Progress report for LS22-363
Project Information
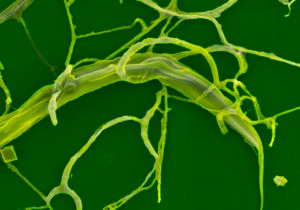
Grazing with the Fun Guy (or fungi). This project will show it is possible to provide a strategy to offer the fungus (see photo of parasite larvae trapped by Duddingtonia flagrans fungus), which controls gastrointestinal worms on pasture leading to improved animal health and productivity, and enhancing sustainability of livestock farms.
Since our first SARE R&E grant in 2002 (LS02-143), we have strived to include nematode-trapping fungus (Duddingtonia flagrans or Df) in the toolbox for livestock producers to control gastrointestinal nematode (GIN) parasites. However, commercialization of the fungus (BioWorma®) took 17 years! GIN are the major health threat for ruminant livestock confounded by dewormer resistance, leading to anemia, poor weight gains and death. The goal of sustainable farms is to eliminate chemical inputs and control GIN; the fungus brings this closer to reality. The fungus can remove much of the GIN on farm. Approximately 90% of GIN are found on the pasture and 10% in the animal; Df can reduce up to 90% of larvae on pasture. The product is considered costly by most farmers (between $0.20 to 0.60 per 100-pound animal per day). Thus, it is imperative to build a strategic program for farmers that considers effectiveness and economics. For example, can Df be fed every other day, or every other week or only in loose mineral supplements? These questions will be answered in research flocks/herds and on farm by examining changes in fecal egg counts, pasture larval counts and/or animal worm counts.
The fungus as part of the farm system will be considered among other management tools to minimize worms and optimize animal health. These include genetic resistance to lower GIN infection and increase economic value (current grant, OS19-124), and use of copper oxide wire particles (COWP) as a dewormer (included in CVs of PIs). These can lead to reduced time spent on GIN management, increased animal growth and production since the immune system is not overburdened, and a greater regenerative agriculture potential without the reliance on chemicals.
We have a rich outreach program through the American Consortium for Small Ruminant Parasite Control (www.wormx.info) which meets twice yearly including virtual meetings. Dr. Whitley is a small ruminant specialist with a strong extension program providing several farmer training sessions yearly (virtual or live) and on an ACSRPC extension committee to produce videos, fact sheets and translation of farmer publications to Spanish. We will communicate with K. Matthews, Delaware State University (LNE21-418), an ACSRPC member also investigating the use of Df. Thus, we can disseminate findings and build recommendations for use of the fungus in the farm system. We will work with Susan Schoenian, University of Maryland (expected to retire) ACSRPC website developer, on progressive development of the ACSRPC website to gauge the frequency of use of materials on the site. We will explore ACSRPC on Facebook and develop more infographics and sound bites of information to lead to more in depth info on the website.
- Examine practical approaches to administer Duddingtonia flagrans (Df) to sheep and goats to obtain good gastrointestinal nematode (GIN) control.
- Examine complementary approaches of using genetics, Df and other GIN control technology within the small ruminant grazing system.
- Develop new outreach materials as fact sheets, videos and webinars in collaboration with farmers, and increase use of available technology for GIN control to disseminate strategies of GIN control that minimize the need for deworming.
Cooperators
- - Technical Advisor
Research
Validation trial. Lambs had access to grass pastures contaminated with parasitic nematode larvae before trial began and were naturally infected. In early January 2023, Katahdin lambs, ~90 days of age, were randomly assigned to 2 treatments (8/treatment; 2 lambs/pen for feeding): 1) control (no D. flagrans or BioWorma), 2) D. flagrans or BioWorma (International Animal Health Prod. Pty. Ltd., Huntingwood, NSW, Aust; Lot no. 3167101, mfr Oct, 2022). All lambs received the same grain supplement and trace mineral mixed into feed fed per kg body weight (Premier Sheep Trace Mineral Premix mixed with salt at a rate of 5:50 per manufacturer recommendations). Lambs were acclimated to pen feeding for 7 days before beginning treatment diets for 7 days. Lambs weighed ~25 kg and were fed 60 mg BioWorma/kg body weight daily. Lambs were housed together and brought into pens in pairs for feeding daily then returned to plots after feeding, and had free choice bermudagrass plus alfalfa hay, water, and access to shelter/shade.
