Final report for ONE17-302
Project Information
Cruciferous crops make up a significant portion of the yearly harvest from Northeastern vegetable and diversified farms. In total, crucifers make up 6% of the total harvested vegetable acreage within the region covering over 16,000 acres. Cabbage root maggot (CRM) is a major pest of cruciferous crops and can impart serious injury on a multitude of cruciferous vegetables.The CRM is particularly a problem for organic growers, as there are few easily deployed organic options available for CRM management. The objective of this project was to improve upon an innovative method to decrease the incidence of damage and reduce yield loss associated with CRM. To accomplish this, we tested the field efficacy of two types of entomopathogenic nematode (EPN) soil applications, commercially available (CA) and regionally adapted (RA), for the control of CRM. In collaboration with Bear Roots Farm, we performed field trials in radish crops to identify the most appropriate application for control and persistence of the EPNs.
Though the project exhibited positive results regarding the persistence of EPNs (success!) in temperate agroecosystems (up to 50% population persistence after 1 winter), our damage assessments were inconclusive (challenge) as there wasn't a disease problem at one site and the disease problem at the second site did not show statistically significant differences between CA and RA EPNs. According to our farm partners, the major barriers to adopting the use of commercially available EPNs are cost and timing of application. Locally adapted EPNs are both inexpensive and only require 1-2 applications to establish a population.
Our outreach program for EPNs extended over the course of almost 3 years and included: two farm partner banquets, three NOFA-VT winter conferences, one national Entomological Society of America conference and a two farm tour/workshops. Over the course of these events, we connected and shared learning opportunities with over 50 commercial growers and market gardeners, and multiple extension agents and research entomologists. From these events, we have been able to adapt the EPN project to multiple cropping systems and has resulted in several additional grower-generated externally funded research projects.
Provided these finding/conclusions, we are currently assessing the “combined” strategy approach in potato agroecosystems. We are currently working on a project that is exploring the combination of beauveria bassiana and EPNs in Andean potato agroecosystems. We are also looking to explore the use of locally adapted EPNs in sweet potato systems. With the recent growth in the sweet potato market within the northeast, we believe that EPNs would be perfectly suited for the wireworm pressure. And, we recently awarded a NE-SARE Research and Education Grant with collaborators at Cornell and UVM to assess the biological control of corn rootworm in conventional and Organic corn production. We believe that the use of persistent populations of EPNs can benefit a wide variety of vegetable growers within the northeastern growing region. Particularly, any grower dealing with a pest insect that resides in the soil at any stage within its life cycle may potentially benefit from the use of EPNs.
The goal of this project was to add to the available IPM toolbox for vegetable growers to decrease the incidence of damage and reduce yield loss associated with CRM. To accomplish this, we tested the field efficacy of two types of entomopathogenic nematode (EPN) soil applications, commercially available (CA) and regionally adapted (RA), for the management of CRM. Specifically, we hoped to determine if soil applications of CA and/or RA will decrease the number of CRM in the radish rhizosphere and increase the market quality (as measured by root damage) and/or yield of radish crops when compared to untreated controls (UC). We also tried to determine if the EPN infection rate varies temporally (i.e. at seeding, post-EPN application and post-harvest) for each treatment.
We will look to answer are the following: Do soil applications of ENPs decrease the amount and severity of damage leading to increases in market quality and yield in radish crops? Do soil applications of each method, (CA, RA and UC) differ in their ability to reduce the amount and severity of damage in radish crops? Do regionally adapted nematodes persist longer as biologically active control agents when compared with commercially available applications?
