Final report for ONE17-300
Project Information
Saprophytic fungi can be paired with companion crops in interplant systems to increase production efficiency. However, fungal species/strain, substrate, and inoculation rate can affect the growth of companion crops. This project investigated the viability of open-field mushroom production by interplanting three strains of oyster mushrooms, Pleurotus ostreatus (Elm A, Elm B, and 8801), with kale (B. oleracea var. acephala) and forage radish (Raphanus raphanistrub sub. sativus), and measured the effect of interplanting on plant yield over two field seasons. In the field, Elm A showed an increase in plant yield (forage radish 500 gm/1.1 lb) at a low inoculation rate (244 gm) and decrease in plant yield (forage radish 104 gm/.23 lb) at a high inoculation rate (488 gm), compared to the untreated (forage radish 286 gm/.63 lb). Conversely, 8801 showed a reduction in plant yield (forage radish 195 gm/.43 lb and 163 gm/.36 lb) at high and low inoculation rates in the field. Elm B at a high rate showed a reduction in plant yield both in the field and greenhouse.
Kale was grown in hydroponics with fungal secretions added at a range of concentrations (10, 100, 1,000 and 10,000 ppm). Elm A showed an overall increase in plant yield in hydroponics (approx. 2.2 gm wet weight), and Elm B showed an overall decrease in plant yield (approx. 1.5 g, wet weight), compared to the untreated (approx. 1.8 gm wet weight).
For practical application, mushroom production was low in field plots and was not a commercially viable option. Pleurotus ostreatus interplanting methods with companion crops need improvement to make this a commercially viable practice.
Results were shared at an on-farm workshop in April 2018 with attendees practicing the inoculation techniques.
Our goal was to evaluate the concept of interplanting oyster mushrooms in-between rows of fall vegetable crops (forage radish and kale). We examined the effects of saprophytic fungal growth and subsequent mushroom production on the soil health and vegetable crop production. We planned to test two different types of oyster mushrooms (Pleurotus spp. and Hypsizygous spp.) with forage radish and kale to evaluate changes in crop yield. In addition, we sought to evaluate the rate of mushroom inoculum needed to produce changes in plant yield and/or acceptable oyster mushrooms. Lastly, we explored the questions of will interplanting oyster mushrooms produce acceptable mushroom yields? This method of outdoor production could see mushroom production in 3-4 weeks, a more timely return than the expected 9-12 month wait in log production (Fungi Ally pers. comm.).
Interplanting oyster mushrooms with vegetables has increased the productivity of the interplanted and subsequent crops (Abdallah et al. 2000; Mohamed et al. 2014). The spent mushroom substrate is turned into the ground as compost, thus returning available nutrients to the soil. Increased biomass and yield has been observed in brussels sprouts, cabbage, eggplant and faba bean; thus suggesting the beneficial impact of nearby mycelial growth and mushroom formation (Stamets and Pischl 1999; Abdallah et al. 2000; Mohamed et al. 2014). These experiments inoculated various substrates with mushroom mycelium and placed it in close proximity to vegetables and reported positive results in the treatments with mushroom production. Abdallah and Mohamed used multiple strains of oyster mushrooms, where Stamets and Pischl tried 9 different species of mushrooms to determine which species had the greatest benefit on production. They found oyster mushrooms to have the most beneficial effects on production of brassicas.
Our research aims to determine the viability of these practices in New England, since all prior research was conducted outside of the northeast region of the United States. However, we are encouraged by the similar fall climate between Massachusetts and the prior research locations for fruiting of oyster mushrooms (Mohamed et al. 2014). Stamets and Pischl concluded that fungi benefit the habitat by decaying dead plants to recycle carbon, hydrogen, nitrogen, phosphorus, and minerals into nutrients for living plants, insects and other organisms (Stamets 2005). If our proposed intercropping system is economically viable, small farmers can use it as a means of increase production and productivity while improving land use efficiency and stimulating local business. Forage Radish (Raphanus sativus var. longipinnatus) also known as Daikon, is a multi purpose crop grown in New England for food and as a nutrient recycling cover crop (Weil et al., 2009). Kale (Brassica oleracea var. sabellica) is another widely produced fall crop in New England. Both crops provide canopy’s that create a more favorable micro-climate between the foliage and the soil. This micro-climate shows promise for outdoor mushroom cultivation by providing shade, increasing moisture retention and humidity. Forage radish roots have been observed growing out of the soil into the interplant microclimate area. Forage radish and kale grow low to the ground and during the same time of year that oyster mushrooms fruit in nature, thus suggesting they are a prime candidate to interplant with mushrooms. This experiment will test a novel form of mushroom production in New England by examining different methods of open-field mushroom production in order to analyze the and the economic impact mushroom production may have for local farmers.
