Final report for ONE20-355
Project Information
Since 2020, the Vermont Bee Lab has engaged in a collaborative effort with one of the largest honey bee breeding operations in Vermont–French Hill Apiaries (FHA)– to improve the quality and availability of locally adapted bee stock in the northeast. By leveraging the capabilities of the Vermont Bee Lab (VBL), we helped to strengthen the existing breeding program by developing more robust selection standards and using novel assays to select for stock that demonstrates pest/pathogen resistance. Starting in 2021, we evaluated colonies based on a suite of metrics that included overwintering success, brood production, pest and pathogen loads, hygienic behavior performance, and honey production. We selected the top performing colonies as breeders and evaluated their daughter colonies in 2022 and 2023. We compared colonies selected through our improved methods against colonies selected through standard methods at FHA. After using these methods for two generations of daughter colonies, we found that colonies reared from new project queens (NPQ) had lower Varroa loads, and were more likely to perform hygienic behavior compared to colonies selected through standard methods at French Hill Apiary (FHA). There was no difference in Nosema prevalence and loads between the two groups. Importantly, all of the top ranking colonies that were selected as our breeders for 2023 descended from colonies selected for our improved metrics. This finding is very promising and indicates that we were successful in refining the selection criteria to better capture top ranking colonies. We also incorporated a novel method to select for hygienic behavior, UBO assays. We saw a significant effect of the selection method on the level of hygienic behavior present in the groups with more high-scoring colonies among the daughters of our improved breeder selections (NPQ) in 2023. We found that high scoring UBO colonies had lower varroa loads by July. There was a trend that nosema load was higher in high scoring UBO colonies compared to low scoring UBO colonies; however, this finding was not statistically significant and reversed in the other bee operations we tested. Here, we demonstrate a possible long-term selective breeding program design which could be adopted by other bee producers in the Northeast and lead to improved, locally adapted, and pest/pathogen resistant honey bee stock. We have developed guidelines for improved selection criteria, including using UBO assays, and expanded our collaborations to other bee breeding operations in the state.
This project seeks to leverage the capabilities and resources of the Vermont Bee Lab at the University of Vermont and French Hill Apiaries, one of the largest Vermont-based bee breeding operation in order to:
- Develop and implement improved metrics by which Northeast honey bee stock is selected, including pest/pathogen resistance,
- Improve the quality and availability of Northeast honey bee stock, and
- Spread awareness to beekeepers regarding the strengths of locally adapted bee stock.
By improving the quality and availability of locally raised bee stock, this project aimed to improve bee health and reduce colony losses for Northeast beekeepers as well as reduce beekeepers’ reliance on imported bees, which are a potential source for pests and pathogens.
Vermont beekeepers have reported losing an average of 59.25% of their hives each year over the past four years and in 2020-21, Vermont’s average annual colony losses ranked second highest across the nation (Bee Informed Partnership, 2023). Colony losses are attributed to a multitude of interacting stressors which are often difficult to control by beekeepers. One sustainable mechanism whereby beekeepers can mitigate colony losses is through breeding programs that select for stock well-adapted to local environments and pest/pathogen resistance. To combat high colony losses, Vermont beekeepers need bees selected for the Northeast climate and resistant to pests/pathogens.
There are very few queen bee breeders in the Northeast who maintain stock locally bred and selected for high production, the ability to overwinter in the North, and pest/pathogen resistance. Selecting for traits such as colony longevity and overwintering success is accomplished over many years, even decades, of selective breeding. Selecting for pest and pathogen resistance requires scientific expertise and laboratory equipment to identify and quantify pathogen loads.
Thousands of colonies are imported each year to Vermont. Most are brought in by migratory commercial beekeeping operations that transport their bees out-of-state to warmer regions during the winter and participate in large pollination activities where the risk of disease transmission is significantly heightened. After providing pollination services, migratory colonies are split into smaller ‘nucleus’ or 'package' colonies and sold to beekeepers. Migratory honey bee colonies host novel pathogens (Runckel et al., 2011), have high virus loads (Welch et al., 2009; Alger et al., 2018) which can spillover into native bee populations (Alger et al., 2019), and are unlikely to be well adapted to the Northeast climate. Improving the availability and strength of locally adapted bee stock will reduce beekeepers’ reliance on imported bees which represent a risk to the health of both managed and wild bee populations.
