Final report for ONE20-372
Project Information
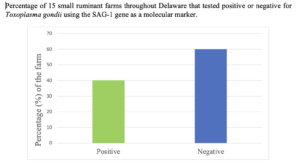
Toxoplasmosis is the zoonotic disease caused by Toxoplasma gondii. The symptoms only become easily noticeable once an animal is immunocompromised (ill or pregnant). Majority of the symptoms occur in females with abortion, fetal mummification, reabsorption, weak kids, and in severe cases, blindness and brain damage. In order to effectively report the incidence and stop the cycle of toxoplasmosis on farms, it is imperative animals with the parasite are identified and treated or culled and preventative measure are taken. Over 40 years ago, molecular detection identified surface antigen 1 (SAG-1) as a major surface antigen with specificity to T. gondii. The SAG-1 gene has been utilized by many to detect T. gondii livestock across the world. Therefore, this project aimed to characterize the level and understand the impact of Toxoplasma gondii infection on small ruminant farms in Delaware. To complete this project, fifteen small ruminant farms (with a minimum of ten animals) in Delaware was selected and blood samples taken from their animals. Utilizing molecular techniques with a gene (SAG-1) specific primer for T. gondii, polymerase chain reaction (PCR) was conducted and the product fractionated to identify SAG-1 gene. The results from this study indicated that 40% (6/15) of the farms had a presence of T. gondii. However, only 15.2% of the animals that were used in this project had the T. gondii. Survey data 78.6% of the farmers had cats on their farms and 91.0% of these farmers allowed their cats to have access to feed and the feed room. This could be the main reason why T. gondii was found on 6 of the 15 farms. The results of this was project presented at the Association of 1890 Research Directors, National Goat Conference, Delaware State University Research Symposium, and the Delaware Agriculture Week Small Ruminant Program.
This project seeks to characterize the level of and understand the impact of Toxoplasma gondii infection on small ruminant farms in Delaware. Molecular detection method (polymerize chain reaction; PCR) will be used to determine the levels of T. gondii parasite in small ruminants in Delaware and surveys will be created to gain information from farmers to understand the impact this parasite had on the reproductive efficiency on their farm. The results from this objective will be used to educate producers of the popularity of T. gondii within Delaware and to help them come up with ways to prevent or limit the impacts of this parasite.
Agriculture is one of the largest enterprises in Delaware with animal-based agriculture the largest and most profitable. Due to the increased immigrant population in the United States (U.S.; specifically, on the East Coast) and the desire for healthier diets, there is an increased demand for small ruminant meat (Ibrahim et al., 2017). The increasing need to meet this demand provides an attractive, affordable, and profitable alternative enterprise for small and beginning farmers on the eastern seaboard including those in Delaware. Although this demand provides an opportunity for many producers, growth of the goat industry is impacted by the prevalence of internal parasite infections that lead to losses in production and increased mortality in many herds/flocks. One parasite that can significantly reduce small ruminant production is the opportunistic zoonotic apicomplexan protozoan parasite, Toxoplasma gondii, that causes the disease toxoplasmosis.
Toxoplasmosis is a disease that is common among animals and humans with up to one-third of the world’s population chronically infected (Halonen and Weiss, 2014). It is transmitted from a definitive host (feral and domestic cats) to an intermediate host (human, sheep, goat, cattle, etc.) or between different intermediate hosts (Dubey and Lindsay, 2006). Sheep and goats generally become infected by eating contaminated feed or drinking contaminated water while the leading cause of infection for human is the consumption of under-cooked/raw meat or unpasteurized goat milk (Dubey and Lindsay, 2006). In the U.S., small ruminants are primarily raised on pastures which are frequently visited by stray cats resulting in high infestation by T. gondii. Toxoplasma gondii infection in sheep and goats is very detrimental to production as it causes abortion, mummification, weak kids, stillbirth, embryonic and fetal death, and neonatal death (Dubey and Lindsay, 2006). Additionally, once this parasite enters the bloodstream it is able to spread to other tissues within the animal and in some cases causing diminished vision and brain damage. Currently, there are limited information in the U.S. on the prevalence of this parasite in small ruminants. However, in a study conduct on lambs in 22 states it was found that 58.8% of the farms tested on the east coast had animals positive for T. gondii infection (APHIS, 2014). Additionally, study conducted at Delaware State University (DSU) after a series of abortions found 18 out of 36 meat-goat does infected with T. gondii (unpublished, 2018).
