Final report for OW15-005
Project Information
Clubroot is a major disease of brassica crops; it causes significant crop losses to Pacific Northwest farmers. The overall goal of this project was to provide farmers, university Extension faculty, and agricultural professionals with the information and resources necessary to implement an effective and economically sustainable integrated clubroot management program.
A major component of the project was identifying commercially available cultivars combining clubroot resistance with desired horticultural characteristics. Using the European Clubroot Differential (ECD) set, only the pathotype 16/2/30 was identified in western Oregon. Through collaborative research, our farmer-scientist team screened twenty-one vegetable cultivars from nine crops (broccoli, cabbage, napa cabbage, etc.) with purported resistance to clubroot for disease incidence and severity in field and greenhouse studies to pathotype 16/2/30. Compared to a crop-specific susceptible check, 17 of 21 cultivars had some resistance to clubroot, and of those, 15 were highly resistant (≤15% incidence with low disease severity). Of the resistant cultivars identified, farmers provided input about their suitability to grower farming systems and whether these cultivars would meet market demands and consumer expectations.
This project focused on soil liming as a principle clubroot management strategy because it is a practice that farmers can easily implement, it is economical, and it has shown to consistently and effectively control clubroot when done correctly. Here are the key findings from multiple collaborative, on-farm research projects:
- Liming did not completely suppress clubroot in the field, but it can significantly reduce infection rate and disease severity when done correctly.
- Liming had little or no effect on incidence and disease severity when a minimum target pH of 7.0 was not reached at planting,
- Soil’s pH response to lime is non-linear above a pH of approximately 6.5. As a result, routine liming recommendations for crop production are not adequate to reach a target at or above pH 7.0. Routine recommendations rarely address pH targets more than 6.5.
- Lime should be applied and incorporated at least one month prior to planting; more time gives better results.
- Thorough lime incorporation is critical to achieve a uniform soil pH, which eliminates localized pH zones below 7.0, where infection can occur.
The results and knowledge gained from these collaborative research projects have been incorporated into a journal article and a peer-reviewed, catalogued Extension publication that is unique from any other Extension clubroot publication currently available in the US. These results have been communicated to clientele through presentations, informational booths, a nationally broadcast eXtension webinar, and electronic media. We have documented that farmers have adopted the clubroot control recommendations generated from this project.
The goal of this project is to provide farmers with the information and resources necessary to implement an effective and economical integrated clubroot management program. The farmer-scientist team will conduct collaborative research and Extension activities:
Objective 1: Conduct on-farm research investigating cultivar resistance to clubroot, and the effectiveness of cultural practices for clubroot suppression.
Performance Target: Based on the findings of variety screening, cultural control, and farm rotation studies, we will develop integrated recommendations for clubroot disease management.
Objective 2: Provide Extension outreach to growers to assist them in developing an integrated approach for clubroot disease control.
Performance Target: During the final year of the project, we will assess changes in farmer understanding, intentions, and practices. Farmers will be asked to identify specific practices they have adopted or intend to adopt as the result of this project. For each strategy, farmers will describe 1) whether or not they adopted it or if they intend to adopt it, 2) if it appears to be effective (if they adopted it), and 3) if it is cost effective. Farmers will also be asked how much damage and lost income they experienced due to clubroot before the project, and how much damage and lost income they anticipate after adopting project findings.
Introduction
Clubroot (causal organism, Plasmodiophora brassicae) is a major disease of brassica crops (broccoli, cabbage, cauliflower, Chinese cabbage, rutabaga, etc.), and causes significant crop losses worldwide. Brassica farmers in the PNW are increasingly concerned about clubroot. Extension personnel, agricultural professionals, and farmers have indicated that the incidence and severity of the disease is increasing. This may be due to
- increased acreage producing forage radish and turnip cover crop seed to meet Midwest demand;
- the increasing number of vegetable farmers with a 15-year or more history of intensive, short-rotation production;
- an increase in the proportion of land planted to brassicas on vegetable farms due to increased demand; and
- increasing acreage planted to overwintering brassica crops as winter markets increase.
Managing clubroot is a challenge. Thick-walled resting spores can remain viable in soil for 15 years or more in the absence of a host, making it very difficult to eliminate the pathogen from an infested field. Therefore, the goal is to manage rather than eradicate the disease once pathogen populations have reached levels that cause economic damage. Rotating out of brassicas for a minimum of five years will often reduce disease severity enough to avoid significant crop loss. However, many smaller, diversified vegetable farms lack sufficient acreage to rotate out of brassicas for this period; nor do they have alternative rotational crops as profitable as brassicas. Although some farmers can lease clubroot-free ground, this may be impossible for organic growers due to a limited supply of organically certified fields. To remain competitive and profitable, they must actively manage clubroot.
A significant body of research has explored strategies to minimize crop loss from clubroot. This work has been compiled into multiple review papers (e.g., Hwang et al., 2014; Donald and Porter, 2009). Some of the management strategies reviewed include the use of bait crops to stimulate spore germination, boron applications, use of biological control agents, production of resistant cultivars, water management to avoid wet areas in a field, and liming to increase soil pH. Many of these strategies are not consistently effective, do not provide sufficient disease suppression, require changes to farming practices, may not be compatible with a farm’s production system, may be prohibitively expensive, or are not allowed in organic systems.
Of the management strategies mentioned, production of resistant cultivars is an attractive option that requires little or no change in farming practices or equipment, gives the farmer flexibility in when and where to plant, and may be significantly less expensive than other options. Besides the in-season benefit of growing resistant cultivars, there is a long-term benefit. Resistant cultivars may act as a bait crop by stimulating resting spore germination, but few or no viable spores are produced. This can decrease spore concentration and ultimately disease incidence and severity in future crops (Murakami et al., 2000 and 2001; Hwuang et al., 2011b). New cultivars have become commercially available in the last decade, but they have not been rigorously screened for resistance to the dominant pathotypes present in the Pacific Northwest. There are many clubroot pathotypes worldwide; some cultivars with putative resistance may not be effective in our region.
Growing resistant cultivars alone will not control clubroot. There are a limited number of cultivars, many of which may not meet the needs of farmers in our region or consumer preferences. In addition, there are no cultivars available for some high-demand crops such as kale (B. oleracea var. acephala), mustard greens (B. juncea), or arugula (Eruca sativa). Too-frequent production of a resistant cultivar in the same field may select for a clubroot population able to overcome resistance.
Another practical, effective, and economical control strategy is liming to raise the soil pH to 7.0 or greater. This approach has been so effective at controlling clubroot in areas such as the Salinas Valley of California, a major brassica production area that the plant pathologist for the region no longer works on the disease (Steve Koike, UC Cooperative Extension plant pathologist, personal communication). Liming does not kill the pathogen but reduces spore germination, thus reducing infection and clubbing (Dixon, 2009). By reducing clubbing, fewer spores are released into the soil. Future infection rates and severity are reduced.
