Final report for ONE22-421
Project Information
Gastrointestinal parasites are a pervasive issue in organic and pasture raised swine that can result in poor productivity and economic losses. There is little research available on organically approved and non-chemical parasite control methods in hogs. The goal of this project was to measure parasite prevalence on farms where hogs were being raised with outdoor access and without the use of synthetic dewormers, and to assess whether the management practices being employed on those farms could impact the level of parasite infection. Seasonal field surveys were conducted on nine pig farms in Pennsylvania, during which fecal, bedding, and soil samples were collected to be analyzed for parasite eggs. A total of 237 fecal samples were collected, and 167 (70.5%) of those samples were positive for parasite infection. Approximately 62% of hogs were infected with Ascaris suum, 26% were infected with strongyle-type parasites, and 10% were infected with Trichuris suis. Some management practices were found to be related to parasite prevalence. Farms that had been raising hogs for more than six years had significantly higher parasite prevalence than those raising hogs for less than six years, and parasite prevalence and intensity was lower on farms that used natural dewormers such as garlic, diatomaceous earth, and apple cider vinegar. These results were shared with participating farmers, as well as hundreds of farmers, students, and consumers at on-farm events and tours as well as at the 2024 Marbleseed Organic Farming Conference.
The ultimate goal of this project is to develop management practices and technical guidelines for farmers for effective control of swine parasites in organic systems. The specific objectives are:
- Evaluate parasite prevalence and intensity on organic and pasture-raised pig farms by conducting a robust survey study (including 10 organic farms in the Northeast region primarily in PA and NY)
- Analyze the effects of management practices (i.e. bedding and housing used, access to pasture, acreage used for pigs, size of the herd, breeds used, etc.) on parasite infections
- Disseminate information to farmers and educate them on the best management practices for control and management of swine parasites on organic farms
Pork is the third-highest selling meat product in the United States, with the average American consuming 51 pounds of pork annually (Davis and Lin 2005). To compensate for this demand, hogs are raised in specialized, indoor confinement facilities that maximize efficiency. These facilities, however, often have poor indoor environments that restrict innate behaviors and are associated with symptoms of chronic stress in pigs (Stolba and Woodgush 1981). There is growing evidence that animal welfare is improved when pigs are raised outdoors (Edwards 2003; Miao et al. 2004; Velazco et al 2013; Weary et al. 2016). Organically raised pigs are required to have outdoor access as stated by the USDA National Organic Program (USDA organic regulations Title 7, Part 205, §205.239).
Allowing outdoor access, however, can bring an additional set of challenges due to greater exposure to the infective stages of parasites in the environment. Conventional farms deploy regular anthelmintic prophylaxis and house animals on slatted floors to avoid parasite infection (Nansen and Roepstorff 1999), but these practices are not allowed in organic production (USDA organic regulations Title 7, Part 205, §205.239). Organic farmers must consider the control of swine parasites without prophylactic treatments, which is challenging considering that semi-free-ranged pigs can be infected with a higher diversity of parasite species at higher abundances relative to those in non-organic, indoor industrialized production operations (Nansen and Roepstorff 1999; Roepstorff at al. 2011).
Surveys of European organic farms have shown that pigs are infected with more types of parasites at a higher prevalence and intensity of infection (Roepstorff et al. 2011). Bedded floors and access to outdoors or pastures that are required by the NOP are the major sources of parasite contamination on organic pig farms. Compared to pigs housed on slatted floors, organic pigs harbor more species of parasites with heavier concentrations (Li 2018). For certain parasite species, prevalence reduced from 95% in pigs housed on bedded floors to 3% in pigs housed on fully slatted floors (Morris et al. 1984).