Sample Collection: Feces were collected on first day of diet acclimation, then, one week later there were three sample day collections every other day to determine fecal egg count (FEC). Feces were cultured to examine L3 recovery rate (L3 larvae/FEC) relative to control group and determine the predominant gastrointestinal parasite (H. contortus or Trichostrongylus spp.; plus Cooperia spp. or Oesophagostomum spp.).
Full study. Katahdin lambs, 3 months of age, had access to grass pastures contaminated with parasitic nematode larvae before trial began and were naturally infected. Lambs were randomly assigned to treatments (8/treatment; 2 lambs/pen for feeding): 1) control (no D. flagrans; CON), 2) D. flagrans as BioWorma (BioWorma dose based on the heaviest animal within the group) as recommended top dressed on grain supplement (DfCON), or 3) D. flagrans as BioWorma (same dose as Trt 2) mixed in 7 g/head of a trace mineral mix (Premier Sheep Trace Mineral Premix mixed with salt at a rate of 5:50 per manufacturer recommendations; DfMIN). The mixed mineral and salt were mixed with BioWorma and stored for 7 days before top dressed onto lamb’s supplement. All lambs received the same supplement and trace mineral mix fed per kg body weight. Lambs were acclimated to pen feeding and diets (control) for 7 days before beginning treatment diets for 21 days. Lambs weighed ~25 kg and were fed 60 mg BioWorma/kg body weight daily for 28 days. Lambs had free choice bermudagrass plus alfalfa hay, water, and access to shelter/shade. A similar study was conducted in the field at Louisiana State University (see below).
Sample Collection: Starting on first day of diet acclimation, blood and feces were collected every 3 days (on Mon or Tuesday, and Thur) for determination of blood packed cell volume (PCV; every 7 d) and FEC. Feces will be cultured throughout the trial to examine L3 recovery rate (L3 larvae/FEC) relative to control group and determine the predominant gastrointestinal parasite (H. contortus or Trichostrongylus spp.; plus Cooperia spp. or Oesophagostomum spp.; still need to be identified). Lambs were weighed at start/end of trial.
Objective 2. Examine complementary approaches of using genetics, fungus and other GIN control technology within the small ruminant grazing system. One of the goals of this objective was to use Df in a mineral on farm by farmer collaborators. We learned through thorough investigation that there were not enough spores in the BioWorma used in the first year of study which set us behind. We were unable to conduct the study on inclusion of Df in mineral on pasture until summer 2023. We were unable to find farmer cooperators (some listed in the grant had sold their farm and others had too low parasite burden to test any hypotheses) to complete this goal. However, both the controlled and field study at LSU showed good results in including Df in a mineral mix for sheep. A similar field study in goats will occur at Fort Valley State University in summer 2024. The second goal was to examine complementary approaches of using genetics, Df and other GIN control technology within the small ruminant grazing system. The two farms (Coffeys and a replacement farm, Heifer Ranch, Perryville, AR) willing to use Df in a trace mineral had a very low incidence of parasitism in their flocks. These farms are good examples of excellent management practices for grazing and parasite control showing that parasites can be managed, even in Arkansas, so as not to cause undue health concerns in sheep. Their management includes rotational grazing, forage management, planting legumes, use of parasite resistant breeds or animals, and multi-species grazing (with cattle). As a follow-up, for the final report and product, we plan to interview these farms for a publication on what has been done well to manage parasites.
Finally, another goal was to consider genetic selection for GIN resistance which is feasible through the National Sheep Improvement Program (NSIP; www.nsip.org) and provides estimated breeding values for parasite resistance and other economic traits associated with farm data. The ARS flock has used NSIP for genetic selection since 2012 and has some of the most resistant sheep in the program. Two other farmer cooperators (J. Morgan and R. Newton) contributed data for this objective. Lambs (all) with NSIP breeding values were monitored between 60 and 150 days of age, the most susceptible time period for worm infection, for FEC, anemia and deworming events (dewormed when FAMACHA > 3 or packed cell volume < 19%) to determine a “safe” estimated breeding value, or a level that requires no deworming. Three farms (ARS, >200 lambs/year; Morgan; Newton) used 2 high FEC resistant breeding value rams to improve genetic resistance on farm compared with at least 2 less resistant or susceptible rams. Fecal samples were collected from all offspring per sire around 60 and 90 days of age to determine FEC, a measure of resistance. Differences in FEC among sire groups will be compared; ARS will have a sufficient data set from previous years and during project to compare outcomes. Analyses is pending.
