Final report for OW22-370
Project Information
Clover seed weevil (CSW), Tychius picirostris Fabricius (Coleoptera: Curculionidae), is a key insect pest in white clover seed production in Oregon. Historically, clover seed growers have relied heavily on broad-spectrum pyrethroid insecticides for CSW management. Since 2017, growers and crop consultants have observed poor efficacy with pyrethroid insecticides, causing serious concern for the white clover seed industry. Moreover, reduced effectiveness of pyrethroid insecticide treatments in a 2021 Oregon State University (OSU) field trial warrants further CSW pyrethroid resistance status evaluation. Available insecticides are limited, leaving few options for growers to rotate between different modes of action (MoA). We aim to i) determine the resistance status to pyrethroids and other MoA using laboratory bioassays, ii) evaluate new MoA and different insecticide timing under field conditions, and iii) develop a scouting method and treatment decision-making guidelines based on the larvae density per seed head. Our education efforts will focus on i) enhancing grower knowledge of CSW biology and optimizing control timing; ii) facilitating training and workshops for calibrating pesticide application equipment and scouting techniques specific to CSW, and iii) gathering input on growers' perceptions of alternative methods of the integrated management plan (IPM). Data from this research will be disseminated during OSU Extension events throughout the project duration. The expected outcomes will include one factsheet, one extension publication, and at least one peer-reviewed journal article. The sustainability goal will be achieved by 1) reducing unnecessary chemical applications and 2) increasing economic return by lowering inputs and improving seed yield.
Our research objectives include:
- Identify the level of pyrethroid resistance development and toxicology of other insecticide modes of action using laboratory bioassays.
- Conduct field efficacy tests using new modes of action and different insecticide timings specifically targeted to control clover seed weevil (CSW) larvae.
- Develop a grower-friendly scouting method and treatment decision-making guidelines based on CSW larvae density per seed head.
The educational objectives of this project will focus on 1) improving current knowledge of clover seed growers and crop advisors on CSW biology and behavior to better time insecticide applications, 2) facilitating the incorporation of insecticide resistance management strategies in clover seed production systems through training and workshops, and 3) gather input from growers and local crop advisors about their perceptions of alternative methods included in the integrated management plan (e.g., host plant resistance, cultural control, and biocontrol).
Cooperators
- - Producer
- - Producer
- - Producer
- - Producer
- - Producer
Research
Insect Collections. Adult T. picirostris populations were collected from commercial white clover seed fields (n = 8 fields) in Linn County, Oregon, in 2022 and 2023. We defined “populations” in this study as field-scale collection locations. Susceptible T. picirostris populations with no prior evidence of insecticide resistance and minimal exposure to foliar insecticides were acquired from commercial alsike clover (Trifolium hybridum) in the Peace River Region, Alberta, Canada, in 2022 and 2023. Adult specimens were captured using sweep net sampling and placed in a 60 × 60 × 60 cm nylon mesh cage for adequate ventilation and transferred directly to the laboratory; insect rearing and all subsequent experiments were conducted at Oregon State University in Corvallis, Oregon. Field-collected T. picirostris in Oregon (2022: n > 5000; 2023: n > 5500) were transferred to the laboratory on the same day of collection; susceptible adult field populations from Canada (2022: n = 1138; 2023: n = 2169) were transferred within 72 h of the collection date. Adult weevils were provided clover heads as a food source and sprayed with a fine mist of water to maintain adequate moisture levels within the cage. To ensure only healthy individuals were selected for assays, weevils were held for ≥24 h before experimentation, and lethargic individuals were excluded. Field collections were conducted between June and August 2022 and 2023 before insecticide applications were applied in respective fields.
Log-Probit Assays.
Surface Contact Toxicity Assays. Log-probit assays were conducted using a standard glass-vial procedure (Miller et al. 2010). Tests were conducted with technical grade bifenthrin [2-methylbiphenyl-3-ylmethyl (Z)-(1RS,3RS)-3-(2-chloro-3,3,3-trifluoroprop-l-enyl)- 2,2-dimethylcyclopropanecarboxylate; 97.4% purity, Chem Service Inc.] and malathion [O,O-dimethyl dithiophosphate of diethyl mercaptosuccinate; 98.6% purity, Chem Service Inc.]. Insecticides were dissolved in acetone and diluted to create a geometric series of concentrations ranging from 0.0125 to 25.6 µg vial-1 (0.00028 to 0.57 µg/cm2). Insecticide solution was then drawn into the repeating pipettor, and 0.5 mL of solution was dispensed into individual 20 mL scintillation vials; vials were rotated using a commercial hot dog roller (heat unit removed) until the solvent had evaporated. Once the vials were dry, five adult T. picirostris were placed into each vial, and a plastic cap was secured loosely to prevent escape and allow sufficient airflow. A minimum of seven concentrations and five replicates per concentration were evaluated for each assay (n ≥ 175 test specimens). A minimum of five replicates were used as the control (n ≥ 25 control specimens; vials treated with 0.5 mL of 100% acetone only). Moribundity was recorded after 24 h and determined based on the ability of adults to maintain an upright posture when gently prodded (Smith and Hammond 2006). Moribundity was corrected using Abbott’s formula, and tests were discarded if control moribundity was greater than 20 percent (Abbott 1925).