Gastrointestinal parasites in swine are a major challenge in organic production systems because they can cause economic losses for farmers. The most common species of parasites in pigs are Ascaris suum (the large intestinal roundworm) and Trichuris suis (the swine whipworm) in growing pigs, and Oesophagostomum spp. (the nodular worm) in breeding sows (Edwards et al. 2014; Lingren et al. 2014). Thousands to millions of eggs are released by these parasites every day and passed in the feces of infected individuals, and infective eggs and larvae can remain viable in the soil for months or years (Roepstorff and Nansen 1998). Infection with gastrointestinal parasites can cause significantly reduced average daily gain and poor feed efficiency, which results in economic losses because more feed is needed to get infected pigs to finishing weight than uninfected pigs (Kipper et al 2011). The growth potential of feeder pigs can be permanently affected by exposure to parasites (Urban et al. 1989). Knecht et al. (2011) found a significant difference between the meatiness of slaughtered pigs that were infected with parasites versus pigs that were not infected. Additional economic loss comes from liver condemnations at slaughter as the result of granulomas from migrating larvae of Ascaris suum (Roepstorff et al. 2011). Overall, the need for additional feed to get the growing stock to finishing weight in a time of rising organic feed costs causes noteworthy economic damage to organic farmers.
Organic pig farmers have a greater need for effective worm control than their conventional counterparts due to the higher incidence of parasite infections, but little scientific data is available on the effectiveness of organically approved anthelmintics. Organic pork producers often employ natural or herbal deworming treatments like garlic, pumpkin seeds, or black walnut husks (Lans et al. 2007). There have been very few scientific studies on the efficacy of these natural methods, especially in pigs, and studies done on other species of livestock often show little to no success. For example, in vivo studies showed that garlic had no significant effect on the fecal egg counts of infected horses or in goats and lambs (Buono et al. 2019; Burke et al. 2009a), two commercially available herbal dewormers were ineffective in goats (Burke et al. 2009b), and diatomaceous earth did not significantly reduce fecal egg counts in grazing ruminants (Fernandez et al. 1998). Effective natural treatments against swine parasites may exist, but more in-depth research is needed to establish a better understanding of organically approved worm management.
With little evidence supporting organically approved deworming methods, farmers need to focus on improving management practices as preventative measures to manage parasites. Pastures are commonly allowed to rest and recover after having pigs, but resting pastures may not be completely effective at controlling parasites because infective eggs can remain viable for over a decade (Roepstorff and Nansen 1998). Proposed natural methods of parasite control via pasture management include planting biofumigants in pastures and the use of predatory or parasitic fungi (Miao 2004). Other farm variables like herd size, stocking density, and whether the operation is a farrowing farm or an all-in/all-out may influence parasite infection.
We plan to address the lack of understanding about parasite prevalence in the northeastern United States by surveying organic and pastured pork production facilities. Prior to 2019, no comprehensive parasite survey of organically managed pig farms existed, and the status of parasite infection on Pennsylvania organic pig farms is widely unknown. In addition to measuring the level of parasite infection on organic farms, we will collect data from each farm on management practices like herd size, stocking density, housing style, and rotation schedule. The parasite infection data will be analyzed with respect to management practices to evaluate the effects of these practices on parasite infection. The results of these analyses will be disseminated to farmers to educate and enable them to make better-informed herd management decisions.
Cooperators
- - Producer
- (Educator and Researcher)
- - Producer
Research
Nine farms participated in this project. Data on farm demographics and management were collected via an in-person written attached survey. Data included housing type, herd size, breeds used, type of outdoor access (concrete pad, dirt lot, pasture, etc.), bedding, organic anthelmintics used, and acreage of dirt lot or pasture if applicable.
Participating farms were chosen based on the following criteria: 1) farms must be certified organic or follow organic management protocols; 2) pigs must have outdoor access and freshly bedded floors. Specifically, farms could not employ chemical prophylaxis and pigs could not be housed on slatted floors or in confinement conditions.
Participating farms were visited seasonally for sampling, which included:
- Fecal samples (n = 10-40 per farm) were collected randomly (Katakam et al., 2016) and labeled. Samples were collected fresh and directly from the rectum.
- Soil samples were collected by walking a ‘W’ route through pastures that had been or were actively occupied by pigs (Roepstorff et al., 2001). Three replicates were collected per area, each consisting of 20 subsamples (approximately 5 g of soil from 0-5 cm depth every 3 m). Subsamples were combined and thoroughly homogenized by hand.
- Bedding materials and manure bedpack were collected based on the methods described by Katakam et al. (2016). Bedded areas were categorized into clean, intermediate, and dirty areas depending on urine and fecal contamination. The clean area appeared dry and minimally contaminated by feces and urine, while the dirty area was wet and heavily contaminated with feces and urine. The intermediate area was between the clean and dirty areas. The top 10 cm of bedding material or bedpack manure was collected along the route of 3 ‘W’ walks. Subsamples were combined and thoroughly homogenized by hand.