Our collaborative bee breeding initiative brought together one of the largest and longest-running bee breeding operations in the Northeast with the expertise and resources of bee disease experts at the University of Vermont- Vermont Bee Lab (VBL). The VBL is an important resource and presents an opportunity for collaboration towards improving current breeding programs. The VBL has the expertise and equipment to test for traits for incorporation into a breeding program to improve pest/pathogen resistance. We have built upon French Hill Apiaries’ existing breeding program and developed selection standards for Northeastern bees that are high producing, adapted to the local climate, and resistant to pests/pathogens. In implementing these standards and selecting for a superior bee stock, we worked to improve the quality of bees available to regional beekeepers and bee breeders who may incorporate this stock in their own breeding programs. We disseminated our research to beekeepers to spread awareness of the strengths of local bee stock. Our work aimed to improve the supply of locally adapted queens throughout the Northeast, and reduce beekeepers’ reliance on imported bees.
Cooperators
- - Producer
- - Producer
Research
Field methods by beekeeping year:
Year 1: In 2020, we had aimed to identify 100 of the best performing production colonies based on honey and brood production as well as overwinter success. However, shut-downs due to the Covid-19 pandemic prohibited our UVM lab and personnel from working at the level we had anticipated. Our partnering beekeeper also experienced staffing shortages. Together, to maintain social distancing and keep everyone safe, we were unable to allow our field teams to work hives together. Secondly, 2020 mite loads put too much pressure on the beekeeping operation, pushing activities well into late autumn. We aimed to conduct the work planned for 2020 in 2021 during Year 2 of the grant.


Year 2: In 2021, we used existing records of 500 colonies, to identify 64 of the best performing production colonies based on overwintering success, as well as honey and brood production. We tested these 64 colonies for the full suite of traits over the season (see ‘description of trait metrics and methodologies’ below), scored the colonies, and in early 2022, selected the 5 top ranking colonies to serve as our queen breeder colonies for the new breeding program (NPQ). We used a reduced set of traits currently used by French Hill Apiaries (FHA) (honey and brood production and overwintering success) to select five colonies which served as queen breeders of the FHA group. Maintaining these two separate groups (NPQ and FHA) enabled us to compare the efficacy of the NPQ with the existing program.
Year 3: In summer 2022, we assigned 50 colonies to the NPQ group and 50 colonies to the FHA group. Colonies in the NPQ group were requeened with NPQ queens (full set of traits). Colonies in the FHA group were requeened with FHA queens (reduced set of traits). The two treatment groups consisted of 10 daughter colonies produced from each of our five NPQ queen breeders and five FHA queen breeders. The 10 daughter colonies descending from each of our 10 total queen breeders were divided across two separated yard locations in Vermont. All nucleus colonies were established by July 1. Besides the origin of the queen, all management practices remained constant across all colonies. Once we requeened all colonies, we tested each throughout the remainder of the season for a reduced suite of metrics, including VSH behavior and pest/disease loads. Other metrics were not tested due to the small size of the nucleus colonies produced this season and the timeline of queen rearing. In 2022, we included one additional test for hygienic behavior using an improved method of synthetic pheromone application– the unhealthy brood odor assay (UBO) (see ‘description of trait metrics and methodologies’).
To raise queens, we use established queen rearing methods practiced by FHA. Queens are grafted from larvae in artificial queen cells. Upon emergence, queens are open mated in an isolated mating yard surrounded by colonies maintained by FHA. We harvest queens after it has been confirmed that the queen has successfully mated by the presence of eggs.
In early 2023, we scored the 2022 NPQ colonies and selected the five top-ranking colonies to serve as our second generation queen breeder colonies for the new breeding program (NPQ) in 2023.
Year 4: In spring of 2023, we returned to the 2022 nucleus colonies to measure their overwintering success and brood production, then transferred them to 10-frame hives. Colonies were moved to two separate yards– one to the north end and one to the south end of French Hill Apiaries’ geographic territory in Vermont, in order to omit yard effect on performance. We ensured that NPQ and FHA colonies were present in equal numbers at each of the two sites. We continued to test the 30 surviving colonies from 2022 for the full suite of traits as full production colonies. Colonies that tested over 1% Varroa load were chemically treated and removed from the program.
