Final report for ONE20-373
Project Information
Environmental change has presented new challenges for hard clam farmers, while a growing demand for limited shellfish leases creates a pressing need to utilize idle farm leases. The issue we addressed in this project is two-fold. First, cownose rays, a major predator of hard clams, have become a considerable threat to hard clam farms as the species has increased in range and abundance. Second, large areas of viable leased bottom is lying fallow because it is in relatively deep water, not suitable for established hard clam predator protection measures - screens. The objective of this project was to use farm-scale, collaborative experiments to assess the application of shell hash as a deterrent of cownose ray predation. If successful, this strategy would provide the means to make hundreds of idle acres productive, while reducing labor costs.
We evaluated hard clam seed survival and growth at three replicate plots of each of three treatments (9 plots total): unshelled bottom, shelled bottom, and predator screens. Results showed that the shell planted on the shelled treatment plots remained stable in terms of fragment distribution over the 20 months of the experiment. Further, the shelled treatments supported twice the survival of planted seed, and twice the recruitment of new clams. Clam seed on shelled plots grew at the same rate as that observed at control and netted plots. Finally, rays were tagged and observed using telemetry within and around the experimental plots, providing evidence that this technique for observing predator behavior is feasible in back bay habitats and on clam farms. These results support the potential for shell hash to serve as predator protection of clam seed, and that the shell, once planted, remains in place and functional for at least 2 years.
This project seeks to test if shell cover protects farmed clam seed from cownose ray predation. The questions we will answer are:
Does clam seed survival increase when shell is applied to the sediment surface, relative to unprotected and screened clam seed?
Does clam growth (shell size and body mass) change when planted beneath shell on the surface?
Do cownose rays avoid feeding on plots covered with shell?
If successful, this project will provide information to help clam farmers prevent cownose ray predation losses, and as a means to use deep water leases that are not suitable for netting. It will also provide important information about potential increases in natural clam recruitment at farm sites planted with shell.
Farmed shellfish production increased 34% in the U.S. from 2013 to 2018 advancing national aquaculture production targets (USDA, 2019, NOAA Fisheries, 2019). Demand for molluscan shellfish is strong and the potential for growth is significant. However, unlike most other farmed shellfish species, the production of hard clams experienced a decline during this same period. In New Jersey, 6,461,000 farmed had clams were produced in 2019 and 20 farms were permitted (Pers, Communication NJDEP, R. Schuster). Barriers to increased growth in the hard clam sector include predation, disease, and limited availability of appropriate farm sites. The problem we will address is increasing predator pressure in the Northeast that is limiting the opportunity for clam farmers to make use of underutilized leases located in deeper water. These leases represent a substantial opportunity for expansion of farm production while limiting additional labor costs, thereby increasing the profitability and sustainability of existing farms. Cownose rays are a major clam predator, often feeding in large schools preferentially on dense patches of clams (Collins et al, 2007), as would be found on farms. These large feeding schools can decimate patches of bivalves (Mann et al., 2016), and anecdotal observations by coastal shellfish farmers support the notion that the abundance and range of cownose rays are expanding, and that predation problems have become more persistent. A number of news articles have focused in recent years on shellfish farmers’ expression of frustration with cownose ray predation, and their calls for a solution. To allow clam farmers to bring the significant amount of viable leased bottom that is lying fallow back into production, we must begin to identify viable solutions to deterring cownose ray losses. These leases tend to be located in relatively deep water, not suitable for use of predator screens which are the established predator protection measures used on shallow water leases. Not only would shelling be a viable protection that could be applied in deeper water lease areas, it also is a predator mitigation measure that would not need the maintenance required for screens, thereby allowing farmers to revitalize these fallow leases with little additional labor and maintenance costs.
Cooperators
- (Researcher)
- (Researcher)
- - Producer
- (Researcher)
- (Researcher)
Research
An experimental site was identified on 5 acres shellfish lease at Cape Horn in Great Bay, NJ, owned by farm partner Dale Parsons. This site was selected because it has sufficient access to deploy and sample the experiment, and it has regular and annual predation pressure by cownose rays .Three replicate plots of each of the three treatment types: shell over clam seed, clam seed under netting, and unprotected clam seed (a total of nine experimental units, Figure 1) were marked on the experimental site using pvc poles on August 11, 2020. Each treatment plot was 20 feet by 14 feet (the equivalent footprint of a clam screen), and was surrounded by a 20 foot buffer on all sides to ensure no edge effects from neighboring plots and to allow the treatment to extend slightly past the edge of the plot for complete plot coverage.
On Sept. 18, 2020 crushed clam shell was distributed on the bottom of the three marked shell treatment plots.