Although liming has been an effective strategy in California, farmers in western Oregon have had mixed success. In 2012, a group of 37 conventional and organic fresh market and processing vegetable farmers in western Oregon who grow significant quantities of brassicas responded to a survey. Eighty-three percent had used lime in an attempt to control clubroot, yet only 21% of these aimed for a pH of at least 6.8, the minimum pH level shown to control the disease. Of those who had used lime, 38% did not determine whether the target pH was reached. Only 26% indicated that liming contributed to clubroot control. These survey results indicate that while there is a general acknowledgement that pH manipulation can control clubroot, Extension faculty, agricultural professionals, and farmers lack the specific information necessary to implement a successful liming program.
Cooperators
Research
Objective 1: Conduct on-farm and greenhouse research investigating cultivar resistance to clubroot, and the effectiveness of cultural practices in suppressing clubroot.
1a. Identification of the dominant clubroot pathotypes present in Oregon.
Clubbed roots were collected from five locations in Oregon’s Willamette Valley. The samples were frozen for not more than 7 months. A clubroot spore suspension was created by masticating clubs with DI water in a blender, filtering through a coarse mesh sieve, quantifying the spore concentration using a hemacytometer, and diluting to 107 spores per ml. The suspensions were refrigerated up to one week before use.
Each clubroot collection was screened against the 15 differential hosts of the ECD set (Warwick Crop Centre, The University of Warwick, Wellesbourne, UK; Table 1). The plants were grown in cone tubes (Ray Leach Cone-tainers; 3.8 cm diameter x 14 cm deep) containing a 3:1 (v/v) potting mix: loam soil with a pH 5.5. Twenty-eight cone tubes per ECD host were seeded in early spring 2016; one milliliter of spore suspension was pipetted into each planting hole before covering the seed (Fig. 1). Plants were grown in the greenhouse (18 to 24 deg C) under natural light, watered twice daily, and fertilized weekly with 1 tablespoon of 24-8-16 per gallon of water.

Five weeks after planting, the potting medium was washed from the roots, and the roots were evaluated for clubroot incidence and severity. Root disease severity was evaluated on the following rating scale: 0= no visible clubbing, 1= small clubs on lateral roots, 2= less than 50% of main root system clubbed, and 3= more than 50% of main root system clubbed. A disease severity index (DSI) was calculated as:
((R1 * 2) + (R2 * 3) + (R3 * 5))/5 [Eq. 1]
Where R1, R2, and R3 are the percentage of plants evaluated with a rating of 1, 2, and 3, respectively (Dixon and Robinson, 1986). This index was used to assign each ECD host to a reaction type as resistant (DI = 0 designated “-“ or 0 < DI < 33 designated “?”) or susceptible DI >33 designated “+”. Each collection was assigned a numerical code designation based on susceptible ECD host as described by Buczacki et al. (1975). Each ECD Brassica spp. group was assigned values of 1, 2, 4, 8, and 16. Summing the values for each susceptible reaction gave a unique virulence designation. For example, 16/2/31 represents positive reactions for hosts 5, 7, and 12-15. All uncertain reactions (?) were treated as resistant.
|
Table 1. European clubroot differential (ECD) series host species and binary values used to identify clubroot pathotypes in western Oregon (Buczacki et al., 1975). |
||
|
ECD No. |
Differential host |
Binary value |
|
Brassica rapa |
||
|
1 |
var. rapifera line aaBBCCz fodder turnip |
1 |
|
2 |
var. rapifera line AAbbCC fodder turnip |
2 |
|
3 |
var. rapifera line AABBcc fodder turnip |
4 |
|
4 |
var. rapifera line AABBCC fodder turnip |
8 |
|
5 |
var. pekinensis 'Granaat' |
16 |
|
Brassica napus |
||
|
6 |
var. napus 'Nevin' |
1 |
|
7 |
var. napus 'Giant Rape' |
2 |
|
8 |
var. napus selection of 'Giant Rape' |
4 |
|
9 |
var. napus New Zealand clubroot resistant rape |
8 |
|
10 |
var. napobrassica 'Wilhemsburger'y |
16 |
|
Brassica oleracea |
||
|
11 |
var. capitata 'Badger Shipper'y |
1 |
|
12 |
var. capitata 'Bindsachsener' |
2 |
|
13 |
var. capitata 'Jersey Queen'y |
4 |
|
14 |
var. capitata 'Septa' |
8 |
|
15 |
var. acephala 'Verheul' |
16 |
|
z aaBBCC, AAbbCC, AABBcc, and AABBCC refer to the allelic state of a set of three resistance genes present in differential lines of turnip. Lower case indicates susceptibility and upper case letters indicates resistance to corresponding races of clubroot. yThese cultivars are part of the Williams clubroot differential set (Williams, 1966).
|
||
1b. Screening brassica cultivars for resistance to western Oregon clubroot pathotypes.
A total of 21 commercially available brassica cultivars with reputed clubroot resistance from nine brassica crops: broccoli (Brassica oleracea var. italica), cauliflower (B. oleracea var. botrytis), Brussels sprouts (B. oleracea var. gemmifera), cabbage (B. oleracea var. capitata), napa cabbage (B. rapa var. pekinensis), pak choi (B. rapa var. chinensis), kohlrabi (B. oleracea var. gongylodes), turnip (B. rapa var. rapa), and rutabaga (B. napus var. napobrassica) were screened in field and greenhouse trials (Table 2). For each crop, a clubroot susceptible cultivar was planted for comparison. Five on-farm trials were initiated with conventional and organic fresh market vegetable growers in fields with a history of clubroot. However, the disease was only uniformly distributed across our field plots at two sites, and only results from these two trials are discussed. Because some farms were organically certified, cultivars with treated seed could not be screened in the field, however they were screened in the greenhouse.