The five top-ranking colonies from 2022 that were selected as breeders in 2022 were removed from the cohort and designated as breeder colonies from which larvae were grafted as queens in 2023. Sixty-six nucleus colonies were produced from the five queen lines, composed of 12-20 daughter colonies per queen line, and divided across two separated yard locations in Vermont. All nucleus colonies were established by August 1. Besides the origin of the queen, all management practices remained constant across all colonies. Once we requeened all colonies, we tested each throughout the remainder of the season for a suite of metrics, including Varroa loads, Nosema loads, and UBO assay performance. The 2023 daughter colonies will be tested for UBO performance in May 2024. We will then make breeder selections for 2024 with the top-ranking 2023 colonies and continue to follow their performance into 2024 and beyond.
While we collected a large amount of data, some data will need to be made available in the spring of 2024 due to lab processing times and logistics. Samples for virus assays were collected and are being analyzed in the winter of 2024. Overwintering success and colony mortality will be determined in spring of 2024. Honey production was recorded on each hive shortly before wrapping the hives for the seasons. Those data will also be recovered once hives are unwrapped in 2024.
Once all data were collected, we compared the two treatment groups on the full suite of metrics to determine the efficacy of the new breeding program in producing high-quality bees with hygienic behavior and pest/pathogen resistance. We also tested whether our improved method for hygienic behavior testing was an accurate method of predicting pest and pathogen prevalence and load.
Description of trait metrics and methodologies.
Brood production: Brood production is indicative of the colony’s population and strength. This metric is measured by the number of frames that contain brood (eggs, larvae, pupae) in the brood chamber. We measured brood production on overwintered nucleus colonies (first-season full production colonies) during colony transfer from nucleus boxes to 10-frame supers in early May.
Bee population: Bee population is indicative of the colony’s health and strength. This metric is measured by the number of frames that are fully covered with adult bees in the brood chamber. We measured bee population on second-season full production colonies during hive reversal in early May.
Honey production: We measured honey production once at the time of honey harvest. We weighed the honey harvested from each colony on a scale to derive the pounds of honey produced.
Overwintering success: We measured overwintering success as the ability of a colony to survive the winter and remain healthy and viable as a production colony the following spring.
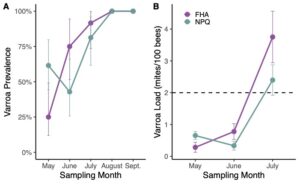
Varroa mite load: Varroa mites are ectoparasites that feed on the fat bodies and hemolymph of bees, suppress the immune system, and vector viruses. Introduced to North America from Asia in the 1980s, Varroa mites are considered the most damaging pest to modern beekeeping. Methods to control mites include chemical treatments, practices that interrupt the mite’s life cycle and utilizing mite resistant honey bee genetics. We measured mite loads in all colonies once a month using the alcohol wash method (Lee et al., 2010). For any colonies that host mite loads above a treatment threshold (Honey Bee Health Coalition, 2018), we treated them with approved mite treatments. Varroa loads are calculated as the number of mites per 100 bees.
Varroa Sensitive Hygienics (VSH) behavior: VSH behavior is an indication of mite resistance (Spivak & Downey, 1998; Wagoner, Spivak & Rueppell, 2018). Honey bees that detect mites under pupal cappings will uncap pupae and remove the pupae and mites, disrupting the mite’s life cycle. We can test for VSH behavior by sacrificing a selected area of brood with liquid nitrogen and replacing it back into the colony for 24 hours. After 24 hours, a colony exhibiting VSH behavior will remove all the sacrificed dead pupae from the cells. VSH behavior may be quantified by counting the number of cells with pupae removed. We conducted this test once per season in 2021 and 2022.
Hygienic behavior by Unhealthy Brood Odor Assay: We can also test for hygienic behavior by conducting Unhealthy Brood Odor Assays (UBO). We apply a synthetic pheromone solution to an isolated section of capped brood and return the frame to the colony for two hours. After two hours, a colony exhibiting hygienic behavior will have damaged the wax cappings of the treated pupae. Hygienic behavior may be quantified by counting the number of cells that show damage to the wax capping (Wagoner et al., 2021). We conducted this test once per season, starting summer 2022.