With the potential of this parasite to be detrimental to animal production and having the ability to cause severe infection in humans, identifying the prevalence and impacts of this parasite within Delaware is of greave importance. Therefore, this research will address a critical area in small ruminant production and human health in Delaware and will enlighten producers on how widespread T. gondii is and how to best limit the effects on their farm. This will lead to different management decisions (such as adding anti-protozoal treatment to feed, limit feral and domestic cat interaction with sheep and goats) and health precautions that could prevent further spread of T. gondii parasites both to animals and humans.
Cooperators
- - Producer
- - Producer
- - Producer
- - Producer
- - Producer
- - Producer
Research
Materials and Methods
In order to conduct this project, Institutional Animal Care and Use Committee (IACUC) and Institutional Review Board (IRB) approvals were acquired to ensure safe use of animals and corporation from humans. Samples were collected from 15 small ruminant farms (goat = 10; sheep = 4; sheep and goat = 1) having a minimum of 10 animals each. Blood sampling were done in duplicated collection from each animal and used for peripheral blood mononuclear cell (PBMC) extraction. After which, genomic deoxyribonucleic acid (DNA) was isolated and used to conduct conventional polymerase chain reaction (PCR). The PCR product was then separated by gel electrophoresis and imaged in order to tell if the animals were positive or negative for Toxoplasma gondii. In order to evaluate farmer knowledge of the parasite and its potential impact a survey was given to all participating farmer and 14 was complete and returned.
Blood Sampling and Peripheral Blood Mononuclear Cells
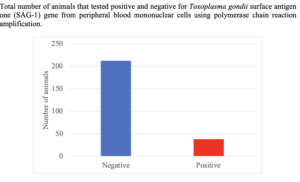
Blood samples were collected at each participating producer farm (n = 15) by jugular venipuncture from individual animals (n = 250) and deposited into blood collection tubes with ethylenediaminetetraacetic acid (EDTA; to prevent clotting). Samples were placed on ice and then transported to Delaware State University’s parasitology laboratory for white blood cell isolation. Individual blood samples (n = 250) were separated into serum, red blood cells and PBMC using Histopaque-1077 (Sigma-Aldrich) following the manufacturer’s instructions. Peripheral blood mononuclear cell were aspirated and washed twice with phosphate buffered saline (PBS) to eradicate red blood cell, histopaque, and plasma residues. The pellets were then transferred to 2 ml centrifuge tubes and stored at -80 °C prior to DNA extraction for T. gondii identification.
Deoxyribonucleic Acid (DNA) Extraction and Conventional Polymerase Chain Reaction (PCR)
In order to identify T. gondii from blood samples, genomic DNA were isolated from individual animal's (n = 250) PBMC and PCR conducted to amplify T. gondii specific surface antigen 1 (SAG-1). Briefly, genomic DNA were isolated by first incubating PBMC in a lysis buffer containing acetic acid, tris base, sodium dodecyl sulfate (SDS) and proteinase K for one hour. After which, a conventional phenol/chloroform extraction method was performed and the isolated DNA pellet resuspended in 20 μL nuclease free water and stored at -20°C for PCR amplification.
The amplification of SAG-1 full coding sequence, was then conducted by conventional PCR. Polymerase chain reaction was conducted utilizing forward and reverse specific oligonucleotide primers designed based on the coding sequences described by Selseleh et al., 2012 and ordered from Integrated DNA Technologies, Coralville, Iowa. After PCR, the amplicons were fractionated on agarose/ethidium bromide (EtBr) gel in 1X Tris-acetate-EDTA (TAE) Buffer for DNA separation based on size and visualized by using a Gel Doc Imager. Once a band was identified on the gel, this allowed researchers to know the animals that were positive for T. gondii and the percent infected on each farm was calculated.
Survey Development
A 22-question survey was designed and approved by the institutional review board (IRB) in order to acquired information on the knowledge and awareness of producers about T. gondii and its impact on small ruminants. Farm demographic data, current disease management practices, presence or absence of cats, handling of cat litter, and abortion history were collected from each farm in order to help understand if there was a spread or impact of toxoplasmosis on these farms. This survey was given to all 15 farms where samples were collected.