Most mushroom production in New England utilizes specialized controlled environment rooms or outdoor production on logs. Indoor production takes specialized rooms and equipment to be successful. Rooms must be outfitted with environmental controls to adjust temperature, light and humidity. Rooms outfitted for mushroom production have significant startup/ maintenance costs and have a large carbon footprint. Carbon levels on Earth have risen above 400 PPM (NOAA) and our population continues to rise. The other form of mushroom production commonly used is outdoor log production. Hardwood logs are harvested and cut into one to four foot sections before they are inoculated with mushroom mycelium. Logs are placed in shade and can take a year or more to colonize the logs and some need to be soaked in water before fruiting bodies (mushrooms) will form. These factors make mushroom production unreasonable for vegetable farmers who specialize in producing local food for New England, where oyster mushrooms retail for $10-15 per pound market/ $5-10 wholesale (Fungi Ally, pers. comm.). This project will investigate a more sustainable approach to mushroom cultivation. Diversified vegetable farmers in New England are faced with the challenge of producing sufficient and unique food offerings while keeping prices low enough to compete with supermarkets. In addition, they are also tasked with meeting the demands of CSA memberships weekly and being environmental stewards. The number of farms in the United States has been decreasing since the mid 1930’s, yet the size in acreage has been steadily increasing; small farms in the United States are being taken over by larger farms. The demand for local food is climbing due to a steady increase in farms Direct-to-Consumer sales on the rise since the early 2000’s (USDA). Local small vegetable producer Teddy Smiarowski from Smiarowski Farm said in an interview that “small vegetable farmers need any competitive advantage we can get, even if it means trying new and novel ideas to increase production and sales” (pers. comm.). On farm mushroom cultivation is an innovative way to meet this demand and increase profitability.
Cooperators
- (Researcher)
Research
Field Trials
Note: a pre-trial was done in 2016 and the results are included here in combination with the 2017 SARE funded trial.
Plant materials
Forage radish (Johnny’s’ Selected Seeds, Winslow, ME) was direct seeded via grain drill with 7.5-inch row spacing at 10 kg ha-1 the third week of August in 2016 and 2017. Every second row was hand-pulled upon germination to create 15-inch row spacing. The kale cultivar Winterbor (Johnny’s Selected Seeds, Winslow, ME) was established from seed in 128-cell trays containing Pro-Mix BX potting soil (Premier Horticulture, Quakertown, PA) 5 weeks prior to transplanting to the field. During the germination and establishment period, kale was maintained under optimal conditions in a greenhouse and fertilized with Peters Professional 20-10-20 fertilizer (JR Peters, Allentown, PA) at 1:100 injector ratio bi-weekly. Following the 5-week period, kale was transplanted into the field by hand with 12-inch plant-spacing and 15-inch row-spacing during the first week of September. The field site was at the University of Massachusetts Crop and Animal Research and Education Farm in South Deerfield, MA and the soil was a Winooski silt-loam soil (Coarse-silty, mixed, superactive, mesic Fluvaquentic Dystrudepts) based on the USDA web soil survey. Field plots were broadcast fertilized with 112 kg ha-1 nitrogen, phosphorus and potassium (13-13-13) and incorporated by hand-tillage the second week of September.