Virus load: Varroa mites transmit a number of viruses to honey bees that cause a multitude of symptoms such as deformed wings, paralysis, and death. Deformed Wing Virus (DWV) is one of the most common and prevalent viruses in honey bees (Zhang et al., 2012). Once per year, we tested colonies for DWV and quantified their viral load. We used established molecular protocols to test samples for the DWV target as well as a housekeeping gene (Actin) (Alger et al., 2018).
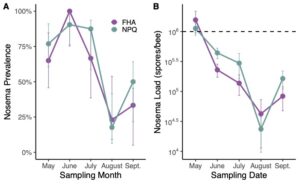
Nosema load: Nosema is a microsporidian parasite that infects the midgut of honey bees and can cause dysentery, poor survival, and reduced honey yield. We tested for Nosema once per season using established microscopy protocols on bee samples (Alger et al., 2018)
Data analysis:
To compare the performance of the two groups of colonies (NPQ and FHA) on their performance across the suite of metrics, we used generalized linear mixed-effects models with each metric as a dependent variable, time, group, and their interaction as predictor variables, and apiary as a random effect. To examine how the suite of metrics predict group membership, we used multivariate statistical analyses.
Comparison of NPQ and FHA colonies
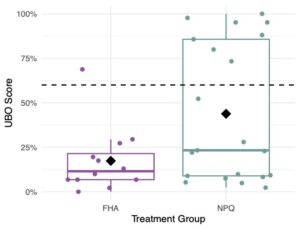
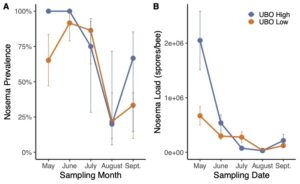
By the end of the project in 2023, we were able to evaluate and compare full production colonies of the two cohorts (colonies selected by our improved breeding program (NPQ) metrics and colonies selected by previous French Hill Apiaries (FHA) standards). We found that NPQ colonies had lower varroa loads compared to FHA colonies (Figure 1). However, all colonies in both NPQ and FHA groups tested above treatment threshold by August and required acaricide application. Nosema prevalence and loads did not differ between the two selection groups (Figure 2).We saw a significant effect of the selection method on the level of hygienic behavior present in the groups with more high-scoring colonies among the daughters of our improved NPQ breeder selections in 2023 (Figure 3). All of the top ranking colonies that were selected as our breeders for 2023 descended from NPQ mothers, indicating a clear improvement over time. This finding is very promising and indicates that we were successful in refining the selection criteria to better capture top ranking colonies.
UBO results
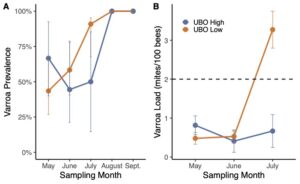
We found that high scoring UBO colonies had significantly lower varroa loads compared to low scoring UBO colonies (Figure 4). Across other bee operations, we found similar results. However, all colonies had to be treated with acaricides by August due to loads exceeding treatment thresholds. Ideally, beekeepers could breed colonies that can resist Varroa mites without chemical intervention. Varroa mites may travel between and among colonies and previous research found that colonies with high mite loads may become sources to neighboring colonies. Thus, we recommend future studies to isolate high UBO scoring colonies from low scoring UBO colonies to determine whether the spillover of mites from low scoring colonies may overwhelm the hygienic behavior and mite resistant abilities of high scoring UBO colonies. There was no significant difference in nosema prevalence and load between high scoring and low scoring UBO colonies. At French Hill Apiaries, there was a trend that nosema loads were higher in high scoring UBO colonies compared to low scoring UBO colonies; however, this finding was not statistically significant and reversed in the other bee operations we tested, which calls for future research. (Figure 5).
From our breeder selections in 2023 (top ranking 2022 colonies), 66 nucleus colonies were established and tested for a reduced suite of metrics including UBO performance and pest/pathogen loads in August and September. Of all the daughter colonies in 2023, 17.2% were high scoring on the UBO assays. Our findings indicate that UBO-linked hygienic traits can be heritable, but daughter colonies are not guaranteed to inherit high UBO scores from their mother. A high level of heritability of UBO-linked hygienic traits is unlikely in an open mated system. Our results indicate the UBO is a promising novel tool for selecting hygienic behavior and colonies that are better able to combat Varroa mites. Due to the limited heritability in this open mated system, we suggest investigating whether heritability can be improved through instrumental insemination to control the patriline genetics.