Entomopathogenic nematodes (EPNs) represent an important alternative to chemical controls for the protection against various agricultural insect pests (Morris 1985; Georgis et al. 2006). Research exploring the application of EPNs for insect pest control is increasing rapidly as EPNs become more accessible and commercially available (Georgis et al. 2006). However, despite several decades of EPN research there remains significant gaps in knowledge regarding the efficacy and cost-effectiveness of EPNs. Management of insect pests using EPNs is often unpredictable as multiple abiotic (e.g. temperature, moisture, etc.) and biotic (e.g. EPN strains, host behavior/biology, etc.) factors must be considered to maximize EPN activity (Chen et al. 2003; Georgis et al. 2006; Shapiro-Ilan et al. 2006). Optimizing EPN infection rates requires focused research and experiments to develop appropriate methods for specific ecological settings. Furthermore, researchers must consider the evolutionary context for deploying EPNs. Lab-reared commercially available EPNs, though convenient, are often genetically depauperate and/or maladapted for the field conditions where they are being deployed (Stuart and Gaugler 1996). The use of non-adapted EPN strains (i.e. commerical) also precludes them from persisting during or over multiple growing seasons (Shields pers. comm.).
Previous studies testing EPNs for CRM management provide important insights for their use. For example, Bracken et al. (1990), Schroeder et al. (1996), and Nielsen (2003), all report improved infection rates in CRM with the EPN, Steinernema feltiae, as compared to other EPN species. In addition, field application of commercially available S. feltiae in cabbage crops displayed reduced CRM damage in EPN treated plots (Schroeder et al. 1996; Beck et al. 2014). Yet, despite these promising results, success may be limited to specific crops or strains, as Simser (1992) showed limited control of CRM in collards plants. Other possible reasons for this variance, may be attributed to environmental mismatches between EPN strains and local ecologies, improper application timing, inhospitable environmental factors and genetic founder effects (Stuart and Gaugler 1996; Fenton et al. 2001; Chen et al. 2003).
Our proposal looks to build upon the current research of EPNs for CRM management in two ways: 1) by testing the efficacy of EPNs in root crops and 2) by exploring the feasibility and utility of small-scale regionally adapted EPN strains. Root crops such as radishes and turnips are particularly sensitive to CRM, as any root damage may lead to reduced market value. However, there is scant research documenting the use of EPNs in these crops. Moreover, the vast majority of contemporary studies utilizing EPNs for CRM primarily use commercially cultured nematodes. Considering the high cost and low genetic variability associated with commercially reared EPNs, our study will aid in the development of more affordable long-term EPN solutions.
Cooperators
- (Educator and Researcher)
- (Researcher)
Research
After consideration and discussion with our farm partners, we decided to replicate our experiment in two locations, Bear Roots Farm in Barre, VT and the UVM Horticultural Research and Education Center (HREC) in South Burlington, VT, as opposed to a single location with temporal replicates (i.e. spring and summer). This alteration to our original experimental design allowed us to assess the performance of EPNs during the most damaging/vulnerable time of the season for CRM infestation. In addition, this setup allowed us to explore the persistence of EPNs in two different soil types (Bear Roots – silty; HREC -sandy). Pink beauty radishes was grown during the spring brassica growing season at each location. On each farm, a field that was cover cropped, plowed, disked, and fertilized using Pro Grow and compost was then formed into 46in wide beds. Radishes were direct seeded with an Earthway seeder with 1” spacing, ½” depth, and 3 rows/bed on June 1st at UVM HREC and June 13th at Bear Roots Farm.
We had five treatments replicated ten times at Bear Roots Farm and four times at UVM HREC, thus we had a total of 70 experimental units, 50 at Bear Roots Farm and 20 at UVM HREC. The plots at Bear Roots Farm were 10ft long with 10ft buffers and 9ft long with 9ft buffers at UVM HREC. Plots in adjacent beds at both sites were staggered such that no plot was immediately adjacent to a plot in an adjacent bed. The five treatments were 1) commercially available H. bacteriaphora and S. feltiae (CA:Hb+Sf); 2) commercially available S. carpocapsae and S. feltiae (CA:Sc+Sf); 3) locally adapted H. bacteriaphora and S. feltiae (LA:Hb+Sf); 4) locally adapted S. carpocapsae and S. feltiae (LA:Sc+Sf); and 5) an untreated control (UC). The treatments were applied immediately after seeding, on June 1st at UVM HREC and June 14th at Bear Roots Farm.