Duddingtonia flagrans included in trace mineral mix or feed for control of gastrointestinal nematodes in sheep
J.M. Burke, K. Petersson, E. Kass, J.E. Miller, A. Vatta, M. Acharya, S. Rohila
Objective was to determine efficacy of Duddingtonia flagrans (Df) included in a mineral mix compared with a feed supplement in Katahdin ewe lambs weaned Jan 2022 (76 ± 2.0 d of age; 21.2 ± 1.1 kg). Lambs were supplemented in pairs with a 12% CP sweet feed (226 g and 6 d later 450 g/lamb) that was thoroughly mixed daily with trace mineral mix [7.1 g salt (87.7%), Sheep Trace Mineral Premix (8.8%), and Vitamin ADE mix (3.5%), Premier 1] and coccidiostat (0.2 g of Decoquinate Type A as Deccox®, Premier 1). Lambs were randomly assigned to one of three treatments (n = 8/treatment; 2/pen): 1) control (CON) of no Df, 2) Df (BioWorma® Int. Anim. Health Prod.) mixed in supplement as recommended (DfC; weighed daily at 1.7 g/lamb), or 3) Df added in mineral (DfM; trace mineral was mixed with 13.6 g Df for 8 lambs then stored for 7 d in a Ziploc bag) which was then added to the supplement. The Df dose used was for the heaviest lamb. Pair feeding lasted 1 h, then lambs had access to free choice bermudagrass hay and water. Fecal samples were collected for fecal egg counts (FEC) and coproculture in pairs twice weekly, and blood collected to determine packed cell volume (PCV) weekly between d -2 and 27. Larval recovery was calculated per pair: (L3/g feces/average FEC) × 100. GIN were 51.3% Trichostrongylus spp., 44.9% Haemonchus contortus and < 2% each of Cooperia spp., Oesophagostomum spp. and Teladorsagia spp. Most samples contained some Strongyloides. FEC were log transformed; FEC, PCV, larval recovery were analyzed using repeated measures over time. There was a treatment × date interaction for FEC (P = 0.02) but DfM was similar to CON throughout. PCV were similar among treatment groups (P > 0.10). Surprisingly, larval recovery was similar among treatments (P > 0.10). In working with the Df manufacturer, it was determined that spore count of the BioWorma of this lot number was ~10-fold lower than anticipated (possibly due to mixing) or required for expected larval recovery. In addition, the coccidiostat mixed in the feed may have had an adverse effect on Df viability and deserves further attention. A subsequent study was conducted using BioWorma from a new lot, validating the spore viability and repeating the objective. Results will be presented in next report. Implications are that Df products should be tested for viability before use. This has prompted us to develop simple in vitro cultures to be conducted before research studies. These in vitro studies will be presented in the next report.
Df in mineral to sheep on pasture at Louisiana State University. The objective was to determine the effectiveness of mixing Df in mineral to sheep on pasture. The Df spores must survive long term exposure to mineral to be successful on pasture. In 2023, at LSU, 150-day old Katahdin and Hampshire lambs of mixed sexes weighing 34 kg (mean) were randomly assigned to 1) a control of no Df, 2) Df to be fed as recommended top dressed on a corn/soybean meal supplement, or 3) incorporated at a rate estimated to be the same in a loose trace mineral mix (based on determined intake of trace mineral mix prior to initiation of study), n = 16/treatment, 2 reps/treatment (48 lambs total). Lambs will be fed for 14 d to acclimate and 28 days on treatment. All groups had free choice mineral and same feed per unit body weight according to NRC recommendations. For the Df/mineral, the combination was “aged” by mixing 7 days before feeding and replaced every 7 days. Measurements: Blood was collected every 7 days for determination of packed cell volume, and feces for FEC and fecal culture twice weekly, described above. As in the last subobjective, larval recovery was the most important measurement for this objective. Lambs were weighed every 7 days and need for deworming was recorded. Data were analyzed using mixed models with repeated measures over time (SAS). Variables in the model included treatment, sex, breed, replicate, day or week, and all possible interactions.