All samples were analyzed to identify parasite species and quantify egg counts for each species. The number of eggs per gram (EPG) of samples was used as a relative measure of infection size. Samples were processed to isolate swine parasite eggs using a concentration McMaster technique (Roepstorff and Nansen, 1998), with a sensitivity of 20 EPG. The flotation fluid used to quantify parasite eggs was a solution of saturated NaCl and glucose (50 g NaCl, 75 g glucose monohydrate, and 131 g water), which has a specific gravity of 1.27 g/mL. This solution is suitable to recover swine intestinal parasite eggs and to assess the relative abundance of coccidia oocysts as few, medium, or many (Carstensen et al. 2002). Lab activities included:
- Fecal samples: 4 g of feces were soaked in 56 mL of tap water for 30 minutes then filtered through a single layer of cheesecloth. 10 mL of filtrate was transferred to a 15 mL Falcon tube and centrifuged at 2000 rpm for 7 minutes. After drawing off and discarding the supernatant, the pellet was resuspended in ~4 mL of flotation solution. Both chambers of a McMaster slide were filled and the slide rested, undisturbed, on the bench top for 5 minutes to allow eggs to float. All eggs under the engraved grids were counted. This egg count was multiplied by 20 to get a calculated eggs per gram (epg) of feces.
- Soil samples: 10 g of homogenized soil was weighed into a 50 mL Falcon tube and the tube was filled to the 50 mL mark with 0.5 M NaOH. The soil soaked for 24 hours. After 24 hours, the tubes were centrifuged at 2000 rpm for 7 minutes. The supernatant was discarded, and the tubes were filled to the 50 mL mark with flotation solution and the pellets resuspended via vortexing. The tubes were centrifuged at 2000 rpm for 7 minutes. The supernatants were collected in pre-labeled glass beakers. The resuspension of pellets in flotation solution and centrifugation was repeated three more times. The collected supernatant in each beaker was then washed with tap water on a 20 µm sieve, and the residue remaining on the sieve was transferred to a 15 mL Falcon tube and centrifuged at 2000 rpm for 7 minutes. All fluid except the bottom 0.5-1 mL was discarded. ~2 mL flotation solution was added, and both chambers of a McMaster slide were filled. The slide was allowed to rest for five minutes on the bench top, and then all eggs in the slide were counted (inside and outside the engraved grid).
- Bedding samples: Bedding material was cut to pieces 1-5 cm in length and homogenized. 5 g of bedding material was soaked in 0.5 M NaOH for 16-18 hours. The sample was then thoroughly washed with tap water on a 212 µm sieve on top of a 20 µm sieve. 10 mL of material retained on the 20 µm sieve was transferred to a 50 mL Falcon tube, then the tube was filled to the 50 mL mark with flotation solution and centrifuged at 2000 rpm for 7 minutes. The supernatant was removed, and the pellet was washed on a 20 µm sieve. Material retained on the sieve was transferred to a 15 mL Falcon tube and centrifuged at 2000 rpm for 7 minutes. The supernatant was discarded and the pellet resuspended in ~2 mL flotation solution. Both chambers of a McMaster slide were filled and the slide was allowed to rest for 5 minutes on the benchtop. All eggs in the slide were counted.
Data from feces was statistically analyzed using R software (R Core Team, 2017). Data from soil and bedding samples are still being statistically analyzed. Shapiro-Wilk normality tests on the fecal EPG of each parasite species showed data for all species was not normal (p<0.05, for all parasites) and we therefore used non-parametric tests in our analysis. We analyzed the prevalence of infection, i.e. the proportion of fecal samples positive for a particular parasite species’ egg, using Chi-squared tests. We used a Kruskal-Wallis rank sum test to compare the average EPG in fecal samples. An alpha of 0.05 was set for all analyses.
Nine Pennsylvania farms raising pigs with outdoor access and without the use of synthetic anthelmintics were identified to collaborate for this project. Four seasonal farm visits and sampling events took place as follows: Fall 2022 (September-November 2022), Winter 2023 (January-February 2023), Spring 2023 (April-May), and Summer 2023 (July-August). Farmers filled out surveys with farm demographic and hog management information, including location, type of hog housing and outdoor access, farm production information (i.e., number of hogs raised per year, length of time raising pigs), non-synthetic anthelmintic remedies used, and manure and pasture management. Survey data is outlined in Table 1.