To gain knowledge on the prevalence and the level of understanding producers have about Toxoplasma gondii infection, producers were given a 22-question IRB approved survey that covered farm demographics, rate of abortion, and knowledge of toxoplasmosis. Based on the survey data, 78.6% of the farmers had cats on their farms and 91.0% of the farms that had cats allowed their cats to have access to feed room and feed products. Additionally, it was found that 35.7% of the farmers had no knowledge about T. gondii while the other 64.3% of farmers had some knowledge about T. gondii.
In order to identify T. gondii on small ruminant farms, blood samples were collected individually from a total of 165 goats and 85 sheep from fifteen producer farms throughout Delaware. White blood cells were isolated from each animal's blood sample and used for genomic deoxyribonuclease acid (DNA) isolation. The isolated DNA was then used in a polymerase chain reaction (PCR) to amplify surface antigen-1 (SAG-1) that is specific to T. gondii. Gel electrophoresis and imaging of the PCR product, indicated that T. gondii was present in 15.2% (38/250) of animals tested and on 40.0% (6/15) of farms tested. Additionally, the data indicated that 33.3% of the farms that had positive animals belonged to sheep producers while 66.7% of the positive animals belonged to goat producers. The detection of T. gondii on these farms can be linked to the fact that several of these farms had cats and most of the cats had access to the feed used for these animals. The cats are the common carrier of T. gondii, especially, kittens that are infected.
It can be concluded that T. gondii was present on several farms in Delaware. However, not all the animals on these farms were positive for T. gondii. Additionally, more research is needed with a larger sample size than 15 farms in order to be more accurate of the status of T. gondii prevalence in the Northeast region.
Education & Outreach Activities and Participation Summary
Participation Summary:
The results of the research project was presented at the National Goat Conference, the Professional Agriculture Workers Conference, at the Delaware State University student research symposium, the Association of 1890 Research Directors, and Delaware Ag Week Small Ruminant Sessions. This data was presented to both farmers, professionals, and students. The data was well accepted by farmers and several farmers were not aware of the implication of Toxoplasma gondii on both animals and humans. Producers indicated by word of mouth that they will have to pay more attention to the cats on their farms and where they are feeding them. If there was more funding, several other farmers indicated interest in this project which will lead to me writing a larger grant to cover a wider range of farms. Currently, the finds from the project is being used to prepare a manuscript for publication which is still under review and will be set out once it is accepted in a peered review journal. Mariline Hilaire ARD Conferences 03.29.2022
Preliminary Poster (Fisrt time data was presented)
Research Update (Ag Week 2023) T gondii portion
Learning Outcomes
The farmers reported changes in knowledge of the impacts of Toxoplasma gondii and management of cats on their farms. Based on the survey given to the farmers that participated in this project, it was noticed that the majority of the farmers had barn cats or wild cats on their farms and they did not contain where these cats had access to. After completing the project and education the farmers about this parasite, the producers all indicated that they will limit cats getting access to their animals' feed and feed room.
Project Outcomes
The farmers that participated in this project indicated that they will no longer leave feed room doors for cats to have access to feed rooms. Several of the farmers never heard about Toxoplasma gondii and is now aware and willing to join any project or workshop discussing T. gondii infection and prevention. This project helped to improve the knowledge of all the farmers that participated on how this parasite operates.
This project was successful because it was directly linked towards identifying an issue that many farmers did not know about and it was linked to both small ruminant and human abortion. The farmers were very interested and willing to not only allow us to collect samples, but they wanted to know the results and how to prevent the spread of this parasite on their farm One limitation was that the funding was not enough to test more farms and animals on other types of farms. One feedback from an Wildlife Biologist was that I needed to test not only the goats on the farm but also the cats to see if there is any direct link. This was great but the funding was limited and getting the permission to test farm cats was not investigated to see if a veterinarian would have to be onsite. One funding limitation was that the funds was not enough to pay for a full time graduate student in order to take the data and so the research student's time was split across other project. Overall, given the conditions of the grant and project limitations, this project was successful. After this project, a larger grant proposal was submitted to a USDA National Institute of Food and Agriculture grant for 300,000 to do this project more in-depth in several northeast states. This proposal is still pending.