Gastrointestinal nematode larvae were predominantly H. contortus. The mean FEC among treatments was 3343 eggs/g and mean PCV was 23.1 +/- 0.8%; no differences were detected among treatments. Importantly, larval recovery of both Df treatments was reduced (P < 0.001) compared with control, and no difference detected between the two Df treatments. Thus, Df remained viable in the aged mineral in a field study in which sheep were allowed ad lib access to a mineral mix that contained Df.
Determining in vivo and in vitro predatory activity of Duddingtonia flagrans and interaction with dietary coccidiostat. (JM Burke, S Rohila, K Petersson, E. Kass, A. Vatta, J.E. Miller). The objective was to validate methods to examine the interaction between Df and dietary coccidiostat. In August 2023, 7 mo old anthelmintic-treated ram lambs (FEC < 500 egg/s) were fed supplement daily with coccidiostat (+C) or without (-C; n = 3/diet) on d -7 to 1, and, within diet, administered 3 g BioWorma®/d in 6 gel capsules (+DF; n = 2/diet) or not (-DF; n = 1) on d -1 and -2. Fecal samples were collected on day 0 (2 g/plate + 300 L3 of H. contortus) and d 1 (6 g/plate + 1000 L3) and placed on water agar plates (n = 3/lamb) at 25°C. Trapping structures were visually assessed after 24, 48, and 144 h, and cultures baermannized on d 7 and 8, respectively for L3 recovery. Data were analyzed by Proc GLM (SAS) as a 2×2 factorial . For the d 0 plates, DF formed loops and traps around L3 by d 6 and reduced L3 recovery (P < 0.001) without an effect (P = 0.38) or interaction (P > 0.05) by coccidiostat. For the d 1 coproculture, feces from one +DF-C ram appeared to have non-H. contortus L3 (which will be confirmed) and was removed from analyses. However, +C-DF reduced L3 recovery (P = 0.003) but did not antagonize DF. This aspect of the study was repeated with additional reps (analyses pending), though it seems clear that Deccox can be added to the diet in the presence of BioWorma®. The in vitro studies allow detection of adverse issues with viability of Df and can reduce the number of animals needed for research studies.
Education
The American Consortium for Small Ruminant Parasite Control performs outreach and teaching through the extension specialists and technology transfer specialists within the group (see www.wormx.info). In addition, knowledge gained through research conducted by the ACSRPC has been integrated into curriculum for veterinary students at the University of Georgia, Louisiana State University, Virginia Tech, Michigan State University, Ohio State University, Tuskegee University, St. George’s University in Grenada, and Ross University in St. Kitts. The website has continued to update fact sheets and other materials available, primarily focused for producers and extension specialists (ACSRPC | Best Management Practices (wormx.info)). The blog has been reactivated and focuses on synopsis of research articles: Weekly Blog | wormx. Several articles are available in Spanish (ACSRPC | En español (wormx.info)).
Other education included the following.
Gave invited presentation at Lincoln University on control of parasites in small ruminants, April 2023. Train-the-trainer event attended by 20 extension specialists.
Small Ruminant Field Day, DBSFRC, Booneville, AR, April 2023. Topics included parasite control in small ruminants, grazing and forages, nutrition, health of sheep and goats. Attended by 50 farmers and extension specialists.
ACSRPC meeting, LSU, Baton Rouge, LA, May 2023, attended by 30 members and students. Included presentation and discussion of the SARE nematode-trapping fungus research, outreach and education, collaboration. Virtual meeting, December 2023. Attended by 20 members. Gave update on SARE research.
NC-214 Efficiency of Sheep Production committee meeting, Dubois, ID, June 2023, attended by 30 sheep researchers, extension specialists, and graduate students. Presented Station Report on SARE nematode-trapping fungus research.
Congressional Field Day, DBSFRC, Booneville, AR, August 2023, attended by 50 USDA administrators, farmers, and extension specialists. Gave presentation on research which included SARE nematode-trapping fungus research.
American Dairy Goat Association Annual Conference, Tulsa, OK, October 2023. Gave invited presentation on control of parasites in goats; attended by 20 farmers.
Small Ruminant Field Day, Heifer International, Heifer Ranch, Perryville, AR, November 2023, attended by 50 farmers. Gave invited presentation on control of parasites in small ruminants.