|
Table 2. Clubroot incidence (CI) and disease severity index (DSI; in parenthesis) of brassica cultivars grown in commercial fields where clubroot was present, or in the greenhouse with inoculated growing media. |
||||||
|
CI (%) and DSI |
||||||
|
Crop |
Source |
Cultivar |
Field site 1 |
Field site 2 |
Greenhouse |
|
|
Broccoli (S)x |
Sakata |
Emerald Crown |
100au (77a) |
- |
90 (90) |
|
|
Broccoli (R) |
Sakata |
Emerald Jewel |
2b (1b) |
- |
0 (0) |
|
|
Brussels sprouts (S) |
Bejo |
Diablo |
- |
- |
100 (100) |
|
|
Brussels sprouts (R) |
Syngenta |
Crispus |
- |
- |
0 (0) |
|
|
Cauliflower (S) |
Harris Moran |
Artica |
- |
- |
100 (100) |
|
|
Cauliflower (R) |
Syngenta |
Clapton |
- |
- |
0 (0) |
|
|
Cauliflower (R) |
Syngenta |
Clarify |
- |
- |
0 (0) |
|
|
Kohlrabi (S) |
Unknown |
Kossack |
96ab (69) |
- |
82 (51) |
|
|
Kohlrabi (R) |
Rijk Zwaan |
Lech |
100a (69) |
- |
100 (88) |
|
|
Kohlrabi (R) |
Hild Samen |
Azur-Star |
77b (51) |
- |
67 (37) |
|
|
Napa Cabbage (S) |
Unknown |
Michihili |
88a (80a) |
- |
100 (100) |
|
|
Napa Cabbage (R) |
Bejo |
Emiko |
- |
- |
0 (0) |
|
|
Napa Cabbage (R) |
Bejo |
Bilko |
10b (6b) |
- |
0 (0) |
|
|
Napa Cabbage (R) |
Bejo |
Pacifico |
- |
- |
0 (0) |
|
|
Napa cabbage (R) |
Sakata |
China Gold |
2b (2b) |
- |
0 (0) |
|
|
Napa cabbage (R) |
Sakata |
Yuki |
5b (3b) |
- |
0 (0) |
|
|
Napa Cabbage (R) |
Seed Science |
Panda |
- |
- |
0 (0) |
|
|
Napa Cabbage (R) |
Asia Seed Co. |
Chun Dae Gil |
- |
- |
60 (60) |
|
|
Pak choi (S) |
Sakata |
Joi Choi |
100 (88) |
- |
90 (86) |
|
|
Pak choi (R) |
Sakata |
Feng Qing Choi |
98 (90) |
- |
100 (100) |
|
|
Pak choi (R) |
Sakata |
Mei Quing Choi |
- |
- |
74 (74) |
|
|
Turnip (S) |
Takii |
Purple Prince |
9b (5b) |
- |
0 (0) |
|
|
Turnip (R) |
Kobayashi Seed Co. |
Scarlet Queen |
98a (89a) |
- |
82 |
|
|
Cabbage (S) |
Bejo |
Farao |
100a (87a) |
- |
100 (100) |
|
|
Cabbage (S) |
Bejo |
Gazelle |
- |
100±0 (84±5)t |
- |
|
|
Cabbage (R) |
Syngenta |
Kilaton |
3b (3b) |
5±5 (2±2) |
0 (0) |
|
|
Cabbage (R) |
Syngenta |
Tekila |
- |
0±0 (0±0) |
0 (0) |
|
|
Cabbage (R) |
Syngenta |
Kilagreg |
5b (4b) |
5±5 (5±5) |
0 (0) |
|
|
Cabbage (R) |
Bejo |
Lodero |
3b (2b) |
15±6 (7±3) |
0 (0) |
|
|
Rutabaga (S) |
Unkown |
American Purple Top |
32 (18) |
- |
0 (0) |
|
|
Rutabaga (R) |
Tozer |
Marion |
26 (13) |
- |
0 (0) |
|
|
x(S) = susceptible check and (R) = cultivar with purported resistance to clubroot.tmean ± SE (n=5 subsamples, each consisting of five plants per subsample); the experimental design was unreplicated, and ANOVA could not be applied. |
||||||
Field site 1 was located on an organic farm on Sauvie Island outside of Portland, OR in a field that had experienced a complete crop failure due to clubroot on broccoli in 2013. The soil texture was a loam with a pH of 6.2. On April 29, 2015, the field was transplanted (two rows per 90 cm bed with 28 cm in-row spacing) in a randomized complete block design with six replicates. Each plot was 3 m long. The field was irrigated with overhead sprinklers. Seven to nine plants per plot were carefully excavated to minimize loss of lateral clubs. Roots of cabbage, broccoli, and all other crops were evaluated at 93, 61, and 52 days after transplanting, respectively, for disease incidence and severity (Eq. 1).
Field site 2 was also located on Sauvie Island outside of Portland, OR on a conventional farm growing cabbage for processing. Three green cabbage cultivars (‘Kilaton’, ‘Tekila,’ and ‘Kilagreg’) and one red cabbage (‘Lodero’) were direct-seeded in 70 m long unreplicated strips (single row of each cultivar planted side by side with 76 cm row spacing) in the farmer’s field on June 24, 2015. The cultivar planted by the grower, ‘Gazelle’, was used as the susceptible check. The soil texture was a silt loam with a pH 6.3, and the crop was watered with overhead sprinklers. On July 15, plants were thinned to an in-row spacing of 30 cm. On September 30, five consecutive plants from each cultivar were excavated across the planting strips, and the roots were evaluated for disease incidence and severity (Fig. 2). This was repeated five times at different locations across the strips.

All cultivars were evaluated in a greenhouse trial. Following the protocols outlined above, a spore suspension (107 spores per ml) was created that contained spores from the five sites. This suspension was used for pathotype determination with the ECD set. Equal weights of root from each clubroot collection were combined and masticated. Using the same cone tubes and growing media, one milliliter of spore suspension was pipetted into each planting hole at seeding. A total of 28 cones tubes of each cultivar were seeded, as were susceptible checks for each crop. Five weeks after planting, roots were washed and evaluated for clubroot incidence and disease severity using the methods described above.
1c. Greenhouse screening of Oregon State University’s archived clubroot resistant germplasm collection for resistance.
- Three broccoli (CR1, 2, and 6) and three European cabbage (Oregon 100, 123, and 142) breeding lines from OSU’s clubroot-resistant archived germplasm collection were evaluated for resistance (Baggett, 1983 and 1985). OSU vegetable breeder James Baggett introduced these lines in the mid ‘80’s. The purpose here was to evaluate these lines’ resistance to the pathotypes prevalent in Oregon today. Dr. Baggett had indicated shortly after their introduction that resistance may have been breaking down.
- Due to poor germination and limited seed availability, the plants were topped at flowering to promote side shoots. These side shoots were cut and placed in a cloning chamber to stimulate root growth. Once sufficient roots formed, the side shoots were planted in the planting medium described above. They were inoculated with two ml of spore suspension containing clubs from all 5 collections. This suspension was pipetted directly into the root zone. A limited number of plants survived the cloning chamber; we could only evaluate between 3 to 12 plants per line.
1d. Liming strategies for clubroot control
The lime used in all our field studies was Ashgrove Limestone Flour with the following characteristics: CCE of 98% (CCE= calcium carbonate equivalent defined as the acid-neutralizing capacity of a liming material expressed as a weight percentage (%) of pure CaCO3), 97% CaCO3, and 91% passing a US #100 mesh screen.
- A disease suppression experiment using lime was conducted at Field site 1, in conjunction with the variety screening. The experiment consisted of 3 treatments: Control (no lime), Lime 1x (2.7 ton/acre), and Lime 2x (5.4 ton/acre). The soil was a sandy loam with a pH= 6.1, SMP buffer pH= 6.4, and CEC= 15 cmol/kg. Three replicates were established in a randomized complete block design. The lime was applied by hand on March 26, 2015 and incorporated to a depth of 8 inches on April 6, 2015 with a spader. Eighteen days after incorporation (DAI), the cabbage variety ‘Farao’ was transplanted with a plant spacing of 8 inches. The field was irrigated with overhead sprinklers. On July 15 (100 DAI), cabbage head yield and root disease severity were evaluated. Soil was sampled 18 and 100 DAI to a depth of 6”, air dried, and analyzed for pH (1:2 water).
