Final report for ONE15-250
Project Information
Since their introduction in 1991, neonicotinoids have quickly become the most popular insecticides worldwide. Within the past decade their use as preventative seed coatings has expanded enormously and now hundreds of millions of acres of field crops are planted annually with neonicotinoid seed treatments (NSTs). Unfortunately, the great majority of NSTs are used outside an IPM framework with little regard for pest populations even though limited unbiased evidence suggests that NSTs improve yield and profitability. Moreover, recent evidence suggests that they can pollute surface water and negatively influence wildlife, including populations of pollinators. In addition, our ongoing research is documenting that NSTs are exacerbating slug populations in no-till crop fields by disrupting biological control. This issue is important because slugs are among of the most challenging crop pests faced by Mid-Atlantic and Northeastern field crop growers.
In the first year of this project we planted corn and then we extended the project for a second year to explore the same questions in soybeans. In the first year, we had corn plots on four farms, while in the second year we had only three of these farms plant soybeans (the fourth farmer found that hosting plots conflicted with their operations). We finished the second year of the project in November 2016 when we harvested soybeans from the three farms. Some of the data (2015 data, and some of the 2016 data, including slug populations and damage) have already been analyzed while others are still a work in progress (e.g. identification of natural enemies and soybean yields; for this latter detail we are waiting for the yield numbers from the farmers).
From our 2015 effort and the 2016 preliminary results, the results appear to indicate that the planted green rye cover crop reduced slug abundance and damage to the subsequent crop. In 2015, slug populations were low, but in 2016 we had sizeable slug populations and we again detected on slug populations and natural enemies a strong influence of planting green (i.e., planting green reduced slug populations while increasing predator populations). Moreover, the neonicotinoid seed treatments resulted in plots having greater slug populations.
To combat slug infestations and over-reliance on NSTs, we are collaborating with four farmers in central Pennsylvania to test an alternative approach that avoids NSTs and uses rye cover crops to provide an alternative food source for slugs while maintaining strong populations of potential predators. Our two specific objectives were to:
- Determine the influence of a fall-established rye cover crop on slug and natural enemy populations and slug damage to corn and soybeans;
- Compare corn and soybean productivity and biological control of slugs in presence and absence of neonicotinoid seed treatments.
Cooperators
Research
On four farms of members of the Pennsylvania No-Till Alliance, we established in 2015 a factorial experiment crossing two levels of seed treatment (treated or untreated seed) with three levels of cover (bare plot, rye cover crop terminated before planting, rye cover crop terminated after planting). Two growers (Mr. Harbach and Mr. Anchor) hosted the full design with six treatments replicated three to four times each, whereas Mr. Criswell was not interested in a bare ground treatment, and Mr. Meyer was not interested in an early termination cover crop; thus two of the farms have a reduced number of treatments (four treatments, 16 plots) that fit within their production systems. Mr. Anchor owns a seed company (Agseed), and sold us the corn for the four farms, so corn variety was consistent across locations, and we have the same corn variety with treated and untreated seeds. During the season, it was apparent that two farms (Anchor and Criswell) had heavier slug infestation, while very little/no slugs were observed at Harbach and Meyer despite history of slug infestations in the fields.
In autumn of 2014, rye was planted on either 15” (Schrack farm) or 30” (the three other farms) rows and established so that rows of rye alternate with empty rows. In the spring 2015, we planted glyphosate-resistant corn without a seed treatment or seeds treated with the high rate of the neonicotinoid clothianidin (1.25 mg per seed). For the conventionally managed cover crops (referred to as “planting brown”), the cooperators terminated the rye in the spring two weeks prior to planting, whereas our cooperators killed the rye cover crop 5 days after planting (referred to as “planting green”).
To quantify damage and understand pest and natural enemy populations, we used a variety of sampling approaches. We measured slug populations every week in June and July for the four farms. Because very few slugs were found at Schrack farm and Meyer's farm, in August we only sampled the two other farms every other week because slugs were less abundant due to higher temperatures. To provide artificial shelters for slugs so we could track their populations, we used 0.1-m2 traps made of white roofing shingles (4 per plot) that reflect heat from the sun. Once corn reached the V3 growth stage, we assessed cover crop/crop residue per plot (i.e., percent cover) using the line-transect method (Laflen et al. 1981). At the V5 growth stages (in June), we assessed slug damage and corn establishment by counting the number of plants on 10 feet transects, per plot, and by using a 0-4 scale with 0 representing no damage; 1: 1-25% damage; 2: 25-50% damage; 3: 50-75% damage; and 4: 75-100% damage.