American Sheep Industry Association Annual Conference, Denver, CO, January 2024. Gave invited presentation on nematode-trapping fungus, including SARE research results. Attended by 70 farmers, veterinarians, and extension specialists.
Southern Section American Society of Animal Science Annual Meeting, Louisville, KY, January 2024. Gave abstract presentation in Small Ruminant Production session on SARE research on nematode-trapping fungus. Attended by 40 animal scientists, students, extension specialists, and educators.
Outreach and Education for LSU:
VATTA, A.F., 2024. Parasites—Anthelmintics in sheep and goats: Making the best use of what we have. 2024 Louisiana Veterinary Medical Association Winter Meeting, Shreveport, Louisiana, January 26-28, 2024.
VATTA, A.F., 2023. Deworming sheep and goats? What you should know about anthelmintic resistance and the correct use of dewormers. American Association of Small Ruminant Practitioners Webinar, November 16, 2023.
VATTA, A.F., 2023. A new old parasite—liver fluke. Oral presentation with article published in: Proceedings of the American Association of Bovine Practitioners 56th Annual Conference, American Association of Small Ruminant Practitioners Session, Milwaukee, Wisconsin, September 21-23, 2024.
Educational & Outreach Activities
Participation Summary:
The website of the American Consortium of Small Ruminant Parasite Control has been updated to include several Spanish translations of fact sheets and infographics: ACSRPC | En español (wormx.info). In addition there were 10 fact sheets published on Other Worms: ACSRPC | Other Worms (wormx.info), and 11 infographics (ACSRPC | Infographics (wormx.info)). An article was written for the Eastern Alliance Katahdins on how selection for parasite resistance in sheep affects other important traits: How Does Selection for Parasite Resistance in Katahdin Sheep Affect Other Important Traits? - Eastern Alliance for Production Katahdins (easternalliancekatahdins.com). The ACSRPC will hold their annual meeting at Louisiana State University in May 2023 to present research reports.
A field day at Dale Bumpers Small Farms Research Center, Booneville, AR occurred on April 29, presenting an update to use of nematode-trapping fungus to control parasites on pasture, and other alternative measures of parasite control. A webinar on worm control in small ruminants was presented at a train-the-trainer event at Lincoln University and at a producer's conference in Texarkana in April 2023. A train-the-trainer event was held at Tuskegee University and PIs presented information on small ruminant parasite control.
Publications:
Abstracts
Burke, J.M., Lewis, R.M., Murphy, T.W., Freking, B.A., 2024. Use of genetics to estimate resistance of gastrointestinal nematode infection in sheep. The 9th Novel Approaches Conference, St. George’s University, Granada, West Indies. March 2024.
Forbes, R.M., Murphy, T.W., Burke, J.M., Lewis, R.M., 2024. Adding parasite resistance to the breeding objective in hair sheep. (abstr. Midwest Sec. ASAS).
Nilson, S.M., Burke, J.M., Becker, G.M., Murdoch, B.M., Petersen, J.L., Lewis, R.M., 2024. Genomic and pedigree diversity of Katahdin sheep: The impacts of breeding and selection. (abstr. Int. Plant Anim Gen.; accepted 11/27/23).
Nilson, S.M., Burke, J.M., Becker, G.M., Murdoch, B.M., Petersen, J.L., Lewis, R.M., 2024. Genomic diversity of Katahdin sheep and impacts on genomic prediction. (abstr. Midwest Sec. ASAS).
Burke, J.M., Rohila, S., Petersson, K., Kass, E. Vatta, A.F., Miller, J.E., 2024. Determining in vivo and in vitro predatory activity of Duddingtonia flagrans and interaction with dietary coccidiostat. J. Anim. Sci. 102 (Suppl. 1), 91-92 (abstr.).
Gunes, H.Y., Howard, R., Fudolig, M., Burke, J.M., Lewis, R.M. 2023. Fit of the zero-inflated negative binomial model to analyze fecal egg counts. J. Anim. Sci. (abstr. National ASAS, July 2023).
Lewis, R.M., Freking, B.A., Heaton, M.P., Gore, K., Burke, J.M., Pejsar, B.G., Burgett, R.L., Brown, D.J., 2023. Determining genetic conditions in U.S. sheep with a medium density ovine bead array. J. Anim. Sci. (abstr. Western Sec. ASAS).