- A second liming experiment was conducted at Field site 3 on a diversified organic vegetable farm near the city of Philomath, OR. The soil texture was a silty clay loam with a pH= 6.8, SMP buffer pH= 6.6, and CEC= 30 cmol/kg. The experiment consisted of the following treatments: 1) No lime 1x tillage, 2) Lime 1x tillage, 3) No lime 2x tillage, and 4) Lime 2x tillage. On July 29, 2015, 4.4 ton/acre of powdered, calcitic lime was hand applied and incorporated with a rotary hoe (1x tillage). The rotary hoe was lifted between plots to prevent movement of lime between plots. Following the rotary hoe, select plots in the 2x tillage treatment were subjected to an additional rototiller pass. Kale (cv. ‘Lacinato’) was transplanted on August 11 (12 DAI) on 1 foot spacing. The field was irrigated with overhead sprinklers. On October 22 (85 DAI), aboveground biomass and root disease severity were evaluated. Soil was sampled at 12 and 85 DAI to a depth of 6 inches, air dried, and analyzed for pH (1:2 water).
The farmer from Field site 3 embarked on an aggressive liming program to control clubroot. In late August to early September 2014, the grower applied approximately 4.0 tons/acre of a very finely ground lime product to six fields using a drop spreader. The lime was incorporation with a disc and/or rotary hoe. Although the lime was applied to spatially diverse fields, the soil textures were either silty loam or silty clay loam. To determine whether a fall liming strategy would effectively achieve the target pH in the spring (i.e., would the pH stay high through the winter), soil pH was monitored over a year.
1a. Identification of the dominant clubroot pathotypes present in Oregon
Despite the geographic separation of the field sites, only a single pathotype, 16/02/30, was identified using the ECD set (Tables 3 and 4). Three of the four hosts in the ECD set are also part of the Williams differential set, which include ECD 10, 11, and 13 (‘Wilhelmsburger’, ‘Badger Shipper’, and ‘Jersey Queen’, respectively). The ECD pathotype identified in this study would correspond either to pathotype 3 or 6 using the Williams set. Pathotypes 3 and 6 (equivalent ECD designations of 16/15/12 and 16/0/14, respectively) have been identified in Canada, (Strelkov et al, 2006).
An evaluation of thirteen clubroot collections from the west coast of the USA identified the presence of pathotypes 16/02/31 and 16/03/31 (results from Oregon shown in Table 4), which the authors designated as pathotype 7 (Dobson et al. 1983). Williams (1966) also identified pathotype 7 as present in a single sample from California. For a collection to be designated as pathotype 7, a positive reaction for ECD 11 (‘Badger Shipper’) is required, which was not observed in our study, however, one collection had a low-incidence response, and was given the uncertain designation (“?,” Table 4).
Clubroot differential sets have their limitations and results must be interpreted carefully. Within field populations, there is a mixture of virulent pathotypes (Crute et al., 1980) and the differential sets may only identify the dominant pathotype. For example, when single spore isolates were taken from field populations, Xue et al. (2008) identified multiple pathotypes within a population. Different pathotypes can even be found inside a single club (Jones et al., 1982). Some pathotypes occur at a very low frequency and infect only a few plants of a susceptible differential host, resulting in an uncertain reaction. This may be what occurred for collections from sites 1, 2, and 3 that showed low disease incidence for ECD 9 and 11 (Table 3).
|
Table 3. Clubroot incidence (CI) and disease severity index (DSI) of European clubroot differential (ECD) set hosts to clubroot collections from western Oregon. |
||||||||||||||||||
|
|
|
CI (%)z / DSI |
||||||||||||||||
|
Collection location |
Host crop |
B. campestris |
|
B. napus |
|
B. oleracea |
||||||||||||
|
01 |
02 |
03 |
04 |
05 |
|
06 |
07 |
08 |
09 |
10 |
|
11 |
12 |
13 |
14 |
15 |
||
|
1-Sauvie Islandx |
Cabbage |
0/0 |
0/0 |
0/0 |
0/0 |
96/95 |
21/14 |
81/75 |
0/0 |
4/4 |
0/0 |
0/0 |
64/38 |
96/93 |
100/99 |
79/36 |
||
|
2-St. Paul |
Cabbage |
0/0 |
0/0 |
0/0 |
0/0 |
100/100 |
14/12 |
100/100 |
0/0 |
0/0 |
0/0 |
12/8 |
100/100 |
96/96 |
96/96 |
85/85 |
||
|
3-Sauvie Islandx |
Broccoli |
0/0 |
0/0 |
0/0 |
0/0 |
100/100 |
21/21 |
100/100 |
0/0 |
4/4 |
0/0 |
0/0 |
77/76 |
100/100 |
100/100 |
56/56 |
||
|
4-Damascus |
Cabbage |
0/0 |
0/0 |
0/0 |
0/0 |
100/94 |
4/4 |
100/98 |
0/0 |
0/0 |
0/0 |
0/0 |
100/98 |
100/100 |
100/100 |
93/76 |
||
|
5-Corvallis |
Kale |
0/0 |
0/0 |
0/0 |
0/0 |
96/95 |
4/4 |
100/99 |
0/0 |
0/0 |
0/0 |
0/0 |
89/83 |
100/95 |
100/100 |
85/85 |
||
|
xThis collection location was also the location of a field trial. |
||||||||||||||||||
Because we identified only a single pathotype in western Oregon, if a cultivar shows resistance on a single farm, it is likely that it would be resistant throughout the region. More detailed results and discussion can be found in the publication “Screening brassica cultivars for resistance to western Oregon clubroot pathotypes” (Heinrich et al., 2017).