At the end of the growing season (November), we harvested the plots for yield using the growers’ field scale equipment. We analyzed damage, stand establishment and yield with analysis of variance (ANOVA). If variation between sites complicates analyses, we will use Z-scores to standardize value at each site relative to the controls and then analyze Z-scores across sites using ANOVA.
We also sampled natural enemy populations using pitfall traps (two per plot) and measured predation services using sentinel prey. Pitfall traps measure arthropod activity-density and comprise 32-oz plastic containers sunk in the soil and filled with small amounts of propylene glycol as a killing agent and preservative. We established the traps with lids to prevent accumulation of rainwater, and then removed the lids monthly (June, July, August) for 48h to capture arthropods and slugs. Because our previous research has identified the main slug predators in central Pennsylvania, our assessment will largely focus on C. tricolor and P. melanarius. We are currently working on identifying the arthropods we captured to the lowest taxonomic level possible.
We also deployed pinned sentinel waxworm caterpillars (six per plot) in June, July, and August for 24h, and then we visited the prey recording the status of the waxworms and any predators present (as in Lundgren et al. 2006). In August, we only sampled the two farms that had high slug infestations (Anchor and Criswell). We used waxworm caterpillars rather than slugs because slugs are uncooperative sentinel prey despite our best efforts; slugs will pull themselves off of pins and even sacrifice themselves if we confine them with rings of salt or copper.
We compared predation rates (Z-scores if necessary) across the two factors (seed treatments and cover) and their interaction using linear mixed models, with location as a random factor (repeated measures if needed). We compared activity density of C. tricolor and P. melanarius among treatments by ANOVA. Field community data will also be analyzed using multivariate statistics because the species are unlikely to be independent of each other. We will use linear discriminant analysis, or redundancy analysis (RDA), which is a linear method of direct ordination similar and complementary to principal components analysis (PCA). Significance of variables will be tested with a permutational analysis of variance (Anderson 2005) and a Monte Carlo simulation (999 iterations with a forward stepwise selection procedure) and ordination analyses will be conducted in CANOCO (V. 4.5; ter Braak and Šmilauer, 2002). We will also use linear regression to explore relationship between predation or predator communities with percent cover, slug densities, and other variables.
In 2016, we established a similar experiment on three of the farms (Anchor, Criswell, and Harbach). The size, footprints, and identity of our plots were the same from the previous year; the same plots were cover crop or not and received seed treatments or not, but were planted with soybeans in May 2016.
Accomplishments
Fall 2014
November
Plant rye in assigned plots (four growers)
Order corn for the four farms (Mr. Anchor)
Spring 2015
May
Terminate rye two weeks before planting corn (four growers)
Plant corn (four growers)
Terminate rye 5 days following planting corn (Mr. Harbach, Mr. Anchor, and Mr. Criswell).
Summer 2015
June through August
Measure slug populations (shingle traps) and scout for early pest every other week (Dr. Le Gall)
-We actually increased the sampling effort and sampled weekly.
June
Collect pitfall trap samples (Dr. Le Gall)
Deploy pinned sentinel waxworm caterpillars (six per plot) for three hours during the early morning, and then again in the early evening (Dr. Le Gall)
-Because of logistical constraints, we only checked the sentinel prey once, after leaving them 24h in the field
Assess cover crop/crop residue per plot (i.e., percent cover) using the line-transect method (Dr. Le Gall)
Corn V5 stage. Assess stand establishment by counting the number of plants in four randomly chosen 10-ft sections of row and measure slug damages (Dr. Le Gall)
July
Collect pitfall trap samples (Dr. Le Gall)
Deploy pinned sentinel waxworm caterpillars (six per plot) for 24h (Dr. Le Gall)
August
Collect pitfall trap samples (Dr Le Gall)
Deploy pinned sentinel waxworm caterpillars (six per plot) for 24h (Dr. Le Gall)
-We only made this effort on the two farms were slugs were actually observed (i.e. Anchor and Criswell)
Fall 2015
September
Schrack farm field day (Dr Tooker and Dr Le Gall), 60 attendees
Anchor farm field day (Dr Tooker), 50 attendees
November
Harvest corn and measure yield (four growers)
Establish rye cover crop (three growers: Anchor, Criswell, and Harbach)
Spring 2015
May
Terminate rye two weeks before planting corn (three growers)
Plant soybean (three growers)
Terminate rye 5 days following planting soybeans (Mr. Harbach, Mr. Anchor, and Mr. Criswell).