Table 1. Participating farm demographic and management data.
|
Farm ID |
Farm Location |
Time Raising Pigs on Farm (Years) |
# of Pigs Raised Annually |
Outdoor Access Type |
Shelter Type |
Non-Synthetic Anthelmintics Used? |
Biosecurity Measures Implemented? |
|
1 |
Fleetwood, PA |
5 |
10-14 |
Pasture |
Mobile |
Y |
N |
|
2 |
Nescopeck, PA |
3 |
40 |
Forest |
Mobile |
Y |
Y |
|
3 |
Barto, PA |
9 |
40 |
Pasture |
Forest Cover |
N |
N |
|
4 |
Oley, PA |
10 |
20 |
Concrete Pad |
Permanent |
Y |
N |
|
5 |
Kingsley, PA |
3 |
15-20 |
Pasture |
Forest Cover |
N |
N |
|
6 |
Newmanstown, PA |
10 |
25 |
Pasture |
Mobile |
Y |
N |
|
7 |
Kutztown, PA |
10 |
40-60 |
Pasture |
Permanent |
N |
Y |
|
8 |
Lenhartsville, PA |
5.5 |
<10 |
Pasture |
Mobile |
Y |
N |
|
9 |
Lancaster, PA |
8 |
20-30 |
Pasture |
Mobile |
N |
Y |
Eight of 9 farms (88.9%) bought-in pigs and followed a feeder-to-finish production system, while 1 farm (11.1%) maintained breeding stock and followed a farrow-to-finish production system. Participating farms had been raising hogs for 3-10 years (average 7 +/- 3 years) and raised between 10-60 hogs (average 29 +/- 16 hogs) per year. Five (55.6%) farms reported using natural deworming methods, which included apple cider vinegar, diatomaceous earth, garlic, and a commercially available herbal deworming powder.
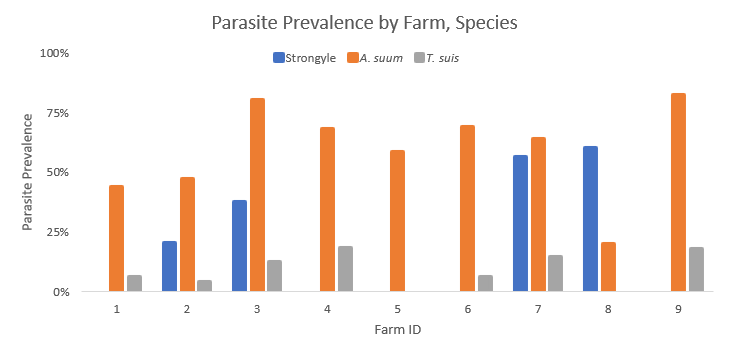
A total of 237 fecal samples were collected throughout the experiment. In the fall, 90 fecal samples were collected from 8 farms (1, 2, 3, 4, 5, 6, 7, and 8), 39 were collected in the winter from 3 farms (2, 3, and 7), 47 were collected in the spring from 3 farms (2, 7, 8, and 9), and 61 were collected in the summer from 7 farms (1, 2, 3, 4, 7, 8, and 9). All 9 farms had hogs that were infected with at least one species of parasite at some point in the study. The prevalence of each parasite species on each farm is illustrated in Figure 1. Parasite prevalence ranged from 43.8-82.4% (average 68 +/- 4.8%). Ascaris suum was the most common parasite species, occurring in all 9 farms (100%), while Trichuris suis was found on 7 farms (77.8%), and strongyles, likely Oesophagostomum spp., were found on 4 farms (44.4%).
Figure 1. Prevalence of parasite species (strongyle, Ascaris suum, and Trichuris suis) on 9 participating farms.
Of 237 fecal samples collected, 167 samples (70.5%) were positive for parasite infection. Overall, 146 fecal samples (61.6%) contained A. suum eggs, 62 samples (26.2%) contained strongyle parasite eggs, and 24 (10.1%) contained T. suis eggs. Among infected samples, 65.9% (n=110) were infected with one parasite species, 29.3% (n=49) were infected with two parasite species, and 3.4% (n=8) were infected with three parasite species.