|
Table 4. Reaction of European clubroot differential (ECD) set hosts to collections of clubroot from western Oregon. |
||||||||||||||||||||
|
|
|
Disease reactionz |
|
Pathotype numerical designationx |
||||||||||||||||
|
Collection location |
Host crop |
B. campestris |
B. napus |
B. oleracea |
|
|||||||||||||||
|
01 |
02 |
03 |
04 |
05 |
|
06 |
07 |
08 |
09 |
10 |
|
11 |
12 |
13 |
14 |
15 |
|
|||
|
1-Sauvie Islandw |
Cabbage |
- |
- |
- |
- |
+ |
? |
+ |
- |
? |
- |
- |
+ |
+ |
+ |
+ |
16/2/30 |
|||
|
2-St. Paul |
Cabbage |
- |
- |
- |
- |
+ |
? |
+ |
- |
- |
- |
? |
+ |
+ |
+ |
+ |
16/2/30 |
|||
|
3-Sauvie Islandw |
Broccoli |
- |
- |
- |
- |
+ |
? |
+ |
- |
? |
- |
- |
+ |
+ |
+ |
+ |
16/2/30 |
|||
|
4-Damascus |
Cabbage |
- |
- |
- |
- |
+ |
? |
+ |
- |
- |
- |
- |
+ |
+ |
+ |
+ |
16/2/30 |
|||
|
5-Corvallis |
Kale |
- |
- |
- |
- |
+ |
? |
+ |
- |
- |
- |
- |
+ |
+ |
+ |
+ |
16/2/30 |
|||
|
Greshamy |
Cauliflower |
- |
- |
- |
- |
+ |
- |
+ |
- |
- |
- |
+ |
+ |
+ |
+ |
+ |
16/2/31 |
|||
|
Corvallisy |
Broccoli |
- |
- |
- |
- |
+ |
? |
+ |
- |
- |
? |
+ |
+ |
+ |
+ |
+ |
16/2/31 |
|||
|
Brooksy |
Cauliflower |
- |
- |
? |
- |
+ |
+ |
+ |
? |
- |
? |
+ |
+ |
+ |
+ |
+ |
16/3/31 |
|||
|
zReaction type based on disease severity index (DSI) cutoff point of 33; susceptible = “+” (DSI ≥ 33); resistant = “-“ (DSI = 0) or “?”(0<DSI<33). DSI was calculated as: (R1*2 + R2*3 + R3*5) ÷ 5 were R1, R2, and R3 are the percentages of total plants evaluated with a rating of 1 (small clubs on lateral roots), 2 (<50% of main root system clubbed), and 3 (>50% of main root system clubbed), respectively. yResults from Dobson et al. (1983) added for comparison. xEach positive (“+”) ECD host of each Brassica spp. group was assigned a corresponding binary value (Table 1), which were then added together to create the pathotype numerical designation. For example, a positive reaction for B. campestris host 5, B. napus hosts 6, 7, 8, 9, and B. oleracea 13, 14 would be designated 16/15/12. |
||||||||||||||||||||
1b. Field and greenhouse screening of commercially available cultivars with reputed resistance to the clubroot pathotypes present in Oregon
Clubroot incidence (CI) and DSI for cultivars with purported resistance, and for susceptible cultivars are given in Table 2. At field site 1, high disease pressure was uniformly distributed across the fieldsite, and the CI and DSI for all crop-specific susceptible cultivars were ≥88% and ≥69%, respectively, except in the case of rutabaga. None of the resistant cultivars were completely resistant to clubroot, however, CI and DSI were significantly less than the susceptible check for most crops. For example, at field site 1, 100% of plants of susceptible cabbage, ‘Farao’, had visible clubbing compared to ≤5% of the resistant cabbage cultivars ‘Kilaton’,’ Kilagreg’, and ‘Lodero’.
Field site 2 also had uniform disease pressure and severity across the designated sampling area, however, this trial was not randomized and consisted of unreplicated strips through the field from which subsamples were collected and evaluated. The CI for the susceptible cabbage cultivar Gazelle was 100% compared to ≤15% for the resistant cultivars. No visible clubroot symptoms were observed for the green cabbage cultivar ‘Tekila’.
In the greenhouse study, almost all susceptible cultivars had 100% CI, all with the highest disease rating (R3; Eq.1) while most resistant cultivars showed no visible symptoms. Conditions in the greenhouse represent a worst-case scenario; warm soil temperature, low soil pH, high inoculumn level next to emerging roots, a diversity of isolates from five farms, and ample moisture. Despite conditions being ideal for disease development, most resistant cultivars had a lower frequency of clubbing in the greenhouse than in the field. The difference in CI and DSI between the field trials and the greenhouse suggest that field screening is a better method for evaluating clubroot resistance due to the greater diversity of virulent pathotypes in a field. However, finding a field site with uniform disease pressure, or environmental conditions conducive to clubbing can be a challenge. For example, trials were initiated on five farms in fields with a history of clubroot, and although clubroot was present in all fields, disease incidence and severity were only uniformly distributed across two field sites. For efficiency, preliminary resistance screening should be initiated in the greenhouse to identify resistant cultivars, followed by evaluation of these cultivars in infested fields to better assess resistance potential.
All the resistant cultivars evaluated from B. oleracea showed high resistance to pathotype 16/02/30 except for kohlrabi (Table 2). Cabbage cultivars ‘Tekila’ and ‘Kilaton’, and brussels sprouts cultivar ‘Crispus’ have shown resistance to pathotype 6 (16/00/14) in Canadian field trials (Saude et al., 2012; Sharma et al., 2013). The following cultivars showed high resistance in our study: ‘Clapton’ and ‘Clarify’ (cauliflower; Fig. 3), ‘Lodero’ (red cabbage), ‘Kilagreg’, ‘Kilaton’, and ‘Tekila’ (green cabbages), and ‘Emerald Jewel’ (broccoli). Purple kohlrabi cultivar ‘Azur Star’ showed low resistance (CI of 77%) compared to the susceptible check (CI or 96%). This level of resistance would likely be unacceptable to farmers, and growing these varieties would not contribute to a significant reduction in soil spore concentrations.

Napa cabbages displayed high levels of resistance to clubroot. The cultivars ‘Bilko’, ‘Yuki’, ‘China Gold’, and ‘Emiko’ have previously shown high resistance to pathotype 6 (16/00/14) in Canadian trials (Adhikari et al., 2012; Saude et al., 2012; Sharma et al., 2013). ‘Panda’, was the only napa cabbage with low-resistance in this study, with 60% disease incidence and the highest disease rating (R3) for every infected plant. By comparison the susceptible check had 100% incidence (Table 2).
No resistance was observed for the pak choi cultivars ‘Feng Quing Choi’ and ‘Mei Quing Choi’. ‘Feng Quing Choi’ has not shown resistance to pathotype 6 (16/00/14; Adhikari et al., 2012) and ‘Mei Quing Choi’ has not shown resistance to pathotypes 6 (16/00/14) and 3 (16/15/12; Sharma et al., 2013).
Of all the crops evaluated at field site 1, clubroot incidence was the most variable for rutabaga. For example, the range in CI for ‘American Purple Top’ was 0 to 78%. In the field, average CI was 32% and 26% for ‘American Purple Top’ and ‘Marian’, respectively, but in the greenhouse, no clubbing was observed. This is surprising given that the spore suspension used in greenhouse trials contained clubs from all sites including from field site 1 where clubbing was observed.
This research demonstrated that western Oregon farmers have several commercially available cultivars with resistance to the dominant pathotype in the region. However, to preserve genetic resistance, planting resistant cultivars must be only one part of an integrated clubroot management strategy, rather than the only strategy. Diversity of virulent phenotypes exists within field populations and some of these may be present only at low frequencies. Reliance on resistant cultivars in a short rotation may result in the selection of pathotypes capable of overcoming resistance. For example, frequent planting of resistant canola cultivars has led to the erosion of resistance in Canada (LeBoldus et al., 2012; Strelkov et al., 2016).