Treatments
Fungal strains were originally supplied by local mushroom producer, and then supplied by the producer’s supplier. Treatments consisted of three strains of P. ostreatus tested at 2 concentrations. Specifically, Elm A, Elm B, and 8801 fungal strains were obtained from Aloha Medicinals Culture Bank (Carson City, NV) and sub-cultured onto Potato Dextrose Agar (PDA) in 100 x 15 mm petri plates. Spawn was made in 2.26 kg blocks using 73.0% hardwood sawdust, 24.6% wheat bran, and 2.4% CaSO4, hydrated to 65% moisture, and sterilized at 121*C for 2 hrs in polypropylene life science bags (Tufpak, Ossipee, NH). Bags were inoculated from 100 x 15 mm colonized PDA plates and were allowed to colonize for 3-4 weeks until the whole substrate was filled with white mycelium. Preliminary tests were conducted to determine the optimal substrate for field mushroom production. Chopped straw soaked in hydrated lime and supplemented with hardwood sawdust was optimal for inoculating fungal strains in the field. Straw substrate was prepared by soaking pre-chopped straw (Semican, Plessisville, Qb) in hydrated lime (Ca(OH)2) at 0.01 g ml-1 H2O for 16 hrs and drained immediately prior to inoculation. Plots with fungal treatments were covered with soaked-straw at 1.5 kg m2 -1, fungal treatment from spawn bags was added on top, then both were covered with straw again at 1.5 kg m2 -1 for a total of 3.0 kg m2 -1 straw plus fungal treatment. Plots were irrigated immediately and received a daily light irrigation for the duration of the experiment. Treatments consisted of three main effects: two inoculation rates (244 g m2 -1 and 488 g m2 -1); three fungal strains (Elm A, Elm B and 8801); and two crop species (kale and forage radish). The 244 g m2 -1 inoculation rate and P. ostreatus 8801 isolate were both used in the 2016 field trial only. Treatments in the 2017 field trial consisted only of 488 g m2 -1 inoculation rate and the isolates used were P. ostreatus Elm A and Elm B.
Soil samples were taken every other week starting at the time of kale transplanting in 2017 only. For each sampling date, three soil plugs were taken per treatment-plot and homogenized. Biomass measurements were taken after 8 weeks. Measurements were made fresh in whole-plant (above soil surface) and harvest-yield (marketable portions) distinctions.
Soil analysis
Orthophosphate, ammonium, and nitrate were analyzed using 96-well microplate colorimetric assays based on the methods of Ringuet et al. (2010), Rhine et al. (1998), and Miranda et al. (2001), respectively and with modifications. Briefly, reagents were used to facilitate chemical reactions with extracted soil-nutrients, and nutrient standards were used to create a calibration curve. K2SO4 was used to extract mineral nutrients from 0.5 grams of dry soil. Conical tubes with soil and K2SO4 solution were shaken for 1 hour at 300 rpm, followed by centrifugation at 4000 G for 1 hour. For orthophosphate, the standard was potassium phosphate monobasic (KH2PO4) and there were 4 reagents. Reagent 1 was 51 mM ammonium molybdate, reagent 2 was 5 N sulfuric acid, reagent 3 was 0.1 M ascorbic acid, and reagent 4 was 1.2 mM antimony potassium tartrate. 1.5 mL reagent 1, 5.0 mL reagent 2, 3.0 mL reagent 3, and 0.5 mL reagent 4 were mixed thoroughly to mage a working reagent immediately prior to analysis. 200 µL of sample or standard was added to microplates. 50 µL of working reagent was added. Microplate was vortexed then shaken for the 30-minute reaction time. The absorbance was read at 880 nm by Epoch microplate spectrophotometer (BioTek, Winooski, VT). For ammonium, reagents were PPS-nitroprusside, 0.17 M citrate, and 0.03 M buffered hypochlorite solution. The standard was ammonium sulfate ((NH4)2SO4). Reagents were added in this order: 50 µL sample or standard, 50 µL citrate reagent was added and allowed to react for 1 minute, 50 µL PPS- nitroprusside, 25 µL buffered hypochlorite reagent, and 100 µL DIH2O. Microplate was shaken on vortex for 30s. Microplate was then sealed and stored in dark for 45-minute reaction time, then absorbance was read at 660nm. The reagents used for nitrate were Griess 1 (N-napthyl ethylenediamine dihydrochloride “NED”), Griess 2 (sulfanilamide), and Vanadium Chloride. 25 mL Griess 1 and 25 mL Griess 2 were mixed before starting. 100 µL of sample or standard was added to microplate, followed by 100 µL VCL3, then 100 µL of combined Griess reagents (quickly). Microplate was incubated at 37C for 60-minute reaction time. The standard used was potassium nitrate (KNO3) and absorbance was read at 540nm.