A total of 202 soil samples were collected from 69 paddocks on eight farms; farm #4 had hogs on a concrete pad and therefore did not have soil to be collected. Soil samples from 63 paddocks (91.3%) contained parasite eggs. Parasite eggs were identified in 93% (n=40) of paddocks that were occupied by hogs at the time of sampling, and in 88.5% (n=23) of paddocks that were previously occupied by pigs. Eggs of A. suum, T. suis, and strongyle parasites were found in 85.5%, 34.8%, and 18.8% of paddocks, respectively. Thirty-two bedding samples were collected from three farms (farms # 2, 4, 7). 62.5% (n=20) of bedding samples contained parasite eggs. A. suum, T. suis, and strongyle eggs were identified in 59.4%, 6.3%, and 3.1% of bedding samples, respectively.
Relation between years farming pigs and parasite infection:
A higher proportion (32.3%) of fecal samples from farms growing hogs more than 6 years were positive for strongyle parasite eggs relative to farms growing hogs for less than 6 years (13%; X2=9.1, DF=1, P=0.002). The average number of strongyle parasite eggs was higher in farms growing hogs more than 6 years (KW X2=11.56, DF=1, p=0.0007). Similarly, a higher proportion (67.7%) of fecal samples from farms growing hogs more than 6 years were positive for A. suum eggs relative to farms growing hogs for less than 6 years (48%; X2=7.67, DF=1, P=0.005), and the average number eggs was higher in farms growing hogs more than 6 years (KW X2=10.12, DF=1, p=0.001). Finally, a higher proportion (13%) of fecal samples from farms growing hogs more than 6 years were positive for T. suis eggs relative to farms growing hogs for less than 6 years (3.9%; X2=3.85, DF=1, P<0.05), and the average number of T. suis eggs was higher in farms growing hogs more than 6 years (KW X2=4.78, DF=1, p=0.03).
Relation between type of outdoor enclosure and parasite infection:
Four types of outdoor enclosures were provided to pigs by farms: concrete paddock, pasture, forest, or a mix of pasture and forest. There was a difference in the proportion of fecal samples positive for strongyle parasite eggs between types of outdoor enclosures (X2=12.71, DF=3, P=0.005), with no strongyle eggs recovered from concrete, and 35.6% positive fecal samples from pastures, 20.4% positive samples from forests, and 26.2% positive from pasture/forest mix. The average number of strongyle parasite eggs differed between types of outdoor enclosures (KW X2=12.36, DF=3, p=0.006), with higher EPG in pasture and pasture/forest mix. There was no difference in the proportion of fecal samples positive for A. suum eggs between types of outdoor enclosures (X2=6.58, DF=3, P=0.09), with 68.2% positive fecal samples from concrete, and 60.9% positive fecal samples from pastures, 46.9% positive samples from forests, and 68.7% positive from pasture/forest mix. The average number of A. suum eggs was not different between types of outdoor enclosures (KW X2=6.97, DF=3, p=0.07). Similarly, there was no difference in the proportion of fecal samples positive for T. suis eggs between types of outdoor enclosures (X2=3.73, DF=3, P=0.29), with 18.2% positive fecal samples from farms with concrete, and 11.5% positive fecal samples from farms with pastures, 4.1% positive samples from farms with forests, and 10% positive from farms with pasture/forest mix. The average number of T. suis eggs was not different between types of outdoor enclosures (KW X2=3.41, DF=3, p=0.33).
Relation between type of shelter provided and parasite infection:
Three types of shelter were provided to pigs by farms: forest, mobile, or a permanent shelter. There was a difference in the proportion of fecal samples positive for strongyle parasite eggs between types of shelter (X2=14.42, DF=2, P=0.0007), with no strongyle eggs recovered from farms with forest shelter, and 20.8% positive fecal samples from farms with mobile shelter, and 40.3% positive from farms with permanent shelter. The average number of strongyle parasite eggs differed between types of shelter provided to pigs by farms (KW X2=12.45, DF=2, p=0.002), with higher EPG in farms providing mobile and permanent shelter. There was no difference in the proportion of fecal samples positive for A. suum eggs between types of shelter (X2=0.63, DF=2, P=0.7), with 58.3% positive fecal samples from farms with forest shelter, 59.7% positive samples from farms with mobile shelter, and 40.3% positive from farms with permanent shelter. The average number of A. suum eggs was not different between types of shelter provided by farms (KW X2=5.73, DF=2, p=0.06). Similarly, there was no difference in the proportion of fecal samples positive for T. suis eggs between types of shelter (X2=4.6, DF=2, P=0.1), with 0% positive fecal samples from farms with forest shelter, 8% positive samples from farms with mobile shelter, and 15.6% positive from farms with permanent shelter. The average number of T. suis eggs was not different between types of shelter provided by farms (KW X2=4.13, DF=2, p=0.13).