More detailed results and discussion can be found in the publication “Screening brassica cultivars for resistance to western Oregon clubroot pathotypes” (Heinrich et al., 2017).
1c. Greenhouse screening of Oregon State University’s archived clubroot resistant germplasm collection for resistance
The broccoli breeding lines CR-1 and CR-2 showed great resistance to western Oregon pathotypes (Table 5). All cabbage breeding lines tested exhibited an infection rate of 33% or more, indicating little resistance. ‘Emerald Jewel’ from Sakata was the only resistant commercially available broccoli cultivar. Many growers did not like its horticultural performance. These breeding lines could be used to create resistant cultivars with more desirable horticultural characteristics from the producer and consumer viewpoint. There is little incentive to breed new European cabbage varieties because multiple seed companies have released cultivars with high resistance to the multiple pathotypes found in Europe, the US, and Canada. This was an initial resistance screening, and these varieties should be evaluated further before forming the basis of a breeding program.
|
Table 5. Clubroot incidence (CI) and disease severity index (DSI) of for 6 OSU clubroot resistant breeding lines (Baggett, J.R. 1983 and 1985). |
|||
|
Infected |
Disease |
||
|
Crop |
Line |
% |
index |
|
Broccoli |
CR-1 |
0 |
0 |
|
Broccoli |
CR-2 |
0 |
0 |
|
Broccoli |
CR-6 |
92 |
78 |
|
Cabbage |
CR-100 |
33 |
11 |
|
Cabbage |
CR-123 |
67 |
22 |
|
Cabbage |
CR-142 |
50 |
50 |
1d. Liming strategies for clubroot control
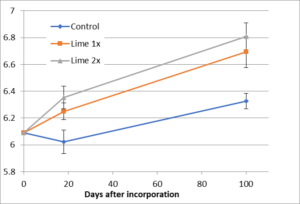
The goal at Field site 1 was to raise soil pH to approximately 6.8 and more than 7.0 with the 1x and 2x lime rates, respectively, so that we could determine whether pH 6.8 was adequate to control clubroot, as the literature suggests, or whether greater control could be achieved at a higher soil pH. However, too little lime was applied to achieve the target pH (Fig. 4), and it was incorporated more deeply than anticipated. The lime rate was based on a 6-inch incorporation depth, but the lime was incorporated to 8 inches. In addition, the lime was not thoroughly incorporated, and after more than three months, unreacted lime was still visible in the soil profile. As a result, the soil pH at transplant was approximately 6.2, well below the level required to suppress infection and disease development. Even though the pH increased over the growing season, and ultimately reached pH 6.7 and 6.8 for the 1x and 2x lime rates, respectively, lime had no effect on reducing infection rate or disease severity. Clubroot incidence was 100% for the susceptible cultivar ‘Farao’.
At Field site 3, the objective of the study was to evaluate the impact of minimal versus thorough lime incorporation on clubroot incidence and severity. The change in soil pH following the addition and incorporation of lime is given in Fig. 5. Tillage had no effect on the no lime treatments (pH 6.8), but the 2x tillage treatment increased the pH by 0.2 - 0.3 units on the two sampling dates compared to the 1x treatment. At 12 days after incorporation (planting), the pH in the 1x treatment was 7.1 and the 2x treatment was 7.3. At 91 days after incorporation (harvest), the pH in the 1x treatment was 7.4 and in the 2x treatment, 7.7. The higher pH in the 2x tillage treatment is due to the action of the rototiller, which breaks up soil clods and more thoroughly mixes the lime into the soil (Fig. 6). As the result, the lime reacts with a greater proportion of the soil particles, potentially generating fewer soil microsites of pH < 7.0.

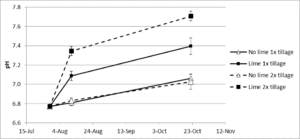
Liming reduced disease incidence in both tillage treatments, but reduced severity only in the 2x tillage (Table 6). The no lime 2x tillage treatment reduced both incidence and severity compared to the no lime 1x tillage treatment. Because rototilling did not increase the pH, it may have changed the soil physical conditions, and that change resulted in a decrease in incidence. The difference between the tillage treatments for the limed plots is likely due to a more homogeneous soil pH with fewer microsites of lower pH. Dobson et al. (1983) showed that soil limed to the same bulk pH but with different degrees of lime mixing affected disease incidence and severity. In the less thoroughly mixed soil, they attributed a higher disease incidence to low pH microsites where spore germination was not inhibited.
|
Table 6. Clubroot disease incidence and severity in the GTF liming and tillage trial. Numbers followed by the same letter are not statistically different (LSD p=0.05). |
||
|
Treatment |
Disease incidence (%) |
Disease severity |
|
No lime 1x tillage |
59a |
39a |
|
Lime 1x tillage |
35b |
20b |
|
No lime 2x tillage |
33b |
22b |
|
Lime 2x tillage |
8c |
5b |
The change in pH over approximately 400 days in 5 fields following application of 4 ton/acre of Microna Ag lime in August is given in Fig. 7. Site 4 was removed from the study as we were unsure whether sampling was performed in the correct location. In all fields, the highest pH reached was ~7.4, and most fields achieved this pH within approximately 90 days after lime incorporation. For all sites, the average difference between the starting pH and the maximum pH measured was 0.7 units (range 0.4 to 1.1). After 1 year, the average pH was 0.5 units higher (range 0.1 to 1.1) than the starting pH. The data suggests that the pH will be high enough in the spring to control clubroot when fields are limed in the fall. Fall lime applications give farmers greater flexibility in timing; farmers may be too busy in the spring/summer to apply lime shortly before brassica planting or to incorporate the lime thoroughly. In addition, fall applications permit farmers to test soil pH in late winter and apply additional lime before a spring planting if soil pH has not yet reached pH 7.1 or above.
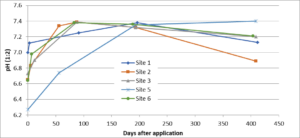
This trial demonstrated that
- soil pH must reach or exceed the minimum threshold of 6.8 at planting to suppress clubroot inoculation, and to positively impact infection rate or disease severity;
- lime must be thoroughly incorporated as it only reacts with the soil with which it is in direct contact;
- estimating the correct rate of lime is critical to achieve the target pH; and
- lime must be applied long enough in advance to react and reach the target pH before planting. Our results suggest applying finely ground lime products at least one month before planting.
