Final report for ONE18-317
Project Information
Haemonchus contortus is the number one Gastrointestinal Nematodes (GIN) affecting small ruminant production world-wide. In the absence of vaccines, the only mode of control of H. contortus is the use of anthelmintic drugs. However, indiscriminate use of available drug classes has led to the emergence of multi-drug resistant parasites which pose a significant challenge to parasite control on small ruminant farms world-wide. Hence, there is an urgent need to determine the resistance level on-farm in order to come up with the best treatment plan possible. The aim of this project was to utilize the larval development assay (DrenchRite® Assay) to characterize the levels of gastrointestinal nematode resistance on small ruminant farms in Delaware. In order to conduct this research, 11 small ruminant farms were identified and individual fecal samples collected. Four grams of feces were held from each animal to conduct an individual fecal egg count and the remaining fecal from each sample collect was pooled, vacuum sealed and shipped to the University of Georgia to for DrenchRite® analysis. Of the eleven farms tested, one farm (sheep farm) did not have enough parasites to be able to conduct the DrenchRite ® Larval Development Assay (LDA) while all other farms not only had enough parasite but had parasites that were resistant to the Benzimidazole class of anthelmintics and Ivermectin. Haemonchus contorts was the most predominant parasite found on 8 of the 10 farms with Trichostrogylus being the most predominant on one farm and one farm having 50% H. contorts and 50% Trichostrongylus. These results indicate that more farms in Delaware need to be tested for parasite resistance and the prevalence of multi-drug resistance in Delaware is higher than expected. Therefore, it is imperative that farmers use the combination deworming technique that is supported by the American Consortium for Small Ruminant Parasite Control and/or do a DrenchRite® to know what dewormers are not effective on there farm in order to determine the best dewormers to use on their farms.
To utilize the larval development assay (DrenchRite® Assay) to characterize the levels of gastrointestinal nematode resistance on small ruminant farms in Delaware.
The in vitro larval development assay method will be used to determine levels of anthelmintic resistance in small ruminant GIN populations in Delaware. This is an alternative to the laborious task of performing fecal egg count reduction tests (FECRT) in order to determine GIN resistance in small ruminants. The results from this objective will be used to educate producers of the resistance levels in Delaware and to identify the most effective drug that can be used to treat small ruminant parasites in Delaware.
The control of parasites in the U.S. rely on the use of broad-spectrum anthelmintics which is administered in some cases every 2-3 weeks (Miller, 1996) without regards to the specific diagnosis or symptoms of parasites. Due to this indiscriminate use of the available dewormers, there have been reports of increased resistance selection for internal parasites of sheep and goats throughout the world (Jackson, 1993; Zajac and Gipson, 2000; Mortensen et al., 2003; Kaplan et al., 2007;Howell et al., 2008; Crook et al., 2016). Research conducted in the southern (Mortensen et al., 2003) and Mid-Atlantic (Crook et al., 2016) United States (U.S.) confirmed that there is a high prevalence of anthelmintic resistance on sheep and goat farms. Five years after the research conducted in southern U.S., another research using the Drenchrite® Larval Development Assay (LDA) to detect resistance in southern states, Puerto Rico, St. Croix, and the U.S. Virgin Islands found that forty-eight percent of the farms tested (11 sheep, 11 goat) had resistance to all three classes of anthelmintics (Howell et al., 2008). Additionally, an even more recently (2016) research conducted on sheep farms in Maryland, Virginia, and Georgia, has indicated a higher prevalence of anthelmintic resistance (S. Schoenian, personal communication). This indicate that the prevalence of anthelmintic resistance is increasing across the U.S. Therefore, it is dire that more information on this topic is researched and producers educated on how to deal with this issue.
The proposed project is geared towards identifying the current status of GIN anthelmintic resistance in Delaware with overall aim of informing producers of the parasites in their herd/flock and telling them the most effective drug that they should be using on their farm. This will lead to producers deworming their herd/flock less times in the year and allowing them to only deworm the animals that actually need drug. This will preserve the population of worms that are not exposed to the drugs, hence, reducing anthelmintic resistance. Due to the implications that increased parasite resistance has on small ruminant production in the Delaware and worldwide, this research could potentially impact not only local (state), regional, national, but also the international sheep and goat industry. Understanding the level of the problem in our area could thus greatly impact profitability and sustainability of small ruminant industries in similar areas nationally and internationally. This project is very similar to several projects that have been conducted in the U.S. However, if the data on anthelmintic resistance is updated every three to four years it allows producers to understand how their current dewormers are controlling the parasite load and give them an insight into the idea of changing the dewormers earlier or using more than one dewormer to the kill the parasites that may be resistant to one specific dewormer.