Relation between use of natural anthelminthics and parasite infection:
A higher proportion (37.1%) of fecal samples from farms not using natural anthelminthics were positive for strongyle parasite eggs relative to farms using natural anthelminthics (14%; X2=15.22, DF=1, P<0.0001). The average number of strongyle parasite eggs counted in fecal samples from farms not using natural anthelminthics was higher than in samples from farms using natural anthelminthics (KW X2=16.65, DF=1, P<0.0001). A higher proportion (71%) of fecal samples from farms not using natural anthelminthics were positive for A. suum eggs relative to farms using natural anthelminthics (51%; X2=9.28, DF=1, P=0.002), and the average number of eggs was also higher in farms not using natural anthelminthics (KW X2=12.1, DF=1, P=0.0005). Similarly, a higher proportion (13%) of fecal samples from farms not using natural anthelminthics were positive for T. suis eggs relative to farms using natural anthelminthics (7%; X2=1.67, DF=1, P=0.2), and the average number of eggs was also higher in farms not using natural anthelminthics (KW X2=2.58, DF=1, P=0.11).
Relation between use of biosecurity measures and parasite infection:
There was no difference in the proportion of fecal samples positive for strongyle parasite eggs between farms with or without biosecurity measures (X2=0.04, DF=1, P=0.84), with 24.7% positive fecal samples from farms with no biosecurity measures, and 26.8% positive from farms with biosecurity measures. There was no difference in the average number of strongyle eggs counted in fecal samples from farms with or without biosecurity measures (KW X2=0.03, DF=1, P=0.87). There was no difference in the proportion of fecal samples positive for A. suum eggs between farms with or without biosecurity measures (X2=0.04, DF=1, P=0.84), with 65.9% positive fecal samples from farms with no biosecurity measures, and 58.8% positive from farms with biosecurity measures. There was no difference in the average number of A. suum eggs counted in fecal samples from farms with or without biosecurity measures (KW X2=0.64, DF=1, P=0.42). Finally, there was no difference in the proportion of fecal samples positive for T. suis eggs between farms with or without biosecurity measures (X2=0.001, DF=1, P=1), with 10.6% positive fecal samples from farms with no biosecurity measures, and 9.8% positive from farms with biosecurity measures. There was no difference in the average number of T. suis eggs counted in fecal samples from farms with or without biosecurity measures (KW X2=0.04, DF=1, P=0.84).
There were a few aspects of the project that differed from the original methods. Sample collection began in fall of 2022 instead of spring 2023 as proposed. Additionally, some farms were not sampled during all four sampling periods due to a lack of material to sample, scheduling conflicts, or discontinuation of communication from farmers. Soil and bedding data are still being analyzed for statistical significance.
The goal of this project was to measure swine parasite prevalence on organic and pastured pig farms, and to evaluate the effects of management practices on parasite infection levels. Fecal, bedding, and soil samples were collected during four seasonal field surveys from nine farms in Pennsylvania raising pigs with outdoor access and without the use of synthetic anthelmintics. Demographic and management data was collected from each farm at the time of the field surveys via written survey. Fecal, bedding, and soil samples were analyzed for the presence of parasite eggs.
Participating farms represented a variety of management practices. Five farms reported using natural deworming methods, including apple cider vinegar, diatomaceous earth, garlic, and a commercially available herbal deworming powder, and three implement biosecurity protocols to prevent disease in their herds. Parasite eggs were found in the feces, bedding, and/or soil on all nine sampled farms. Parasite prevalence on farms ranged between 43.8-82.4% (average 68 +/- 4.8%), and 70.5% of all fecal samples collected were positive for parasite infection. Parasite eggs were demonstrated in soil and bedding of both occupied and previous housing and pasture areas, indicating on-farm transmission is possible. The prevalence of parasite infection on sampled farms indicates a high occurrence of parasitosis in Pennsylvania swine and identifies a need for further research on non-synthetic and organically approved parasite prevention methods.