Metric Results by Year
In 2021, we gathered data on a full suite of metrics including overwintering success, honey/brood production, hygienic behavior, and pest/disease loads. Our results are as follows: Overwintering success was determined by the number of frames of bees or brood that were present in early May. Only colonies with six or more frames of bees/brood were selected for the study. Varroa mite and nosema loads were assessed on a monthly basis. Varroa mite counts increased consistently throughout the season, growing from an average of 0.14 mites/100 bees across all colonies in May to an average of 5.01 mites/100 bees in September when we treated any colonies above treatment threshold (2 mites per 100 bees; 2%) with acaricide. Nosema peaked in late-May with an average of 825,000 spores/bee across all colonies (remaining under the treatment threshold), then the abundance decreased to a very tolerable level for the rest of the season. In most colonies, the fungus was completely eradicated by July. Sixteen colonies were considered to be ‘hygienic’ based on our lowered threshold, demonstrating 80% removal of freeze-killed pupae. Honey yield ranged from 55 pounds to 206 pounds of honey stores. October weight ranged from 75 pounds to 195 pounds of fall nectar/honey stores. Using R-programming language, the top 15 colonies were identified to monitor for overwintering success in spring 2022, which informed the selection of the final five breeder queens.
In 2022, we gathered data on a reduced suite of metrics including hygienic behavior and pest/disease loads from our NPQ and FHA nucleus colonies. Our overall results are as follows: Varroa mite counts increased consistently throughout the season, growing from an average of 0.24 mites/100 bees across all colonies in July to an average of 0.80 mites/100 bees in September when we treated any colonies above treatment threshold (2 mites per 100 bees; 2%) with acaricide. Average Nosema load was highest in late-July when we began sampling, with an average of 162,000 spores/bee across all colonies (well below the treatment threshold). Minimal Nosema was found in September. From a reduced group size of 50 randomly selected colonies, twenty-nine (58%) were considered to be ‘hygienic’ when tested with freeze-kill brood assays (threshold of 95%), while only eleven colonies out of the full group of 100 colonies (11%) were considered to be ‘hygienic’ based on our improved UBO method (threshold of >=60%).
In 2023, we repeated sampling the 2022 colonies and gathered data on overwintering success, brood production, hygienic behavior, and pest/disease loads as full production colonies. Our overall results are as follows: Overwintering success was determined by survival and the number of frames of bees that were present in early May. Only 30% of our project colonies survived over winter, ranging from 3-12 frames of bees in size. Varroa mite and nosema loads were assessed on a monthly basis. Varroa mite counts increased consistently throughout the season, growing from an average of 0.49 mites/100 bees across all colonies in May to an average of 11.07 mites/100 bees in September. All full production colonies from 2022, monitored in 2023, measured over the varroa treatment threshold by September and were treated with acaricide. Treatment thresholds were reduced to 1% due to high mortality levels over winter in 2023. Nosema peaked in late-May with an average of 1,318,478 spores/bee across all colonies, then the abundance decreased to a tolerable level for the rest of the season. In 2023, we replaced the freeze-kill method with the improved UBO method. Nine colonies out of 30 (30%) were high scoring and considered to be ‘hygienic’. Five out of the nine high scoring colonies in 2023 were not high-scoring when tested as nucleus colonies in 2022, indicating that UBO tests in early spring on full production colonies (as opposed to late summer on nucleus colonies) may produce more reliable results.
In 2023, we also monitored the 66 nucleus daughter colonies made from NPQ breeder colonies. Varroa mite counts increased from August to September, from an average of 0.17 mites/100 bees to 1.5 mites/100 bees in September when we treated any colonies above treatment threshold (1 mite per 100 bees; 1%) with acaricide. Nosema load was higher late-September than in August, with an average of 130,952 spores/bee across all colonies (well below the treatment threshold). As a fungal pathogen, Nosema prevalence is linked to wet cool conditions, typically found in spring. Late season Nosema prevalence, like we witnessed in 2023, is unusual and we attribute this to the extreme rainfall we received that year.