A video of the shell being deployed to the experimental plots is attached. PlantingShell
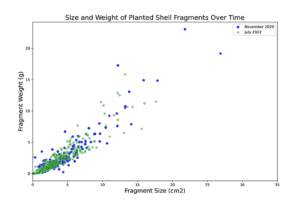
The shell was brought to the site by barge and washed overboard with a hose. On Nov. 10, sediment cores were taken at each of the nine treatment plots. These cores were frozen for subsequent sediment grain size analysis. Thickness of the shell layer on the shelled plots was also measured, and a sample of the shell fragments was collected from each shelled plot. Additional sediment cores and shell samples were taken throughout the duration of the experiment at each of the nine treatment plots on 6/16/2021, 7/22/2021, and 7/6/2022). Sediments were frozen for storage and later processed to evaluate sediment grain size and shell fragment size (area was measured using photographs and imageJ) and weight (Figure 2).
Hard clam seed (20mm shell length) was planted at a density of 10 clams/ft2 in each of the nine treatment plots on Nov. 25, 2020, and predator screens were installed over the three netted treatment plots. A subsample of the clam seed (n=80 randomly selected individuals) were returned to the lab to evaluate size at planting (i.e. the initial size at the start of the experiment).
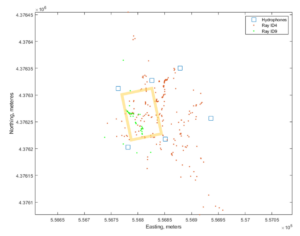
Six Lotek hydrophones were deployed at the four corners of the experimental site, plus 2 more beyond the experimental site creating 2 boxed areas encompassing the experiment and an adjacent control area. Shortly thereafter, one bullnose and one cownose ray were surgically tagged (Figure 3). Tagged rays were tracked within and around the experiment site using the hydrophone array.
At the conclusion of the experiment (7/6/2022) each of the nine plots were sampled. A subsample of each of the nine treatment plots was harvested using a suction dredge and the contents passed through a 9 mm mesh. The area and depth of sediments sampled with the suction dredge at each plot was measured and recorded. Each sample was sorted and live clams, clam hinges (these are what often remains after a cownose ray eats a clam), and whole dead clam shells counted. All live clams collected from the subsample were measured for shell length, and sucked to measure meat weight.
All of the data collected were analyzed statistically (using analysis of variance, Student's t-test, or Kolmogorov–Smirnov statistic) to evaluate differences in planted clam density, clam recruitment, clam growth, or shell fragment size and weight among the three treatment types.
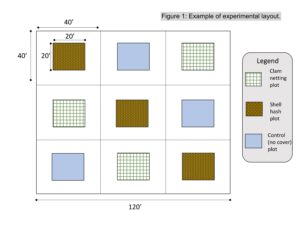
Figure 2: Shell fragments were measured (area) and weighed individually.
Figure 3. Ray tagging surgery.
Sediment and Shell Characteristics
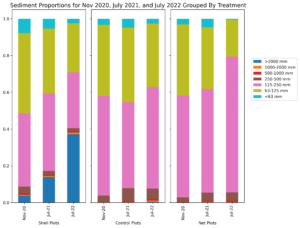
Initially, sediment characteristics among the treatment types was similar, with majority of sediment grain size between 63-250 mm (Figure 4). Over time, sediment grain size at the netted and control plots remained consistent; however at the shelled plots, the proportion of large size particles increased (Figure 5). This increase in grain size distribution is likely due to the planted shell fragments (which tend to be larger than most soft-bottom sediments) on these plots. Shell fragments used for shell planting ranged in size, however the distribution of sizes and the weight of fragments of a given size remained consistent throughout the 20 months of the experiment (Figure 5). This result suggests that shell planted by clam farmers should remain in place and consistent for at least 2 years after planting.
Figure 4: Sediment grain size distribution for shelled plots (left), Control plots (middle), and netted plots (right) over the duration of the experiment.
Figure 5: Distribution of individual shell fragment sizes and weights from the start (blue) and end (green) of the experiment.
Clam Survival and Growth
Final seeded clam density at the shelled treatment plots were 4 clams/ft2 , twice the survival seen on the netted (1.6 clams/ft2) and control (2 clams/ft2) plots (Figure 6). Newly recruited clams (from wild settlement) was also highest on shelled treatment plots (0.9 clams/ft2) compared to netted (0 clams/ft2) and control (0.5 clams/ft2); however, it should be noted that the mesh used in the field (9mm mesh) would have allowed some new recruits to be washed through the mesh. Clam seed on shelled plots grew at the same rate as that observed at control and netted plots. Seeded clams at all three treatment types grew from an average size of 20 mm at the start of the experiment, to an average size of 40 mm at the end: no difference in growth was observed among treatments (Figure 6). The final size frequency distributions show that the shelled and control plots had new recruits (clams smaller than 30 mm), and at the netted plots some clams were observed that were larger than would be expected from planted seed (Figure 7) which may have migrated into the experiment or been present before the plots were seeded. It should be noted that at the time of final sampling, one of the netted treatment plots had been lost and therefore all final clam density and growth results derive from 2 replicate netted plots.