Results indicate a relationship exists between some management practices and parasite prevalence and infection levels. Prevalence and egg counts were higher for all three parasite species on farms that have been raising hogs for more than six years. Strongyle parasites were found significantly less on farms where hogs were on concrete and where forest cover was used as shelter. Farms using natural deworming methods had significantly lower prevalence for all parasite species than on farms where natural anthelmintics were not employed. There was no difference in prevalence or number of parasite eggs between farms that did or did not employ biosecurity measures. These results will be shared with both participating farmers and the public to enhance informed decision-making regarding management of hogs.
Education & Outreach Activities and Participation Summary
Participation Summary:
Participating farmers were consulted during each farm visit, as well as via e-mail, text, and phone call regarding parasite infection status on their farm and project results. Project goals and preliminary results were shared with the public at on-farm events and tours at Rodale Institute, like the 2023 Field Day, which brought in over 400 farmers, educators, and consumers from 16 states and 4 countries. Project and parasite information was also shared with farmers and students during 5+ tours at Rodale Institute’s hog facility during 2023, which included college students studying agriculture and farmers looking to begin pastured hog production on their farms. A guest lecture on organic hog production and research, which included an overview of project objectives and results, was given to Penn State University’s Principles and Practices of Organic Agriculture class. A similar lecture was presented to the 2023 class of Rodale Institute Farmer Training students. A poster outlining parasite prevalence and survey results was presented at the 2024 Marbleseed Organic Farming Conference, which is the largest organic farming conference in the United States. Finally, a manuscript is in preparation that will include results from the field survey and the effects of management practices on parasite infection. This manuscript will be submitted to Veterinary Parasitology: Regional Studies and Reports, or other relevant peer-reviewed journals.
Learning Outcomes
Participating farmers were more aware of the prevalence of swine parasites on their own farm, as well as on farms following similar practices in their region. Participating farmers, as well as farmers consulted during tours and at the Marbleseed Organic Farming Conference, were educated on parasite species, life cycles, risk factors, and current scientific knowledge. Farmers were also informed of potential relationships between management practices and parasite infections, as well as the need for further research to explore organic and non-chemical parasite control methods.
Project Outcomes
By the end of the project, cooperating farmers were more educated on swine parasites and the prevalence of parasites on their farm. Data analysis is in progress, but preliminary results have begun to be shared with farmers. Although farmers did not change management practices during this project, they were more informed on the prevalence of parasites and better recognized the need for scientific research focusing on swine parasite reduction and control.
Overall, this project concluded with several successes and challenges. As proposed, swine parasite prevalence was successfully studied on farms in the Northeast where hogs were being raised with outdoor access and without the use of synthetic anthelmintics. This is only the second study of this kind to take place on organic and pastured pig farms in the United States. Participating farmers showed great interest in understanding the risks of gastrointestinal parasite infection in their herds, as well as the potential for parasite control practices and methods.
Challenges faced during the course of the project largely involved the project’s scale and partner farm participation. It was challenging to find farms that met our criteria for eligibility to participate and who were willing to be a participant in the study. Over 40 hog farms in Pennsylvania, New York, New Jersey, and Maryland were contacted via phone call and/or email to inquire about interest in participation, but only 9 farms in Eastern-Central Pennsylvania were eligible and interested. Maintaining communication with all 9 farms throughout the course of a year was an additional challenge, and only 7 farms participated in the final (summer) survey.
Despite challenges and barriers, the project successfully demonstrated high parasite prevalence on swine farms and bolsters the need for research on natural parasite control and treatment methods. Data also indicates that a relationship between parasite infection and some management practices exists. This information will be used to develop additional projects to further assess these relationships and to establish a better understanding of the pros and cons of different management practices.
Hog growers both regionally and nationally can benefit from the results of this project by using the data to implement different management practices to potentially control parasites in their swine herd. The prevalence data found might not be representative of parasite infections in other areas of the United States, particularly those with different climatic conditions.
Information Products
- Relating hog management practices to parasite infection on organic and pastured pig farms (Conference/Presentation Material)