Research Outcomes
Education and Outreach
Participation Summary:
To disseminate the project results to a national audience, Aaron Heinrich and Alex Stone, both of Oregon State University, presented Integrated Clubroot Management Strategies for Brassica Crops, a webinar, to the eOrganic community of practice, eXtension on February 15, 2017. Most of the 80 attendees were from the US (attendees from 26 states), 12 were from Canada (attendees from 4 provinces), and one each from Estonia and the United Kingdom. The audience included 15 agriculture professionals, 10 extension agents, 20 farmers, 10 government agency staff, 3 nonprofits, 12 Others, and 10 University researchers or educators. The webinar is available: http://articles.extension.org/pages/74054/integrated-clubroot-management-strategies-for-brassica-crops
Other outreach efforts included:
- Clubroot resistant field demonstration. This was part of the OSU North Willamette Research and Extension variety field day. September 2017, Aurora, OR. 59 participants. Figure 8

- Disease Management in Organic Vegetable Brassicas. OSU Extension Service Small Farms Conference. Feb. 18, 2017. Corvallis, OR. In this session, we integrated two years of project results to provide attendees with a holistic clubroot management program. Color copies of the extension publication were made available to project participants.
- Don’t Get Clubbed! Grow Clubroot Resistant Varieties. (Informational booth on results of clubroot resistant variety trials). OSU Extension Service Small Farms Conference. Feb. 20, 2016. Corvallis, OR. Sixty seed packets of resistant varieties for organic and conventional farmers were distributed. This outreach effort resulted in many one-on-one conversations about disease scouting and identification, prevention, containment, and mitigation strategies.
- Don’t Get Clubbed! Grow Clubroot Resistant Varieties. (Informational booth on results of clubroot resistant variety trials). North Willamette Horticultural Society Annual Meeting. Jan. 12-13, 2016. Canby, OR. Sixty seed packets containing resistant varieties for organic and conventional farmers were distributed.
- Clubroot management strategies for brassica production. North Willamette Horticultural Society annual meeting. Canby, OR. January 13, 2015. 139 participants
IMPACT OF RESULTS and OUTCOMES
Economic Analysis
Project outputs – the management recommendations - have the potential to reduce in-season and future economic losses. They give farmers greater flexibility in deciding when and where to plant, and which crop mixture will be most profitable. Currently, no data exists to quantify economic loss due to clubroot in western Oregon. However, grower comments listed here give some sense of the economic impact of clubroot on organic and conventional brassica growers of any size.
- In the past 3 years [2009–2012], we have had a 25% loss in our brassica crops due to clubroot. It cost us between $60,000 to $80,000 in lost revenue per year. We are running out of clubroot-free ground on which to rotate brassica crops. —Mid-acreage fresh market vegetable grower, Portland, OR
- We have stopped growing brassicas on 15% of our land due to clubroot. —Large-acreage processed vegetable grower, Mt. Angel, OR
- We experienced a 30 to 50% loss in five of our highest-yielding brassica crops in 2013, totaling [an economic loss of] $20,000. Three years ago, we played out this scenario, knowing that our future looked quite bleak. So, we started to search for [organically certified] land that hadn’t grown brassicas. So far we are still looking. We need to figure out a way to grow brassicas in fields that have a high clubroot population. —Small-acreage diversified organic vegetable grower, Portland, OR
For most farms, the benefit of implementing a clubroot control program far outweigh the cost.
Farmer Adoption
This WSARE funded project provided multiple opportunities for farmer outreach leading to changes in grower practice and/or adoption of effective clubroot control strategies. These outreach events were evaluated, and we determined that considerable learning took place (short term learning outcomes). Further, growers adopted project recommendations, or indicated an intent to adopt new practices (medium term behavioral outcomes). Here we summarize their evaluation of the value of this project to them.
Producer Participant Survey
An online survey was sent to the 10 producer participants in our research. Five responded to questions about improved knowledge of clubroot. All respondents stated that their knowledge of clubroot management strategies had increased because of their participation in this work. (Short term learning outcome). All respondents were familiar with clubroot, and reported over the past five years having lost from 0-20% of the crop from any infested field, though loss was as high as 100% on one farm. Because these five had multiple interactions with project researchers over an extended period, we were able to measure medium-term outcomes and overall see greater behavioral change, or intent to change behavior, among the group.
Adoption of Seven Recommended Practices to Control Clubroot
This research emphasized seven practices for the control of clubroot; we queried producer participants about their adoption of these practices.
- All grower participants intend to lime their brassica fields to pH 7 or greater.
- Scouting and record keeping has been or will be adopted by 3 of the 5; the additional two report always having done so.
- Planting resistant cultivars has been or will be adopted by 3 of the 5; 1 has always done so, and the remaining producer does not intend to adopt this practice.
- Cleaning equipment between infested and “clean” fields has or will be adopted by 3 of the 5; the remaining two do not intend to adopt this practice.
- Two of the 5 producer participants intend to begin rotating out of brassicas for a minimum of 4 years; 1 has always done so, and 2 do not intend to adopt this behavior.
- Two of the 5 intend to adopt water management to avoid waterlogging and runoff; two have always done so, and one respondent does not now practice this technique, nor does he intend to.
- Four of the 5 respondents do not now eliminate weedy host plants, nor do they intend to adopt the practice. The remaining participant has always done so.
Adoption of Five Recommendations Regarding Liming to Control Clubroot
This research emphasized five liming-related practices for the control of clubroot; we queried producer participants about their adoption of these practices.
- All grower participants intend to lime their brassica fields to pH 7 or greater.
- Because of this project, 3 of the 5 producer-participants have begun using an OSU Extension publication to calculate liming rate to reach target pH, and the other 2 indicated the intent to do so.
- Three survey respondents indicated that, because of this project, they have adopted the practice of liming and incorporating lime a minimum of one month in advance of planting; the other 2 currently engage in this practice.
- Two producer participants indicated that they currently make additional tillage passes to incorporate lime thoroughly; 2 intend to adopt this practice because of this project; the fifth neither does nor intends to adopt this practice.
- Two respondents indicated that, because of this project, they have adopted the practice of confirming target pH with a pre-plant soil pH test; another one intends to adopt this practice because of this project; the remaining two neither do so now nor intend to do so.
Practice efficacy
- One hundred percent of project producer respondents indicated that liming to pH greater than 7 appears to be an effective strategy to manage clubroot.
- When asked whether scouting and record keeping seems to have made a difference, four of the five participants indicated “yes,” and the remaining respondent has not yet implemented the practices.
- When asked whether cleaning equipment between fields seems to have made a difference, four of the five indicated that they have not yet implemented the practice and the remaining respondent is not yet sure.
- When asked whether planting resistant hosts seems to have made a difference, three of the five indicated that they have not yet implemented the practice and the remaining two said “yes.”
- When asked whether water management to eliminate waterlogging and runoff seems to have made a difference, two of the five indicated “yes;” two are not yet sure, and the remaining respondent has not yet implemented the practice.
- When asked whether eliminating weedy hosts seems to have made a difference, four of the five indicated that they have not yet implemented the practice and the remaining respondent is not yet sure.
- Responses were more variable when participants were asked whether rotation out of brassicas for 4 years seems to have made a difference. One participant indicated “yes,” one “no,” one “not sure yet,” one “have not implemented,” and one “not compatible with my farming system.”
Project dissemination by word of mouth
Two respondents have spoken to more than 10 people about some aspect of this project, one has spoken to 5 to 10, and the additional two respondents have spoken to 1 to 4 people.
