Final report for OS22-153
Project Information
Sugarcane borer management in sugarcane and rice in the EAA relies on biological control provided by parasitic wasps released during biological control programs in the 1930s, 1950s, and 1990s (Roldán et al. 2020b). Ants, including the red imported fire ant, are also thought to participate in sugarcane borer control in sugarcane. Currently, the sugarcane borer does not need to be managed with insecticides in either sugarcane or rice. Encouraged by the success of sugarcane borer management in the EAA, the proposed project aims to improve rice stink bug management by initiating the development of a rice stink bug biological control program in Florida.
Parasitoids using O. pugnax eggs as hosts have been observed in the United States (Bhavanam et al. 2021). These parasitoids include Telenomus podisi (Hymenoptera: Scelionidae), which parasitizes O. pugnax in Arkansas and Georgia, with parasitism levels as high as 90% (Sudarsono et al. 1992, Tillman 2010). In Central America, T. podisi parasitizes O. insularis in rice and weedy habitats (Zachrisson and Polanco 2017). Telenomus podisi occurs in Florida (Buschman and Whitcomb 1980). Thus, we hypothesize that T. podisi parasitizes rice stink bugs in Florida and might be used in a biological control program for conventional and organic rice. The commercial use of augmentative releases of T. podisi produced by Koppert Biological Systems (www.koppert.com.br/podisibug/) for stink bug management in soybean in Brazil supports our approach for the development and evaluation of a biological control strategy in Florida rice.
The proposed project will first identify parasitoids attacking O. pugnax and O. insularis, which are the two main rice stink bug species in Florida rice. Parasitism rate will be quantified in rice fields and adjacent habitats on conventional and organic farms. Subsequently, T. podisi, which is expected to be the most abundant parasitoid of Oebalus spp., will be reared and released in field cages on commercial farms to determine whether parasitoids can effectively suppress rice stink bug populations under Florida conditions. The solution resulting from the proposed project will be a foundation for an augmentative biological control strategy in Florida rice.
The proposed project will represent a first step towards biological control in rice in the EAA, which is a region dominated by sugarcane production. Although biological control of the sugarcane borer in sugarcane in the EAA has been the focus of studies since the 1930s, biological control of rice stink bugs has never been studied. Thus, results of the proposed project are unlikely to provide a short-term solution to the rice stink bug issue. However, the two years of on-farm research will provide critical results for future, more comprehensive studies. In addition, early involvement of growers will generate interest in and support for biological control as a way to achieve greater sustainability.
The ultimate goal of the proposed project is to protect conventional and organic rice yields in Florida while decreasing reliance on pyrethroid applications, which are potentially contributing to the development of pesticide resistance, the disruption of sugarcane borer biological control, environmental contamination, and the public's perception that modern agriculture is addicted to pesticides.
Objectives
The proposed on-farm research project will have two objectives:
- Identify rice stink bug parasitoids and quantify parasitism rates in Florida
- Determine whether parasitoid releases in field cages can control rice stink bugs under Florida conditions
Objective 1. Identify rice stink bug parasitoids and quantify parasitism rates in Florida
Surveys will be conducted during the two years of the proposed project to determine rice stink bug egg parasitism and predation rates, and to identify parasitoids emerging from stink bug eggs and adults. These surveys will represent the first research step towards understanding the role of natural enemies and how they can be manipulated for rice stink bug management. Each year, four surveys will be conducted between June and September (i.e., 1 survey/month). For each survey, four conventional and four organic fields between the flowering and hard dough stages will be sampled. Fields will be >1 mile apart and the same field might be sampled more than once if crop phenology allows or if a ratoon crop is produced.