Summer 2016
June through August
Measure slug populations (shingle traps) and scout for early pest every other week (Dr. Le Gall)
June
Collect pitfall trap samples (Dr. Le Gall)
Deploy pinned sentinel waxworm caterpillars (six per plot), leaving them 24h in the field, then returned to assess survival.
Assess cover crop/crop residue per plot (i.e., percent cover) using the line-transect method (Dr. Le Gall) during the v5 stage of soybeans. Assess stand establishment by counting the number of plants in four randomly chosen 10-ft sections of row and measure slug damages (Dr. Le Gall)
July
Collect pitfall trap samples (Dr. Le Gall)
Deploy pinned sentinel waxworm caterpillars (six per plot) for 24h (Dr. Le Gall)
August
Collect pitfall trap samples (Dr Le Gall)
Deploy pinned sentinel waxworm caterpillars (six per plot) for 24h (Dr. Le Gall)
Fall 2016
September/November
Harvest corn and measure yield (Mr. Harbach, Mr. Anchor, and Mr. Criswell)
Impacts
In 2015, two farms had heavier slug infestations, Criswell and Anchor farms. Very few slugs were observed at Meyer and Schrack farms.
Considering 2015 (corn) and 2016 (soybeans) separately over the whole seasons, our results clearly show that slugs were more abundant where neonicotinoid seed treatments were used. This effect was very evident for the gray garden slug (Deroceras reticulatum), which is the most important pest species farmers experience, and less so for the marsh slug (Deroceras laeve), which rarely causes economic damage.
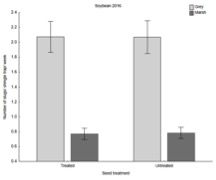
1) Corn damage (June 2015)
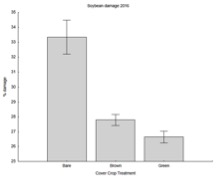
Across all farms, there was significantly less damage by slugs in the planting green treatment.

The effect is particularly true at the Criswell farm where 49% of the plants were damaged by slugs when the rye was killed prior to planting, while only 19% were when the rye was killed after planting. Interestingly, the level of damage in planting green (20%) is similar/lower than the levels observed in farms where there was no heavy slug infestation (Meyer and Schrack farms).
In 2015, the Anchor farm harbored a good population of slugs, providing a good assessment of the influence of planting green and seed treatments on slug populations. As hypothesized, slug population were highest where we had no cover crop, but used insecticidal seed treatments, but were statistically lower where we planted green and used untreated seeds!
Fig1-slugs planting green anchor
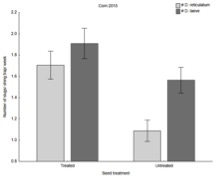
In 2016, slug populations were stronger than those in 2015, providing a more robust test of our hypothesis. Similar to our results in 2015, we continued to see a strong effect of cover crop treatment on slug populations. In fact, we again saw that the crop planted in to standing green cover had significantly less damage from slugs.
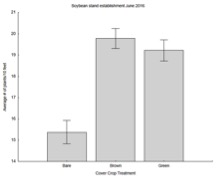
Combining our two main findings (i.e., neonicotinoid seed treatment and cover crop influence slug populations), our soybean experiment at Schrack Farms revealed a significant effect of both cover crop and seed treatment (see below); untreated plots consistently had lower populations of slugs, and cover cropped plots had fewer slugs than bare plots, and planting green harbored fewer populations than planting brown, which is the traditional approach to managing cover crops.
Planting green also harbored more predatory insects that can eat slugs. As an example, see the figure below, which summarizes the number of Chlaenius tricolor, an avid slug feeder. On Criswell Acres, planting green indeed fostered larger populations of this valuable predatory species. Unfortunately, we still do not have final numbers for our predator populations because the postdoc on this project had to leave for another job prior to wrapping up her work. I am hopeful this information will still be obtained.
If we consider stand establishment in corn and soybeans, we find that cover crops inconsistently influence the amount of damage that the two crops received. In corn in 2015, the average number of plants in 10 ft of row was about equal across the three cover crop treatments.
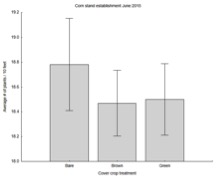
In soybeans, however, there were significantly more plants in June per ft of row in plots with cover crops (both brown and green) than plots with no cover crops, supporting our conclusion that cover crops can help mitigate the influence of slug damage. This detail is consistent with our season long results, and suggest that farmers will benefit from having cover crops in place to help control slug populations.