Greenhouse Trials
Plant materials
Kale cultivar seeds were seeded into germination trays in Fafard premium topsoil (Sun Gro Horticulture, Agawam, MA). Seedlings were transplanted into 1020 trays filled 80% with topsoil with even plant spacing, 10 plants per tray. P. ostreatus Elm A and 8801 fungal strains and chopped straw were used and prepared the same as the field trials. Kale was inoculated with fungal treatments when plants had 2-3 sets of true leaves. Biomass measurements were taken 6-weeks after seeding.
Hydroponic Trials
Plant materials
Kale (B. oleracea var. acephala) cultivar Winterbor (Johnny’s Selected Seeds, Winslow, ME). Seeds were germinated on germination paper for 5 days, and young seedlings were transplanted to 50% Hoagland modified basal salt solution (PhytoTechnology Laboratories, Shawnee Mission, KS) for 3 days, before moving into treatment media and kept there for 10 days before harvest. Plants were grown in small reservoirs that held 250 mL media. Reservoirs were not aerated, but media were changed every third day to replenish dissolved oxygen saturation.
Treatments
Fungal strains P. ostreatus Elm A and Elm B (supplied and cultured per experiment 1) were grown on Potato Dextrose Agar in 100 x 15 mm Petri dishes. When the plates were fully colonized (10-14 days), mycelium and agar were transferred into 1 quart wide-mouth mason jars. Jars contained 200 g of dried rye berries in 200 ml H2O (1:1 rye, water). Jars with contents were autoclaved at 121*C for 1 hour, shaken and left to cool. Mycelium completely colonizes jars within 2 weeks and secretions start to collect around the base 4 weeks after inoculation. Secretions were collected 6 weeks after inoculation and vacuum filtered through 9 cm P8 filter paper. Filtered secretions were collected into 50ml conical tubes and cold sterilization was performed using 2x centrifugation at 4000 G for 1 hour. The secretions were then decanted into clean conical tubes and stored at 4C. Treatments were made by adding mycelial secretions to 100% Hoagland’s solution at levels 10 ppm, 100 ppm, 1000 ppm, and 10,000 ppm. Final solutions were diluted 50% with water prior to planting to make a modified 50% Hoagland’s solution.
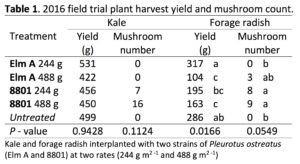
2016 Field Experiment: The treatments measured in the 2016 field experiment were fungal strain (Elm A and 8801), inoculation rate (244 g m2 -1 and 488 g m2 -1) and crop (kale and forage radish) (Table 1). Significant harvest yield differences were observed among treatments for forage radish (P = 0.0166). The Elm A strain (244 g m2 -1) recorded the highest yield, and the 488 g m2 -1 rate recorded the lowest yield (Fig. 1b). The 8801 strain (244 g m2 -1 and 488 g m2 -1) recorded yields lower than the untreated. Strain 8801 produced higher mushroom yields than Elm A for both crops (Table 1). However, there were no significant differences in plant yield for kale (p=0.9428), however for kale, the Elm A strain (244 g m2 -1) recorded the highest yield, and the Elm A 488 g m2 -1 rate recorded the lowest yield. The 8801 strain (244 g m2 -1 and 488 g m2 -1) recorded yields lower than the untreated (Table 1).
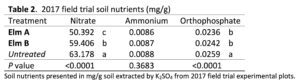
2017 Field Experiment: Strains Elm A and Elm B were interplanted with kale in the field at the 488 g m2 -1 rate. Significant harvest yield differences were observed between fungal strains (P = 0.0117). Elm A showed the lowest harvest yield and was significantly lower than the untreated. Also significant differences in nutrient level for orthophosphate (P < 0.0001) and nitrate (P < 0.0001) were observed in the soil, with Elm A and Elm B plots both having lower levels of orthophosphate and nitrate, but not ammonium (P = 0.3683) (Table 1). No significant differences were observed in soil pH (P = 0.3480). Mushroom count showed Elm B to produce significantly higher mushroom yields than Elm A.