Popular
Burke, J.M., Lewis, R.M., Notter, D.R., 2023. How does selection for parasite resistance in Katahdin sheep affect other important traits? Eastern Alliance for Production Katahdins Newsletter.
Use of genetics to estimate resistance of gastrointestinal nematode infection in sheep (presented at the Novel Approaches of parasite control in livestock, March 2024, Grenada, West Indies (virtual)
J.M. Burke, R.M. Lewis, T.W. Murphy, B.A. Freking, L.F. Brito
The most sustainable defense against gastrointestinal nematodes (GIN) in small ruminants is genetic resistance and/or tolerance. While other tools will be necessary, with good management and nutrition, deworming will be minimized. Genetic evaluation programs exist throughout the world. The National Sheep Improvement Program (NSIP) in the U.S. combines phenotypes and pedigrees to calculate estimated breeding values (EBV). Genomic data have been incorporated to predict genomically-enhanced EBV (GEBV). More than 20 years of collecting fecal egg counts (FEC) resulted in genetic gains in parasite resistance in Katahdin sheep, and other traits of economic importance. More than 11,000 Katahdins in NSIP have been genotyped using a 50k SNP array; incorporating those data into GEBV has increased the accuracy of prediction of traits. Heritability of FEC is moderate at ~0.25. Producers should record animals from well-structured contemporary groups representing multiple sires and collect FEC around the time of weaning and postweaning with a group average of ≥500 eggs/g to obtain accurate FEC GEBV. Other traits of interest with moderate levels of heritability include FAMACHA© scores and packed cell volume, though farmers may not practically be able to collect the latter measure of tolerance. Because of a favorable genetic correlation with body weight, selection for parasite resistance can lead to increases in weaning and postweaning weights. However, there is a slight antagonism with prolificacy. Still, since this reproductive trait is lowly heritable, losses can be overcome with good nutrition at the time of breeding and co-selection for both traits. Whole genome scans failed to identify loci with large effects, indicating a trait influenced by a large number of genes with small effects. Thus, for breeding stock, it will be imperative to continue collecting FEC and other phenotypic traits and genotyping animals with phenotypic records, which in turn, is important as genetic trends favorably evolve.
Learning Outcomes
Farmers gained knowledge in use of copper oxide wire particles as an alternative to resistant dewormers to control parasites, and proper use of feeding fungus for pasture control of worms.
Farmers gained knowledge on practical application of feeding a nematode-trapping fungus, and an understanding of how the product works, what it does and does not do.
Farmers gained knowledge on the genetics of parasite resistance and its importance in controlling parasites within a flock/herd.
Project Outcomes
In the first year, there were issues with the Duddingtonia flagrans (fungus) product used and the initial research study had to be repeated three times, including a study at University of Rhode Island. Once a new lot of the fungus became available, we determined that the fungal spores were viable and the product worked as expected in reducing larval recovery when animals were fed according to manufacturer's directions, and the spores included in a loose mineral worked even better than that treatment. In vitro techniques were learned in the lab, which reduced turn-around time to validate the quality of the fungal spores used for research (and that can be use on-farm), reduced the number of research animals needed for studies to examine interactions with common feed ingredients such as coccidiostats. This led to the successful completion of field studies conducted at LSU and ARS, and this summer will be conducted at FVSU. In sheep, it was determined that the nematode-trapping fungus can be included in the mineral offered to sheep to reduce larvae on pasture. This practice worked as effectively as including the product in feed, but allows farms that do not feed grain to use the product for parasite control.
The two producer collaborators who were to work on the genetics objective failed to meet the requirements outlined. We have found additional farmers to contribute data for the genetics objective (one from Arkansas and two from Georgia). There will be three year's worth of data on examining differences in offspring parasite infection of known parasite resistant rams and unknown sires. An extension publication geared toward farmers will be forthcoming.
It is very difficult to find livestock farmers to contribute meaningful research data since their farm objectives can be quite fluid. I found two farms willing to feed BioWorma in the mineral, but their parasite load was not high enough for meaningful observations. It's been a common frustration over the last 20 years trying to include farmers on my grants in meaningful ways, other than the education component. Appreciate everything SARE has done for my research program!!