Megan Muehlbauer gave an overview of the project, and our initial results. The audience included 25 extension professionals.
Our NJ field site tour was held on May 22nd. At this meeting, Megan Muehlbauer gave an overview of the project and our initial results. In addition, Ashley Asdal and Jamie Bourgeois gave an overview of the farm, and researchers and extension agents provided seasonal pest management information. This meeting was attended by 30growers.
Our Eastern NY field site tour was held on August 15th. At this meeting, Mike Basedow gave an overview of the project and our initial results. In addition, Deborah Aller presented on orchard soil health, and Kitty O'Neil presented on orchard climate resilience. This meeting was attended by 25 people. 20 of them were farmers or farmworkers, of which three were Jamaican.
Our NH field site tour was held on August 21st. At this meeting, Jeremy DeLisle gave an overview of the project and our initial results. In addition, a tour and taste test of a cold hardy peach trial were conducted, along with presentations on seasonal IPM topics from UNH extension specialists. This meeting was attended by 97 people. 83 of them were farmers or workers, of which 21 were Jamaican.
The project was also discussed at the Snyder Farm Fruit and Nut Crop tour that was held on August 22 in Pittstown NJ. Megan Muehlbauer gave an overview of the project and our initial results. This tour was attended by 75 members of the American Farmland Trust.
Our Western NY bilingual meeting was scheduled to be held on June 18th. Due to poor registration, we rescheduled the meeting for August 22nd. We once again had very poor registration for this meeting (three attendees), so we decided to cancel the meeting. Our plan is to hold a final meeting in the summer of 2025, once we have additional data to share.
Learning Outcomes
- Arbuscular mycorrhizal fungi (AMF) and their potential benefits to orchards
- Orchard Soil Health