Greenhouse Experiment: Strains Elm A and 8801 at the 488 g m2 -1 rate were interplanted with kale in the greenhouse. Significant harvest yield differences were observed among fungal strains (P = 0.0370). Lower harvest yields were recorded for both strains (Elm A and 8801) compared to the untreated, but only Elm A was significantly lower than the untreated (Figure 1).
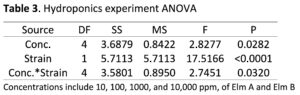
Hydroponics Experiment: Significant harvest yield differences were observed among the main effects strain (P < 0.0001), fungal exudate concentration (P = 0.0282), and the strain*concentration interaction (P = 0.0320). Strain Elm A was significantly higher than the untreated, and Elm B was significantly lower than the untreated (Figure 4a). When the interaction was partitioned, significant harvest yield differences among concentrations within both Elm A (P = 0.0543) and Elm B (P = 0.0091) were observed. Significant harvest yield differences were observed among both strains within fungal exudate concentrations 100 ppm (P < 0.0001), 1000 ppm (P = 0.0225) and 10,000 ppm (P = 0.0002).
The results from all trials (field, greenhouse and hydroponic) showed promotion and inhibition of plant growth depending on the strain and rate of P. ostreatus. These inconsistencies were reported in the literature and are likely due to limited knowledge on the strains being used and other unknown interactions. Abdullah et al. (2000) and Mohamed et al. (2014) observed an increase in crop yield using P. ostreatus in field production of cabbage and faba bean, respectively, yet Stamets (2005) showed a decrease in brussel sprout yield. Mohamed and Abdullah both used straw as a substrate for fungal growth, while Stamets used predominantly sawdust with straw used to retain moisture. This can be a source of variation among the results of these studies. Salama (2016) showed increased nutrient levels in sawdust substrate after inoculation and fruiting of P. ostreatus compared to straw types. Fungal substrates’ effect on plant yield was not shown in this study and is still unknown.
Another source of variation among the data in this study and the previous research of Mohamed, Abdullah, and Stamets is a fungal strain. Although each study contained P. ostreatus, strains were only identified to the species level and their sequence data was not provided. Strain differences at the subspecies level can lead to differences in plant yield, as indicated by results in this study. The amount of different P. ostreatus strains on the market has not been identified, however keeping the same strain across research is important. At the onset of this research project, the objective was to compare the Pleurotus spp. to Hypsizygus spp. oyster mushrooms’ effect on plant growth. From the culture supplier, the Elm A strain was originally identified as Hypsizugus ulmarius, which Stamets (2005) showed to have an improvement of crop yield, and the 8801 strain was identified as P. ostreatus, which Stamets (2005) showed to reduce crop yield. The 2016 field experiment used Elm A and 8801 strains to compare H. ulmarius to P. ostreatus. However, after the 2016 field experiment the culture supplier informed that Elm A was actually P. ostreatus, and provided a new strain Elm B, as H. ulmarius. The strains used in 2017 were Elm A and Elm B, again to test P. ostreatus vs. H. ulmarius. After the 2017 field experiment when both strains showed a reduction in plant yield, isolates were sequenced (no information on how in materials and methods) to confirm species. Sequence results indicated that both Elm A and Elm B were both P. ostreatus (data not shown) and that different strains within P. ostreatus have different effects on harvest yields.