The primary objective of this project was to use improved selection criteria to establish a NPQ population and compare our selected cohort with a cohort of colonies that were selected using FHA’s previous selection metrics. In just two generations, we were able to develop colonies with lower Varroa loads. We were also successful in breeding more high scoring UBO colonies in the operation through novel selection methods. All of the top ranking colonies that were selected as our breeders for 2023 descended from NPQ mothers, indicating a clear improvement over time. This finding is very promising and indicates that we were successful in refining the selection criteria to better capture top ranking colonies. This selection program may contribute to further developing pest and pathogen resistant bee stock if continued in subsequent years. Since we were only able to raise and monitor two generations of daughter colonies during this 3-year project, our findings are limited in their scope and predictive power for future generations. However, these generations of daughter colonies have laid the groundwork for continuing this improved breeding program at French Hill Apiaries and disseminating improved honey bee stock to other operations into the future.
The introduction of UBO hygienic behavior testing to this program was not anticipated at the onset of the project, but has become a major component of the study. We have contributed greatly to the research and development of this technology, as an improved method to the previously used, freeze-kill brood assay. In spring 2024, UBO will become commercially available for bee producers. From this research, we are able to provide more specific guidelines for Vermont bee producers that are interested in incorporating UBO testing into their operations, including how many high-performing colonies to expect in a previously unselected population (first generation of breeder candidates), pest and pathogen loads of high-scoring colonies compared with their low-scoring counterparts, and the best time of year to test for hygienic behavior using UBO given our climate conditions and seasonal nectar flows in the Northeast. We were also able to investigate the heritability of UBO-linked traits to daughter colonies in an open mated system. Our findings offer practical knowledge for beekeepers who want to learn more about UBO. We have developed and disseminated informational factsheets to interested beekeepers, both for producers looking to utilize the technology for making breeder selections and also for hobbyists looking to purchase UBO-tested bees and incorporate the hygienic genetics into their apiaries.
In 2023, we hosted an in-person meeting at the University of Vermont with four Vermont bee breeders to learn how this project could be extended to other operations. We also gathered information on impressions of our project and lessons learned. We plan to repeat this work in 2024.
Education & Outreach Activities and Participation Summary
Participation Summary:
From 2021-2023, Alger shared the progress of the research to date with several beekeeping clubs during virtual and in-person meetings (Franklin County Beekeepers, Southern Adirondack Beekeepers, Chittenen County Beekeepers) and the Vermont Beekeepers Association (VBA). Alger also presented our findings to VBA members during annual summer and winter meetings in 2021, 2022, and 2023. With nearly 646 members, these events provided an opportunity to reach the majority of the state's beekeepers. In 2023, one of her students (now lead bee lab technician, Sydney Miller) presented this work at the University of Vermont Student Research Conference. To share our results with the broader beekeeping community, we presented our results at an international conference in Santiago, Chile (Apimondia) in 2023 and a national conference (American Bee Research Conference) in 2024. Alger and Miller were also able to disseminate our work to the public, through presenting at a local library (Waterbury Public Library), being featured in a published article in American Bee Journal (ABJ-Bee-Lab-article, and interviewing on multiple occasions for a local news channel (WCAX Across the Fence, Burlington VT). We have also highlighted our research on the Vermont Bee Lab website,vermontbeelab.com, and social media accounts. We estimate that this project has reached upwards of 15,000 people through our continuing education and outreach efforts.
During the summers of 2021, 2022, and 2023, Alger and Miller taught online and in-person beekeeping courses. Participants included UVM students and community members. In these courses, Alger and Miller shared the results of this study with beginner beekeepers and incorporated related hands-on activities to share how these procedures may be introduced to bee breeding programs that result in sustainable locally-raised stock. Activities included queen grafting/rearing, mite load testing, and VSH testing. In 2023, Alger and Miller added an activity where students conduct UBO assays on colonies and evaluate their hygienic behavior results. Students in the hands-on summer courses were also invited to French Hill Apiary ‘queen catch’ days where students participated in queen rearing and learned, first-hand, about local bee breeding efforts. Enrollment in 2021 was 11 for the online course and nine for the hands-on course. Enrollment in 2022 was 16 for the online course and eight for the hands-on course. Enrollment in 2023 was 11 for the online course and five for the hands-on course.