For each field, sampling for rice stink bug adults and egg masses will be conducted. For adults, four 25-sweep samples will be collected using a 15-inch sweep net. Adults will be placed in a popup screen cage (12”*12”*12”), inspected for tachinid parasitoid eggs, and maintained in a climate-controlled room at 25°C, 40-60% RH, 12:12 (L:D) until parasitoid emergence. For eggs, all rice plants within ten 1-square meter quadrats randomly selected along a zigzag pattern will be inspected. Egg masses will be individually placed in glass scintillation vials capped with a cotton ball and maintained in a climate-controlled room until parasitoid or stink bug nymph emergence.
Sentinel egg masses will be produced in the laboratory. In short, field-collected O. pugnax and O. insularis adults will be maintained in popup screen cages in a climate-controlled room, fed with rice panicles, and allowed to lay eggs on paper towels. Within 24 hours of oviposition, egg masses will be frozen at -18°C. For each field of each survey, five egg masses for each O. pugnax and O. insularis will be placed in the rice field and in an adjacent weedy border. Sentinel egg masses will be collected into glass scintillation vials capped with a cotton ball 48 to 72 hours after deployment in the field. Egg masses will be observed for signs of predation and will be maintained in a climate-controlled room until parasitoids or stink bug nymphs emerge. Rice stink bug parasitoids will be counted and identified to species with the assistance of taxonomists at the Florida Department of Agriculture and Consumer Services – Division of Plant Industry (FDACS-DPI) in Gainesville, FL.
Parasitism rate of adults, parasitism rate of sentinel eggs, and predation rate of sentinel eggs for each survey will be compared for each stink bug species among production systems (conventional vs. organic) and habitats (field vs. border) using generalized linear mixed models with a binomial distribution (PROC GLIMMIX, SAS Institute 2016). Parasitism of natural eggs might also be compared if the sample size is appropriate.
Objective 2. Determine whether parasitoid releases in field cages can control rice stink bugs under Florida conditions
Field experiments will be conducted during late summer 2023 to determine whether parasitoids released in field cages have the potential to control rice stink bugs under Florida conditions. These experiments will be preliminary, representing a proof of concept demonstrating that parasitoid releases might be used for rice stink bug management in Florida.
Telenomus podisi is expected to be the most abundant parasitoid species attacking O. pugnax and O. insularis in Florida rice fields and adjacent habitats. Thus, T. podisi collected using sentinel eggs (objective 1) will be reared in the laboratory using a method comparable to that of Braz et al. (2021). In short, O. pugnax and O. insularis eggs produced in a climate-controlled room (objective 1) will be collected and frozen within 48 hours of oviposition. Previously frozen eggs will be placed in 60-ml glass culture tubes with eggs parasitized 12-14 days earlier. Honey droplets will be used as a food source for adult parasitoids. Upon adult parasitoid emergence, oviposition into previously frozen eggs will be allowed for 24-48 hours to maintain the laboratory population or to provide adults aged 24-36 hours for releases in field cages.
Two field cages will be deployed simultaneously in each of two organic rice fields. In each field, the two field cages will be placed 10 ft apart on rice at the heading stage. Each cage will be infested with 20 O. pugnax and 20 O. insularis adults (1:1 sex ratio), which will be allowed to lay eggs for 2 days. Subsequently, 10 T. podisi adults will be released in one cage, the other cage serving as a control without parasitoids. This number of parasitoids is five times greater than the release rate used in studies conducted in Brazil for Euschistus heros management in soybeans (de Freitas Bueno et al. 2020). Two days after T. podisi release, stink bug eggs will be collected in each cage and maintained in a climate-controlled room until parasitoid or stink bug nymph emergence.
Parasitism rates between cages with and without parasitoids will be compared using generalized linear mixed models with a binomial distribution (PROC GLIMMIX, SAS Institute 2016). Parasitism rates between 25% and 75% will be considered encouraging results strongly supporting additional laboratory and on-farm research. In case of parasitism rates <5%, releases will be repeated using greater parasitoid release rates.
The technician partially supported by the proposed project will work with a graduate student who has experience in rearing and handling egg parasitoids such as Trichogramma spp., Telenomus remus, and T. podisi at the Insect Biology Laboratory, University of São Paulo/Luiz de Queiroz College of Agriculture, in Brazil. Thus, the personnel involved in this proposed on-farm project are qualified.