In addition to P. ostreatus strain being a major influence on crop growth, the inoculation rate of the strain also shows importance in multiple studies. The Elm A 488 g/m2 treatment was associated with a significant decrease in crop yield in both field studies and the greenhouse study (Table 1; Figure 1, 2). In addition, the Elm B strain significantly reduced yields in the 2017 field experiment (Figure 3) and in the hydroponic trials (Figure 4). In contrast, the Elm A strain (244 g/m2) increased forage radish yield in the 2016 field trial compared to the other oyster strains tested, but not the untreated. Furthermore, the Elm A strain in hydroponics showed a significant increase in kale yield compared to the Elm B and the untreated (Figure 3). Differences in plant yield response of the Elm A strain in the field and in hydroponics were influenced by the inoculation rate or treatment concentration, respectively. However, an expanded range of concentrations has not been tested in hydroponics, and concentrations above 10,000 ppm should be evaluated to determine full dose-response range. The treatment concentration of Elm A (10 - 10,000 ppm) in hydroponics could be representative of a low application rate in the field, but further experiments are needed to confirm how field inoculation and crude exudate rates would relate. Overall, these results suggest the fungal strain and rate of application in intercropping systems can both effect plant growth.
Previous research did not provide supporting data nor discuss mechanisms on how saprophytic fungi affect crop yield; these mechanisms are still widely unknown. Results from 2017 soil nutrient analysis show a significant reduction in nitrate and orthophosphate from Elm A and Elm B treatments (Table 2). Although the reduction in orthophosphate is significant in plots treated with Elm A and Elm B, the change in concentration is not substantial (-8%). The recommended rate of phosphorus for brassica crop production in New England is 112 – 168 kg ha-1 (NEVMG). An 8% loss keeps phosphorus within the optimum range. However, the reduction in nitrate among the Elm A plots in 2017 is 25%, bringing the nitrogen levels below the recommended rate. A reduction in the soluble nitrate at the time of measurement could be a main factor affecting crop yield, but closer examination is needed to further understand the main effect causing changes in plant growth.
Finally, we observed that mushroom production in field and greenhouse experimental plots was less than optimal (Table 1). We also observed that plots with increased mushroom production seemed to have lower crop yields. Fungal substrate and inoculation rate need to be optimized before Pleurotus spp. interplanting with vegetable crops is a viable option in commercial production.
The effect of Pleurotus on crop production in an interplant system seems to be dependent on fungal strain, inoculation rate, and likely substrates used. Oyster mushrooms can be used in agricultural systems when correctly prescribed, however, the methods used in our experiments did not produce enough marketable mushrooms nor increase crop production enough to justify this as a viable practice for production farms. However, this research lays the groundwork needed for further improvement of the methods described and may one day be a suitable method of double cropping and increasing a grower’s profitability. Although it has been shown that an increase in crop yields can be made, methods should be refined before this practice should be utilized by farmers in a production setting. In the field, further experiments are needed to focus on testing a range of fungal strains/species with sequences assisted accurate identification, substrates, inoculation rates, and companion crops. In the lab, further experiments are needed to identify interacting factors and understand effects at the cellular level. Co-culture techniques described by Goers et al. (2014) should be used to remove all possible variables and to examine a complex interaction between plant and fungal cells. Fungi and plants are highly complex organisms whose phenotypes depend on genetics, environment, their interactions, and how they are managed. Fine tuning independent variables to unravel a symbiotic relation between the two systems is no trivial task, but can have valuable upside in a future with uncertain food security and increased scrutiny on synthetic inputs.
LITERATURE CITED
Abdallah, M. M. F., Emara, M.F.Z., and T.F. Mohammady. (2000). Open field interplanting of oyster mushroom with cabbage and its effect on the subsequent eggplant crop. Annals of Agricultural Science Cairo 45(1): 281-293.
Bezalel L, Hadar Y., and C.E., Cerniglia. (1996). Mineralization of polycyclic aromatic hydrocarbons by the white rot fungus Pleurotus ostreatus. Appl Environ Microbiol., 62(1): 292-295.
Gianotti, B. Cleaver, M. Cleaver, P. C. Bailey, and J. C. Holliday. (2009). Diversified agriculture part 1: Simplified and lower cost methods for mushroom cultivation in Africa.
Chaparro J.M., Badri D.V., Bakker M.G., Sugiyama A., Manter D.K., and Vivanco J.M. (2013). Root exudation of phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS ONE, 8:e55731.
Dashtban, G., Schraft, H., Syed, T., and Qin W. (2010). Fungal biodegradation and enzymatic modification of lignin. International Journal of Biochemistry and Molecular Biology, 1(1): 36-50.