Figure 6: Seeded clam and recruit density (left), image of live clams recovered during final sampling (middle), and clam size distributions (right).
Cownose Ray Telemetry
Both of the tagged rays utilized the clam farm in proportion to the number of days since tagging and were tracked in the farm and beyond the farm at high precision and sufficient temporal and spatial resolution to identify changes in swimming speed potentially indicative of foraging. Capture and tagging of rays was met with lower success than originally planned due to seasonal migration issues (rays move through the study region faster than in previous years), and due to challenges in capture and handling for surgeries. Nonetheless, this technology appears promising for studies of predator behavior on and around clam farms.
Figure 7: Ray detections shown in brown and green dots. Blue squares show the locations of the hydrophones, and yellow box outlines the boundaries of the experimental plots.
Does clam seed survival increase when shell is applied to the sediment surface, relative to unprotected and screened clam seed? Yes, our results suggest that planted clams survive twice as well as clams without protection or under screens. Additionally, it appears that recruitment of wild seed is higher on shelled grounds.
Does clam growth (shell size and body mass) change when planted beneath shell on the surface? No. These results suggest that clams on shelled plots grow at the same rate as those on control or netted plots.
Do cownose rays avoid feeding on plots covered with shell? Telemetry was able to discern ray paths in and well beyond the experimental plots at sub-meter spatial resolution; however, data quantity was insufficient for conclusion about foraging habitat choice.
Shell fragments planted remained in place and in the same size and weight distribution throughout the 20 month experiment.
Education & Outreach Activities and Participation Summary
Participation Summary:
Project investigators have presented the project overview, approach, and preliminary findings at the following venues:
- American Fisheries Society MidAtlantic Chapter Meeting, Nov. 15-16, 2022, Asbury Park NJ (primary audience are fishery/aquaculture scientists and state agency managers).
- Northeastern IPM Center Research Webinar, Virtual, March 31, 2021 (primary audience was agricultural research community).
- The Five Senses webinar on Apr 1, 2021, hosted by the Jacques Cousteau National Estuarine Research Reserve (primary audience was public, scientists and educators).
- The National Estuarine Research Reserve association annual meeting Nov 15 - 18, 2021 (primary audience was conservation educators, science outreach specialists, researchers).
The field efforts engaged two graduate student and 4 undergraduate interns. These students were trained in field methods, experimental design and data collection during these campaigns.
Results will also be presented at the Milford Aquaculture Seminar, a regional annual conference that attracts shellfish aquaculturists, researchers, and government agents from the Mid-Atlantic and northeast region. Additionally, a FAQ sheet was developed and shared via relevant email lists, including the New Jersey Aquaculture Association listserv, and via post to the Haskin Shellfish Research Laboratory website. The FAQ sheet provides a summary of this project and main results.
FAQ sheet
Clams Shell Rays Poster
Learning Outcomes
Our farm partner gained important knowledge about how well planted shell will perform to protect planted clam seed from predation. He also learned that this shell will not degrade (break down) over the course of 2 years and this will improve his ability to use these techniques efficiently and economically. Additionally, he learned that planted shell can also help enhance wild recruitment of clams to his farm. The farmer participated in all field activities and in doing so, gained an awareness about the scientific method and in conducting field experiments.
Project Outcomes
na
Keys to success were full collaboration among farm partner and science teams. The farm partner was engaged in experimental planning and initial execution, and all subsequent steps. This ensured that the results were relevant at a farm-scale, and applicable to the farm setting. It also allowed the farmers' ecological knowledge to be leveraged for project planning which further increased success.
A major challenge faced by the project was the ability to tag sufficient numbers of rays given the amount of effort allocated to that part of the project, and given anomalies in seasonal migration patterns of the rays. Nonetheless, tags were deployed and data collected demonstrated the potential value of future efforts at characterizing predation behavior of cownose rays at clam farms using telemetry.
Information Products
- Shell Hash Cover as a Deterrent of Ray Predation on Hard Clam Farms (Conference/Presentation Material)
- Shell Hash Cover as a Deterrent of Cownose Ray Predation on Hard Clam Farms - Summary Report (Conference/Presentation Material)