Two fact sheets were produced:
UBO-Factsheet-Hobbyists-Final UBO-Factsheet-Producers-Final
UBO-Factsheet-Producers-Final
Learning Outcomes
- By evaluating colonies for a full suite of metrics including Nosema and Varroa prevalence/load, top performing breeder colonies can be accurately identified.
- Chemical treatments for Varroa should occur after mite testing shows the need and colonies that test above treatment threshold should be pulled from breeding programs to better select for mite resistant colonies.
- Bee breeders can work together and share information to improve Vermont honey bee stock on a broader scale.
- UBO assays are a promising new tool for bee breeders and can predict lower Varroa loads in many situations.
- UBO assays can be more sensitive at identifying hygienic colonies compared to Freeze Kill Brood assays.
- UBO heritability is limited in open mated systems; UBO scores of daughter colonies should be verified prior to marketing and sale of “high scoring UBO queen/colony.”
- UBO testing is most reliable in spring and early summer or during a nectar flow.
Project Outcomes
This 3-year project has provided proof of concept for how our lab can leverage its resources and capabilities to improve queen selection metrics and strengthen long-term bee breeding programs in Vermont. We have expanded our initiatives across three other bee breeding operations in the state, using the selection criteria that we developed during this project. The relationship formed between Vermont Bee Lab and French Hill Apiaries has laid the foundation for other collaborations and has created a ‘bee breeding collective’ in Vermont, where we currently have four operations sharing techniques, results, and bee genetics with an overarching goal to improve the quality and availability of honey bee stock throughout the state. We have promoted French Hill Apiaries as one of our collaborating operations, in hopes of expanding the market for locally adapted bee stock. French Hill Apiaries and our other partnering bee breeders have reported customers expressing increased interest in their honey bee stock, which has been tested against rigorous criteria. Additionally, the promise of UBO assays has drawn a lot of attention from the beekeeping community. In February, we plan to meet with our four collaborating operations and discuss program design and logistics for next season. Our team was successful in obtaining a Specialty Crop Block Grant that will allow us to continue to support this work well beyond 2024.
Through evaluating of our study's approach and methods, we believe that the project's key successes include our strong collaboration with French Hill Apiaries that has extended to three other bee breeding operations in Vermont, accurate pest and pathogen identification and quantification using VBL's lab capabilities, and developing improved criteria for selecting breeder colonies that are locally bred and tolerant/resistant of pests and pathogens in the Northeast. Our major challenges were posed by the Freeze Kill Brood assay, to test for hygienic behavior, which was often cumbersome to perform in the field and lacked sensitivity. As a solution, we replaced this method with our improved UBO assay, which we found to be a better predictor of pest and pathogen loads in some cases. Additionally, challenges arose with coordinating sample collections with ongoing beekeeper management practices. We overcame many of these challenges by fostering frequent and clear communication and hosting in-person annual meetings to discuss lessons learned and challenges. Due to challenges with lab processing times, we are still working to analyze virus loads from 2023 colonies. In 2022, we found no difference in deformed wing virus (DWV) load between our experimental groups. We decided to expand the virus panel under investigation. This change has resulted in additional processing time but the data will provide insight on eight viruses instead of one. Final data for overwintering success and honey production will be collected in spring 2024 once hives are assessed and unwrapped.
We quickly discovered that the original question we planned to study– whether our added metrics for selecting colonies showed improvement when compared with colonies selected with standard metrics used at French Hill Apiaries– requires multiple generations to answer. In the three years of this project, we were only able to rear two daughter generations of bees, and only one generation that was monitored through its first season as full production colonies. In our preliminary results, we see a trend that using UBO assays has increased the level of hygienic behavior present in the population. We also found that our evaluation and selection processes resulted in colonies with lower Varroa loads. We anticipate that given subsequent generations, the increased level of hygienic behavior in the population will confer additional pest and pathogen resistance. We will continue to monitor and evaluate daughter colonies based on our developed metrics in order to compare results with previous years. We plan to promote these practices to other breeding operations in our region and have already begun collaborations with three other bee breeders in Vermont in 2023. We believe that Northeast commercial beekeepers will benefit the most from our findings in this study. However, hobbyist beekeepers may also benefit by choosing to purchase improved honey bee stock from local suppliers into their apiaries, knowing that breeding programs like this exist.