Mohamed, M., Nassef, D. M. T., A. Waly, E., and M. Kotb, A. (2014). Production of oyster mushroom (Pleurotus spp.) intercropped with field grown faba bean (Vicia faba L.). Asian Journal of Crop Science, 6(1): 27-37.
Goers, L., Freemont, P., and K.M. Polizzi. (2014). Co-culture systems and technologies: taking synthetic biology to the next level. J. R. Soc. Interface, 11: 20140065.
Goodell, B., Qian, Y., and J. Jellison. (2008). Fungal Decay of Wood: Soft Rot-Brown Rot-White Rot. Development of Commercial Wood Preservatives, 2: 9-31.
Jonathan, S.G., Lawal, M.M., and O.J. Oyetunji. (2011a). Effect of spent mushroom compost of Pleurotus pulmonarius on growth performance of four Nigerian vegetables. Mycobiology, 39(3): 164-169.
Jordan, S.N., Mullen, G.J., and M.C. Murphy. (2008) Composition variability of spent mushroom compost in Ireland. Bioresour Technol., 99: 411–418.
Miranda, K., Espey, G.M., and D. Wink. (2001). A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide, 5(1): 62-71.
New England Vegetable Management Guide. (2014). University of Massachusetts Extension Vegetable Program. www.nevegetable.org
Ringuet, S., Sassano, L., and Z. Johnson. (2011). A suite of microplate reader-based colorimetric methods to quantify ammonium, nitrate, orthophosphate and silicate concentrations for aquatic nutrient monitoring. J. Environ. Monit., 13: 370-376.
Rhine, E.D., G.K. Sims, R.L. Mulvaney, and E.J. Pratt. (1998). Improving the Berthelot reaction for determining ammonium in soil extracts and water. Soil Science Society of America Journal, 62(2): 473-480.
Salama A.N.A., Abdou A.A.K., Helaly A.A., and E.A. Salem. (2016). Effect of Residues Agricultural Wastes on the Productivity and Quality of Pleurotus colombinus L. by Using Polyethylene Bags Wall Technique. Adv. Plants Agric. Res., 5(3): 00181.
Stamets, P. (2005). Mycelium running: How mushrooms can help save the world. Berkeley, Calif: Ten Speed Press.
Education & Outreach Activities and Participation Summary
Participation Summary:
On April 25, 2018 an on-farm demonstration / workshop was given at Many Hands Farm Corp in Amherst, MA in partnership with Fungi Ally mushroom farm titled Farming and Gardening with Oyster Mushrooms. Around 20 attendees were informed about basic mycology - the differences between spores, spawn, and mycelium, as well as methods of mushroom production - log, spawn bag, and open field. The group was highly interactive and an open conversation was had about the details of inoculation, questions about why attendees previously attempted projects may or may not have worked, and more detail about mycology.
The ONE17-300 project details were then thoroughly explained from the background to idea conception, execution and results. Specifically methods of mushroom interplanting were outlined. Aquiring spawn, substrate preparation, field inoculation and maintenance were explained in detail with many interacting questions and comments. Followed by an interactive field demonstration of field inoculation was done with all participants. Finally, attendees inoculated their own straw bags to take home and produce mushrooms themselves.
Learning Outcomes
Project Outcomes
Although the methods tested in these experiments did not produce the increase in crop production and the subsequent production of mushrooms that we intended when starting this work, we found indications that the practice is viable when fine-tuned correctly, and clearly illustrated areas of research needed to further our understanding. We approached this project with the assumption that the previous research was ready for expansion, but found that the groundwork had not been laid. We uncovered this foundation to discover that fungal strain, inoculation rate, and substrate play vital roles in the interaction between fungi and the plants that surround them. In the field, further experiments are needed to focus on testing a range of fungal strains/species with sequences assisted accurate identification, substrates, inoculation rates, and companion crops. In the lab, further experiments are needed to identify interacting factors and understand effects at the cellular level. Co-culture techniques described by Goers et al. (2014) should be used to remove all possible variables and to examine a complex interaction between plant and fungal cells.