Locally adapted EPNs, S. feltiae (Sf), S. carpocapsae (Sc), and H. bacteriaphora (Hb), were sent from the Shields lab at Cornell. Approximately 2 weeks after inoculation, EPNs were separated from rearing materials by washing through a 20 mesh wire screen (841 μm openings) and then washed through 40 mesh (400 μm opening) screen with a large volume of non–chlorinated water following the protocol developed by the Shields lab. Locally adapted EPNs were applied as a soil drench at a dosage of approximately 23,200 I Js per ft2. Commercially available (CA) EPNs, NemAttack Pro™ - Sf, NemAttack Pro™ - Sc and NemaSeek Pro ™ - Hb, were purchased through Arbico Organics (www.arbico-organics.com) and applied at the labelled dosage of 3,125 I Js per ft2. The untreated controls received no nematode treatments, simply an equal amount of non-cholonated water that was used in the application of the nematode treatments.
Approximately 40 days after seeding (7/12/17 at UVM HREC and 7/26/17 at Bear Roots) ten plants per plot were chosen randomly and evaluated using a root damage index. Individual root damage scores were 0, no visible injury present; 1, superficial feeding scars present (no wounds reaching the root cortex); 2, deep scars or wounds present but tap root intact; 3, tap root severed or girdled but plant was alive; and 4, plant was dead.
A waxworm bioassay was used to measure EPN activity in the field at seeding, prior to EPN application, and at harvest. At each time interval, three soil samples were taken with a soil core (2.5 cm diameter by 30 cm deep) per treatment per block. The samples were then split into 0-10cm and 10-30cm sections. Each soil sample was thoroughly mixed and placed into a 0.5liter plastic cup. Ten waxworms (Grubco) were placed in each cup of soil and incubated in darkness for approximately 6 days at room temperature at UVM. After the incubation period, each cup was examined for nematode-infected waxworms (showing typical symptoms of nematode infection coincident with the presence of infective juvenile nematodes) and the infected waxworms counted. We confirmed the persistence of EPNs through the winter by conducting the same bioassays in April 2018.
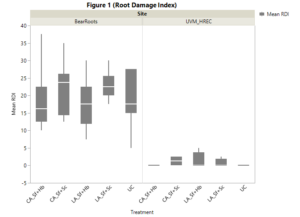
We measured incidence of CRM damage (Figure 1) and EPN presence (Figure 2) in all tests plots at each location. Site effects factored significantly in relation to damage incidence. UVM HREC displayed little to no pest pressure (Mean RDI < 5) in all experimental plots. As a result, we did not see any trends associated with our EPN treatments at the HREC. Conversely, plots located at Bear Roots farm did indicate high levels of CRM damage with both commercially available and locally adapted Sf+Hb treatments showing the lowest mean damage (CA:Sf+HB = 18.5; LA:Sf+Hb = 17.3; CA:Sf+Sc = 21.8; LA:Sf+Sc = 23; UC = 18.8), though differences were not statistically significant (p > 0.05).
Our post-harvest bioassays indicated the presence of EPNs in all treatments excluding the untreated control at the HREC. Untreated controls at Bear Roots Farm did indicate the presence of EPNs within the soil despite negative bioassay results prior to EPN application. According to our post-harvest bioassays, EPN treatment plots showed high levels of persistence (> 50%) at both locations and treatment types. Only CA_Sf-Hb plots at Bear Roots displayed a lower than 50% infection rate (2/10 plots) for the EPN bioassays.