Cooperators
- (Educator)
Research
Year 1
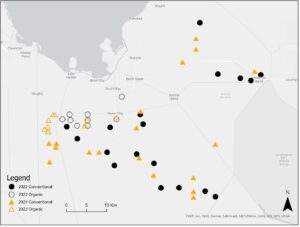
Six conventional and three organic rice fields were selected in early July, late July-early August, and early September 2022 for collection of rice stink bug parasitoids (Fig.1). In each of the 27 commercial fields, adults were collected using a 15-inch sweep net in a 100-sweep (organic fields) or 200-sweep (conventional fields) sample. Adults were separated by species and kept in screen cages in the laboratory for parasitoid emergence.
Wild eggs naturally deposited in rice fields were collected by inspecting all plants in 10 1-m2 quadrat samples selected throughout each field. Plant parts containing eggs were placed in 20-ml glass scintillation vials and kept in a climate-controlled room for three weeks. Egg masses were observed for parasitoid emergence, and parasitoids were counted and identified. Eggs that did not produce parasitoids or stink bug nymphs were observed for the presence of parasitoid emergence holes or dissected to detect the presence of dead parasitoids. Additional wild eggs observed outside of quadrats while walking between quadrat sampling locations were also collected and observed for parasitoid emergence to increase sample size.
Eggs of O. insularis and O. pugnax produced in the laboratory were also deployed in rice fields and field borders as sentinel eggs complementing the observation of wild eggs. The egg masses were first frozen at -18°C for 2 to 14 days to inhibit nymph development. Then, egg masses containing 25-30 eggs, consistent with the average size of a rice stink bug egg mass, were glued to pieces of waterproof paper with transparent non-toxic mounting tape. Fine play sand was sprinkled on top to cover the sticky borders of the tape around the eggs and avoid trapping natural enemies. In each field and border, sentinel egg masses were placed at five locations approximately 50 m apart. At each location, two sentinel egg masses placed 1 m apart were pinned on rice plants or weeds 30 cm above the soil surface. The sentinel egg masses were attached flat to the abaxial surface of a leaf using two entomological pins (Fig. 2). The proportion of O. insularis and O. pugnax egg masses was the same for a field-field border pair but varied among pairs of fields and field borders. These sentinel egg masses were collected after 72 h and kept in glass vials in the laboratory for parasitoid emergence.
Year 2
Twenty-seven commercial fields were selected consistent with Year 1, with field collections conducted in mid-June, mid-July, and mid-August 2023 (Fig. 1). Stink bug adults and associated parasitoids were collected following the same methods as for Year 1. Wild eggs naturally deposited in rice fields and associated parasitoids were also collected following the same methods as for Year 1. However, 20 1-m2 quadrat samples were selected in each field instead of 10 to increase sample size.
Sentinel eggs of O. insularis and O. pugnax were deployed in rice fields and field borders consistent with Year 1. However, the egg masses used for sentinel egg preparation were 48 h old maximum and stored at 7°C instead of being frozen. In addition, the sentinel egg masses were attached to the abaxial surface of leaves within a circular fold of the waterproof paper using a single pin (Fig. 3). These sentinel egg masses were collected after 48 h. The changes relative to Year 1 were made to deploy fresher eggs that were more protected against rubbing on plant surfaces and exposed for a shorter time to potentially adverse weather conditions.
In addition to field surveys, Telenomus podisi adults obtained from two parasitized egg masses collected in mid-August 2023 (approximately 30 wasps/egg mass) were used to initiate a colony maintained under laboratory conditions. Adults from the two parasitized egg masses were kept separately in two transparent plastic bags with droplets of raw honey provided as food and a moist cotton wick to maintain high humidity. Previously frozen and live O. insularis and O. pugnax eggs were provided in Petri dishes for oviposition. The colony was maintained for five generations until approximately 200 live adults were obtained. However, adult releases in field cages were not conducted as proposed because of a lack of suitable field sites in late summer 2023.