From our 2015 effort and the 2016 preliminary results, the results appear to indicate that the planted green rye cover crop reduced slug abundance and damage to the subsequent crop. In 2015, slug populations were low, but in 2016 we had sizeable slug populations and we again detected on slug populations and natural enemies a strong influence of planting green (i.e., planting green reduced slug populations while increasing predator populations). Moreover, the neonicotinoid seed treatments resulted in plots having greater slug populations.
Across all sites where we grew corn in 2015, we detected a significant yield drag from planting green; yield was about 10 bushels lower in our planting green treatment, whereas planting brown and no cover crops were equal. This was surprising because damage to planted green corn was less and slug populations are higher. This result is contradicted by experience of our cooperating growers in other fields where they claim to see a yield benefit from planting green.
Frustratingly, we have yet to pry yield results from our soybeans in 2016 from our cooperating growers and their crop consultants. Busy times for those guys, but we hope to get this information for the winter extension season.
Education & outreach activities and participation summary
Participation summary:
We featured our work exploring the value of planting green and using IPM at three field days on two of the participating farms, those of Jim Harbach (1 Sept 2015 [60 attendees], 22 Sept 2016 [120]) and Joe Anchor (17 Sept 2015 [50], 15 Sept 2016 [50]). We also presented the topic at other field days, including those presented by Penn State Extension or NRCS: (Penn State Farming for Success Field Day, 25 June 2015, Landisville, PA [200 attendees]; Penn State Diagnostic Clinic, 29-30 July 2015 , Rock Springs, PA 165 attendees]; NRCS Field Day, 17 Sept 2015, Drums, PA [40 attendees]; NRCS Field Day, 29 Oct 2015, Somerset, PA [35 attendees]; PA No-Till Alliance Field Day, 26 July 2016, St. Thomas, PA [75 attendees]; NRCS Warren County Producer Meeting, 1 Sept 2016, Asbury, NJ [60 attendees]).
We also presented on planting green and the valeue of IPM at many winter extension meetings for the past three years, exposing many farmers to the topic. These presentations were at various locations across most regions of Pennsylvania (about 16 meetings) and reached around 1300 grower and other agricultural professionals.
In addition to these local presentations, we also discussed this work in visits to Oregon, Virginia, Vermont, Maine, Ontario, Canada. Ontario, Oregon, and Virginia already suffer from slugs infestations, whereas growers in northern New England are concerned that slug populations might develop as more and more acres transition to no-till practices. In these locations, we gave presentations a nine winter meetings, reaching about 800 growers and associated agricultural professionals.
Following all these presentations, individual farmers would approach us to discuss the situations that they experienced. Other farmers would use the telephone to contact us at a later date. From these interactions, I estimated the number of consultations at around 50, but this number is hard to dtermine.
In addition to these extension presentations, we also gave two oral presentations of our research at two annual meetings of the Entomological Society of America. We have also published one research article that included data from our NE-SARE project (Marion Le Gall, John F. Tooker. (2017) Developing ecologically based pest management programs for terrestrial molluscs in field and forage crops. Journal of Pest Science 90: 825-838) and have another in development that we expect to submit before the end of 2017.
Learning Outcomes
These farmers changed knowledge about the threat from slugs, the range of pests that neonicotinoid seed treatments will control, and the value of cover cropping and pest control for pest control.
Project Outcomes
Three of our four cooperating farmers continue to plant green and use untreated seeds. Scores of other farmers have started using these practices to control slugs in PA, VA, and OH.
Our project success is attributable to understanding the biology of the system. Our previous research demonstrated that slug populations were exacerbated by neonicotinoid seed treatments. We also learned that slugs will feed upon green cover crops, rather than emerging cash crops, so the value of planting green for slug control had a biological basis. The biggest challenge have faced is push back from seed and chemical companies on the value of neonicotinoid seed treatments. Industry and many growers view seed treatments as vital to production, and dismiss concerns about them exacerbating slug problems because they assume on balance that the treatments provide a benefit when it comes to insect control. We have also faced a challenge because growers that want to try untreated seeds can have difficulty getting them. The restricted supply of untreated seed limits farmers' ability to follow our recommendation and use untreated seeds where they historically suffer from slugs. Nevertheless, we continue to research this system and will promote untreated seeds and planting green until our data say otherwise.
I strongly feel that more farmers would benefit from being exposed to our results. Even though I have an extension appointment and can research farmers, my reach and that of my colleagues is not long or wide enough to reach all the farmers that would appreciate the significance of our findings.