Optimism concerning clubroot control
Three of the five project participants are highly optimistic that they can reduce their economic loss due to clubroot by implementing practices recommended by this project; the other two are somewhat optimistic.
Clubroot Presentation Attendee Survey
We presented this clubroot work in the “Disease Management in Organic Vegetable Brassicas” session at the Oregon State University Small Farms Program 2017 Conference. WSARE funded clubroot researchers distributed a paper-and-pencil survey at the end of their presentation to assess the short term learning outcomes of session participants.
These participants responded to the six practices, which were the emphasis of this research project. For brevity, the liming practice was not included here, but as a separate question devoted specifically to liming practices. The survey items were designed to capture post- and then pre-knowledge levels on a scale of 1 to 5 (1 being low knowledge and 5 being a great deal of knowledge. Post- and then pre- presents question in this fashion: Now that you have attended this session, how much do you know about the topic? Respondents are then asked: How much did you know before? This approach allows respondents to recognize their learning and not respond before the presentation based on erroneous belief.
Twenty-nine session participants submitted responses. They were 24 growers, 2 wholesalers, 1 retailer, and 2 educators. A scant 14% of those characterized clubroot as a problem on their farms, and 72% indicated they were not concerned; 28% were moderately concerned. They estimated losses from $500 to $1,000-5,000 due to Clubroot in 2016. Because this was a single, brief interaction, we emphasized short term learning outcomes in the session evaluation questions.
|
Table 7: Clubroot short-term learning outcomes, 2017 Small Farms Conference Session – General Management Practices (1 = very little knowledge; 5 = a lot of knowledge) |
||||||||||
|
Practice |
Average pre-session knowledge |
Average post-session knowledge |
||||||||
|
1 |
2 |
3 |
4 |
5 |
1 |
2 |
3 |
4 |
5 |
|
|
Scouting, record keeping |
62 |
21 |
10 |
7 |
0 |
0 |
7 |
38 |
38 |
14 |
|
Cleaning equipment |
59 |
28 |
10 |
0 |
3 |
0 |
0 |
31 |
35 |
31 |
|
Rotation length |
69 |
14 |
17 |
0 |
0 |
0 |
0 |
24 |
48 |
24 |
|
Eliminate non-crop hosts |
59 |
17 |
17 |
3 |
0 |
7 |
10 |
28 |
31 |
17 |
|
Water management |
59 |
17 |
21 |
3 |
0 |
0 |
7 |
35 |
38 |
17 |
|
Resistant cultivars |
59 |
21 |
21 |
0 |
0 |
0 |
3 |
55 |
31 |
7 |
We offer as evidence of learning, Tables 1 and 2 summarizing responses to the session evaluation. Notice that in Table 7, only 7, 3, 0, 3, and 0 percent of participants assessed their pre-session knowledge of the general management practices, respectively, within the top two quintiles. By comparison, their post-session knowledge placed 52, 66, 72.3, 49, 55, and 38 percent ranked themselves in the top two quintiles. Similar observations can be drawn from Table 8, a summary of grower short-term learning outcomes about the specific liming practices emphasized in this project.
|
Table 8: Clubroot short-term learning outcomes, 2017 Small Farms Conference Session – Liming Management Practices (1 = very little knowledge; 5 = a lot of knowledge) |
||||||||||
|
Practice |
Average pre-session knowledge |
Average post-session knowledge |
||||||||
|
1 |
2 |
3 |
4 |
5 |
1 |
2 |
3 |
4 |
5 |
|
|
Target pH |
79 |
10 |
7 |
0 |
0 |
0 |
0 |
28 |
28 |
38 |
|
Calculate lime rate |
59 |
24 |
7 |
7 |
0 |
0 |
7 |
41 |
24 |
21 |
|
Effective application timing |
66 |
24 |
3 |
3 |
0 |
0 |
3 |
35 |
28 |
28 |
|
Effective incorporation |
69 |
21 |
0 |
3 |
3 |
0 |
3 |
31 |
24 |
35 |
|
Pre-plant pH monitoring |
62 |
14 |
3 |
10 |
7 |
0 |
0 |
35 |
21 |
38 |
eOrganic Webinar
Results of this WSARE funded project also were disseminated via an eXtension-hosted webinar on 2/17/17. Eighty persons participated. Most were from the US although 12 were from Canada, with 1 each from Estonia and the United Kingdom. The audience included 15 agriculture professionals, 10 county-based extension faculty, 20 farmers, 10 government agency staff, 10 university researchers or educators, participants from 3 nonprofits and 12 other individuals. The webinar is archived here: http://articles.extension.org/pages/74054/integrated-clubroot-management-strategies-for-brassica-crops. The webinar has had 358 views on YouTube as of 8/15/17.
Thirty-six participants responded to a survey, which was disseminated via email after the webinar. The survey, prepared by eXtension staff, seems intent on capturing current practice rather than intent to change. Of the 33 responding to a question about negative economic impact, two reported severe economic damage, 12 reported moderate economic damage, and 19 reported no loss from clubroot.
Of the 36 responses about the efficacy of lime in controlling clubroot, one used lime and was very successful, six used lime and were somewhat successful, three used lime and were not successful; the remaining 26 did not use lime. The question did not allow for elaboration on what participants meant by “using lime.”
Webinar participants were asked about the length of their brassica rotations. Sixteen reported 1 year in brassicas, with 3 years out; seven indicated 1 year in and 2 years out; three indicated 1 year in and 1 year out; ten indicated that their rotations are longer.
Following the event, an extension agent submitted this remark:
“Very informative webinar. Clubroot is becoming a big problem in western Washington, so this is most timely. Thanks to all involved and especially your farmer cooperators!”
Education and Outreach Outcomes
Areas for Additional Study
Managing the clubroot requires a multifaceted approach. This project focused primarily on growing clubroot resistant varieties and pH management using agricultural lime. Additional research needs to be done in the following areas for a more integrated approach:
- Durability of resistance genes in resistant cultivars. Research is needed to determine whether resistance can be overcome by growing these cultivars repeatedly in the same field, and if such is the case, how many cropping cycles are necessary to do so.
- Better understanding of how clubroot is transported and ways to minimize transport. There is some evidence that clubroot can be spread in floodwaters and by dust. An organized effort to contain clubroot and prevent its spread throughout western Oregon would benefit growers.
- Determine whether clubroot infection and severity can be reduced when planted at a specific soil temperature that would minimize disease spore germination. With this information, farmers could plant brassicas before warming soils increased spore germination, resulting in increased disease severity.
- Determine whether a clubroot-resistant brassica bait crop, is a viable strategy for decreasing spore concentrations. By stimulating the germination of spores without producing disease-forming clubs, there is the possibility of reducing disease incidence and severity in future rotations.
- Determine whether a combination of liming and boron is an effective control strategy. In greenhouse studies, we found boron reduced disease severity but not infection rate, but had no effect when applied in the field. In combination with lime, there could potentially be some benefit.