Fig. 1. Location of conventional and organic rice fields sampled in 2022 and 2023 in southern Florida.
Fig. 2. Sentinel egg mass deployed in 2022 (Credit: Carolina Tieppo Camarozano, UF/IFAS).
Fig. 3. Sentinel egg mass deployed in 2023 (Credit: Carolina Tieppo Camarozano, UF/IFAS).
Year 1
We collected 2,996 rice stink bug adults (936 O. pugnax, 2,060 O. insularis). Differences in adult abundance were not detected between conventional and organic fields for O. pugnax; however, the number of O. insularis collected in organic fields was 2.4 times greater than in conventional fields. Adult parasitism rate was as high as 7.0% for O. pugnax in organic fields. However, differences in parasitism rates were not detected between conventional and organic fields for adults of the two rice stink bug species. A total of 37 parasitoids emerged from O. pugnax and O. insularis adults. The tachinid fly Beskia aelops was the only species collected.
We collected 1,129 wild eggs (55 egg masses) during quadrat sampling. Differences in egg mass and egg abundance on a per m2 basis were not detected between conventional and organic fields. The majority of the eggs were parasitized, with 55.7% and 83.2% egg parasitism observed in conventional and organic fields, respectively (Table 1). However, differences in egg parasitism rate were not detected between the two production systems. Parasitoid emergence, which was as high as 89.1% in organic fields, did not differ between conventional and organic fields (Table 1). In total, 398 parasitoid wasps were observed (261 from quadrats, 137 from outside quadrats). Identification revealed that 386 specimens were T. podisi and 12 specimens were Hadronotus obesus.
Table 1. Number of Oebalus spp. egg masses and eggs, egg parasitism, and parasitoid emergence observed in conventional and organic rice fields in southern Florida, 2022.
|
|
2022 |
|||
|
|
Egg masses/ m2 |
Eggs/m2 |
% Parasitism |
% Emergence |
|
Conventional |
1.6 |
34.2 |
55.7 |
89.1 |
|
Organic |
3.0 |
56.8 |
83.2 |
69.9 |
|
F |
2.1 |
1.5 |
2.0 |
3.2 |
|
df |
1, 23 |
1, 23 |
1, 15 |
1, 10 |
|
P > F |
0.16 |
0.23 |
0.18 |
0.11 |
Nearly half of the sentinel eggs were lost during the 72 h of exposition in rice fields. Egg parasitism rate ranged from 7.2% in conventional field borders to 10.3% within organic fields; however, differences between production systems and habitats were not detected. Similarly, there were no differences between production systems and habitats for parasitoid emergence, which was as high as 31.0% in organic field borders. All 52 parasitoid wasps emerging from sentinel eggs were T. podisi.
Year 2
We collected 3,204 rice stink bug adults (1,290 O. pugnax, 1,914 O. insularis). Differences in adult abundance were not detected between conventional and organic fields for either species. Adult parasitism rate was as high as 4.1% for O. pugnax in organic fields. However, differences in parasitism rates were not detected between conventional and organic fields for adults of the two rice stink bug species. A total of 38 parasitoids emerged from O. pugnax and O. insularis adults, with B. aelops being the only parasitoid species observed.
We collected 3,643 wild eggs (180 egg masses) during quadrat sampling. The number of egg masses and eggs collected did not differ between conventional and organic fields (Table 2). Egg parasitism rate was as high as 75.7% (conventional fields) and parasitoid emergence was as high as 63.0% (organic fields); however, differences between the two production systems were not detected (Table 2). In total, 1,022 parasitoid wasps were observed (593 from quadrats, 429 from outside quadrats). T. podisi was the most prevalent species with 910 specimens, followed by H. obesus with 58 specimens, and Ooencyrtus sp. with 54 specimens.