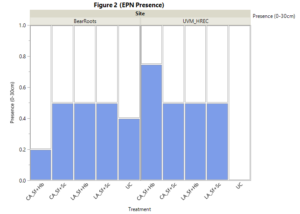
According to our results, evidence for the effective use of EPNs as biological control agents for CRM remains inconclusive. A couple of factors likely played a role in these suboptimal results. First, in the HREC plots, the lack of pest pressure precluded the collection of useful data for the assessment of CRM control. Second, due to early season flooding at the Bear Roots test plots, our EPN application did not coincide with the best application timing. We were racing against the clock and would have ideally applied the EPNs earlier in the season to better establish in the field. Finally, differences between the commercially available and locally adapted EPNs cannot fully be realized in a single season. The locally adapted strains of EPNs generally take longer to establish as their population dynamics need to equilibrate under new environmental conditions. This establishment can take up to two years before seeing widespread control. This is a stark contrast from the commercially available EPNs. These lab reared populations are presumably unable to overwinter in temperate soil and cannot survive without high concentrations of hosts. We believe that the lack of divergence between the two EPN results is the result of this short-term data collection rather than an indication of similarity in utility.
Although both locally adapted and commercially available strains of EPNs persisted from application to harvest in plots at both locations, the long-term persistence of EPNs following an overwintering period did occur at close to 50% for the locally adapted applications.
Education & Outreach Activities and Participation Summary
Participation Summary:
Learning Outcomes
Project Outcomes
Following our outreach events (e.g. On-Farm Workshops/Farm Tours, NOFA-VT Annual Winter Conferences, Vermont Vegetable and Berry Growers Association Annual Conferences) and our Farmer Partner banquets, it became clear that the application of locally adapted nematodes is a viable and useful practice that many of our farm partners are interested in continuing. The adoption of regional EPNs is particularly advantageous in temperate regions, as our data show the potential of single inoculations leading to establishment of EPN populations. The high persistence of the nematodes following the winter provides growers with a low investment application that can lead to resilient soil and a more biologically active rhizosphere. As one farmer noted, "Adding another persistence control option may not be the silver bullet, but if it reduces the pest pressure 10-20% that will compliment the other control tactics that I'm currently utilizing!" Another farmer expressed that he was intrigued by the simplicity of the application regime and looked forward to learning more about rearing the nematode populations himself.
Though the project exhibited positive results regarding the persistence of EPNs (success!) in temperate agroecosystems, our damage assessments were inconclusive (challenge). Upon initial review, this may seem to indicate that locally adapted EPNs are not a viable solution for deterring root maggots or reducing the incidence of CRM damage. However, it is more likely that endemic EPN populations require a longer assessment than commercially available EPNs. Following establishment, it is common for EPN populations to expand both in density and distribution. According to estimations from the Shields lab, populations of locally adapted EPNs expand their range roughly 3ft/year. Consequently, to better assess the efficacy and utility of using these regionally reared populations of EPNs as bioconttol options, we would suggest that experiments span more than a single year evaluation. In addition, it is also possible that EPNs are more of a complimentary strategy, rather than a "silver bullet" tactic. Combining EPNs and other IPM tactics may reduce the incidence of pest resistance to any single strategy. There is a large body of evidence that supports the utility of EPNs in general and we have provided support for the long term persistence of locally adapted EPNs. The next step is to optimize the possible experimental designs for assessing the potential of these region populations of EPNs.
Provided these findings/conclusions, we are currently assessing the "combined" strategy approach in potato agroecosystems. We are currently working on a project that is exploring the combination of beauveria bassiana and EPNs in Andean potato agroecosystems. We are also looking to explore the use of locally adapted EPNs in sweet potato systems. With the recent growth in the sweet potato market within the northeast, we believe that EPNs would be perfectly suited for the wireworm pressure. And, we recently awarded a NE-SARE Research and Education Grant with collaborators at Cornell and UVM to assess the biological control of corn rootworm in conventional and Organic corn production.
Finally, we believe that the use of persistent populations of EPNs can benefit a wide variety of growers in the Northeast region. Particularly, any grower dealing with a pest insect that resides in the soil at any stage within its life cycle may potentially benefit from the use of EPNs. According to our farm partners, the major barriers to adopting the use of commercially available EPNs are cost and timing of application. Locally adapted EPNs are both inexpensive and only require 1-2 applications to establish a population.