Cooperators
Research
To fulfill the objectives of this study, nine farms were visited over the summer and fall of 2018 and 2019. The farms were visited on July 26, 2018 (Wayside Farm), August 07, 2018 (Union Ridge Farm), September 27, 2018 (Cornerstone Acres), October 2, 2018 (Delaware Nature Society: Coverdale Farm Preserve), October 15, 2018 (Sandyland Boer Goat Farm), June 12, 2019 (Water Girl Farm), June 24, 2019 (NAN-E Goat Farm), July 15, 2019 (Shepherds Hope Sheep Farm), and October 17, 2019 (Triple J Farm). Fecal samples were collected rectally from individual animals with a FAMACHA© score of 3, 4 or 5 on each participating farm (Wayside Farm = 19 goats; Union Ridge Farm = 12 goats; Cornerstone Acres = 20 goats; Coverdale Farm Preserve = 9 sheep; Sandyland Boer Goat Farm = 15 goats; Water Girl Farm = 15 Sheep; NAN-E Goat Farm = 25 Goats; Shepherds Hope Sheep Farm = 16 Sheep; Triple J Farm = 13 Sheep) and placed in labeled plastic zippered bags in a cooler on the opposite side of the ice to prevent direct contact. Once the samples were back at Delaware State University, a pooled sample was created by adding fecal from individual animals into one bag to weigh at least 20 grams (This was done for each farm on day samples were collected). The remaining fecal material in the individual bags were then refrigerated for individual fecal egg count (FEC) analysis and the composite samples were vacuum sealed and shipped off to the University of Georgia Infectious Disease laboratory for analysis. Individual fecal samples were analyzed for FEC using the modified McMaster technique (Henricksen and Aagard, 1986) and reported as eggs per gram (epg). Pooled samples made up of fecal from individual animals from each farm with greater than 500 epg were used for DrenchRite ® Larval Development Assay (LDA) analysis of anthelmintic resistance. The LDAs were conducted in the parasitology laboratory of Dr. Ray Kaplan at UGA.
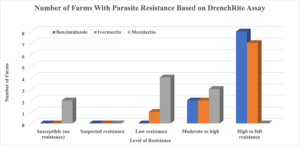
Of the eleven (11) farms tested, one farm (sheep farm) did not have enough parasites to be able to conduct the DrenchRite ® Larval Development Assay while the other farms (goat and sheep farms) had adequate parasite loads to conduct the LDA. Haemonchus contorts was found to be the most predominant parasite on nine (9) of the farms with enough parasite for the test while one (1) farm had Trichostrogylus as the most predominant parasite. The LDA results indicated that all the farms tested had parasite with resistance to the Benzimidazole (BZ; Panacur, Safeguard, Valbazen) class of dewormer and Ivermectin (IVM) while Moxidectin (MOX) was found to still be susceptible on two (2) farms but resistant on seven (7) of the farms tested. The level of resistance varied based on dewormers, with eight farms having total resistance to BZ and two having moderate to high resistance. As for IVM, seven farms had total resistance, two had moderate to high resistance, and one had low resistance. Additionally, the DrenchRite ® analysis for MOX indicated that three farms had moderate to high resistance, four farms had low resistance, and two farms had susceptibility (effective in killing the parasite). There were only nine farms evaluated for MOX resistance as this procedure was not effective on the farm that had predominantly Trichostrogylus. The data from this study were similar to that found Schoenian and colleagues (2017). This research was conducted on sheep farms in Maryland, Virginia, and Georgia and found that 100% of the farms in all three states had resistance to BZ. Additionally, 100% of the farms in Virginia and Georgia had resistance to both IVM and MOX while 90% and 60% of the farms had resistance to IVM and MOX, respectively. The IVM data is similar to that found in Delaware, however, MOX data was contrary to Virginia and George but closely related to Maryland. The DrenchRite® analysis for Levamisole (LEV) indicated that one farm had total resistance, one farm had moderate to high resistance, two farms had low resistance, and three farms had suspected resistance. However, data from three of the farms were inconclusive as the Levamisole on the plates were too old.
Based on the conditions of this study, all farms tested showed some level of resistance to all classes of dewormer and majority of the farms had Haemonchus contortus as their number one parasite population. Therefore, more studies should be done to include more farms for anthelmintic resistance testing in the Mid-Atlantic region of the of the USA. This will give stakeholders the knowledge needed to help with combating this issue.
Education & Outreach Activities and Participation Summary
Participation Summary:
The first time this project was presented, it was presented at a Delmarva goat association meeting to enlighten goat farmers of the project in order to acquire participants. Two of the five goat farmers in this study was at the meeting and volunteered to use their farm as a site for collection. This project has been presented at the 2019 and 2020 Delaware Agriculture Week Small Ruminant session as a means to have producers participate in the study. From the 2019 presentation, seven producers were interested and four of the producer farms were involved. The other three producers were very busy and could not align their schedules with days that the samples could be taken in order to be shipped to the University of Georgia. At the 2020 session 1 more sheep producer indicate interest, however, the granting time may be over before samples can be collected for this study.
The preliminary data from this project was presented to small ruminant specialist and researchers at the Sustainable Small Ruminant Production in the Southeastern U.S. annual planning meeting. Once the project has been completed the data will be used to create a written publication and then used as preliminary results to acquire a larger grant.
Final data from this project will be used to create a small publication and presented at different animal science or extension conferences.
Learning Outcomes
All of the farmers indicated that it was interesting to gain knowledge of the parasite resistance level on their farms to different dewormers/anthelmintics. They all were willing to utilize the assay in order to make their decision on how to deworm their animals. Of all the farmers, one purchased a scale and is now utilizing it for his farm in order to deworm his animals.
Project Outcomes
As a project outcome, one of the producers on this study purchased a livestock scale to weigh his animals in order to give the correct dose of the dewormer (anthelmintic) that is adequate to kill these parasites.
Additionally, this producer started the use of combination deworming on his farm to reduce the spread of anthelmintic resistance.
Looking back at this project, it would be most beneficial to have a lab technician that can help with scheduling and collecting data from farms as the farms often have conflicting times with my schedule. Additionally, COVID-19 impacted the sample collection and submission to the University of Georgia for analysis. The methodology used for this project was very good and should be done on every small ruminant farm in the United States as the information garnered is critical for management purposes on a farm. Even though combination deworming is recommended by many veterinarians, knowing the level of current resistance on your farm will help with the decision making as far as if the combination treatment may need to be coupled with alternative treatment. This project needs more participation from farmers in order to give a more accurate conclusion on the current status of resistance in the state of Delaware.