Table 2. Number of Oebalus spp. egg masses and eggs, egg parasitism, and parasitoid emergence observed in conventional and organic rice fields in southern Florida, 2023.
|
|
2023 |
|||
|
|
Egg masses/m2 |
Eggs/ m2 |
% Parasitism |
% Emergence |
|
Conventional |
3.7 |
75.8 |
75.7 |
54.5 |
|
Organic |
2.5 |
50.9 |
61.5 |
63.0 |
|
F |
0.7 |
0.9 |
1.3 |
0.4 |
|
df |
1, 23 |
1, 23 |
1, 21 |
1, 19 |
|
P > F |
0.40 |
0.34 |
0.27 |
0.52 |
Nearly 20% of the sentinel eggs were lost during the 48 h of exposition in rice fields. Egg parasitism rate across production systems was 53.2% greater within fields than in borders. Parasitoid emergence was as high as 26.2% in organic field borders; however, differences between production systems and habitats were not detected. All 298 parasitoid wasps emerging from sentinel eggs were T. podisi.
Educational & Outreach Activities
Participation Summary:
Year 1
One meeting was conducted in May 2022 at initiation of this project with the Director of Rice and Organic Farming and a Crop Protection Specialist for Florida Crystals Corporation, which grows >80% of the rice produced in Florida. This meeting, which also involved Palm Beach County Extension Agent Dr. Matthew VanWeelden and Graduate Student Carolina Tieppo Camarozano, assisted in developing a specific plan for on-farm sampling during summer 2022. Aspects of biological control for conventional and organic farming were also discussed.
At least three informal in-person updates on the progress of the project were given to Dr. VanWeelden during summer 2022.
Educational events held in Belle Glade, FL included the 5th Florida Rice Field Day (August 16, 2022 - 62 participants) and two meetings of Florida Rice Growers Inc. (December 12, 2022 and March 1, 2023 - 20 participants per meeting). Results of on-farm sampling were discussed, and the value of a potential rice stink bug biological control program was promoted.
Year 2
At least three informal in-person updates on the progress of the project were given to Dr. VanWeelden during summer 2023.
Educational events held in Belle Glade, FL included the 6th Florida Rice Field Day (August 22, 53 participants) and two meetings of Florida Rice Growers Inc. (December 19, 2023 and February 29, 2024 - 20 participants per meeting). Results of on-farm sampling were discussed, and the value of a potential rice stink bug biological control program was further promoted.
One phone call was conducted in May 2024 to discuss the impacts of the project with the Director of Rice and Organic Farming for Florida Crystals Corporation.
Learning Outcomes
Biological control of insect pests
Project Outcomes
This on-farm project revealed that the scelionid wasp T. podisi parasitized rice stink bug eggs in Florida rice and that parasitism rates were relatively high. Upon completion of this on-farm project, the Director of Rice and Organic Farming for Florida Crystals Corporation indicated that the project had increased his knowledge of insect biological control, especially knowledge of stink bug egg parasitoid diversity, behavior, and abundance in the Florida rice agroecosystem. He indicated that although pyrethroid applications for rice stink bug management are effective and relatively inexpensive, a biological control program might be considered if effective. However, a cost greater than twice the cost of pyrethroid applications would represent a barrier to adoption.
T. podisi has been commercially reared and used in biological control programs in other crop systems in other countries. Thus, a biological control program relying on the release of T. podisi to increase rice stink bug egg parasitism and subsequently adult stink bug populations might represent a viable areawide management strategy in southern Florida where conventional and organic rice are produced.
This project resulted in one new working collaboration for Dr. Beuzelin and graduate student Ms. Carolina Tieppo Camarozano who worked with scelionid taxonomist Dr. Elijah Talamas (Florida Department of Agriculture and Consumer Services Division of Plant Industry) to identify egg parasitoids. Ms. Tieppo Camarozano intends to pursue a PhD focusing on T. podisi under Dr. Beuzelin’s supervision and with Dr. Talamas assistance for parasitoid identification.