Oral Toxicity Assays. The toxicity of technical grade chlorantraniliprole (anthranilic diamide) [3-bromo-4'-chloro-1-(3-chloro-2-pyridyl)-2'-methyl-6'-(methylcarbamoyl)pyrazole-5-carboxanilide; 98.38% purity, FMC Corporation] was investigated using a honey water solvent pipetted onto floral foam plugs (Miller et al. 2010). The minimum number of concentrations and adult replicates were the same as the contact toxicity assay procedure described above. Since chlorantraniliprole is insoluble in water, it was prepared as a 1000 μg mL–1 stock solution in 2.5 mL acetone and subsequently diluted in a 10% honey-water solution to achieve the desired concentration range. Control treatments were also prepared with the same rate of acetone to ensure negligible toxicity of the solvent. A geometric series of concentrations ranging from 0.025 to 12.8 µg vial-1 (0.00055 to 0.28 µg/cm2) was used for the assay. Insecticide concentrations were dispensed (0.5 mL) onto OASISÒ floral foam (12 ×12 mm) plugs placed within each 20 mL scintillation vial using a repeater pipette. Moribundity, as defined previously, was observed at 48 h, and adults were contained in vials by loosely securing a plastic cap.
Fig 1: Glass vial bioassay.
Synergistic Assays. Synergistic assays were conducted in this study following the contact assay procedure described above. A geometric series of bifenthrin concentrations ranging from 0.8 to 102.4 µg vial-1 (0.018 to 2.26 µg/cm2) was prepared, and 5 µg vial-1 (0.11 µg/cm2) of one of three synergists was added to each concentration (Dorman et al. 2020). Two common phase-I metabolic detoxification synergists (PBO and DEF) and one phase-II synergist (DEM) were evaluated, including a mixed-function oxidase inhibitor (piperonyl butoxide; PBO 98.5% purity, Chem Service Inc.), a general esterase inhibitor (S,S,S-tributyl phosphotrithioate; DEF 97.6% purity, Chem Service Inc), and a glutathione S-transferase (GST) inhibitor (dimethyl maleate; DEM 97%, Sigma Aldrich). The number of replicates (per concentration and vial) and the test response evaluation (moribundity) were the same as the surface contact assay procedure.
Fig 2: A researcher conducting glass vial bioassay.
Topical Assays. Formulated products, Brigade® 2EC (bifenthrin; FMC Corporation), Malathion 8 Aquamul®(malathion; Loveland Products), and Vantacor® (chlorantraniliprole, FMC Corporation) were evaluated in topical assays. Four serial dilutions of 0.5, 1, 2, and 4 times the product label's highest recommended rate and water control were applied to three replications of ten T. picirostris adults placed on 140 mm diameter Petri dishes. For bifenthrin (Brigade® 2EC) assays only, 5 times the product label rate was also evaluated. Stock solutions were prepared at 23, 46, 92, 184, and 230 ppm for bifenthrin; 234, 469, 938, and 1877 ppm for malathion; and 17, 34, 68, and 137 ppm for chlorantraniliprole. Before treatment, T. picirostris adults were flash chilled at 0○C for 45 seconds to immobilize them. Treatments were topically applied using a Potter spray tower (Burkard Manufacturing Co. Ltd., England). Each petri dish replicate was sprayed with 1.5 mL of insecticide solution or water (for the control group) using an air pressure of 47 kPa (6.8 psi) and a spray distance of 22 cm (Kaur et al. 2020). The Potter spray tower delivered the insecticide solution as a fine mist, which formed a uniform layer on T. picirostris adults without creating any visible droplets. Moribundity of treated T. picirostriswas recorded after 24 h and 48 h for contact and systemic insecticides, respectively.
Fig 3: Potter spray tower bioassay.
Statistical Analysis. For log-probit and topical assays, percent moribundity was corrected using Abbott’s formula. The Proc Probit procedure in SAS 9.4 (SAS Institute Inc., Cary, NC) was used to analyze log-probit assay data to compute the median lethal concentration (LC50), 95% fiducial limits, and other descriptive statistics. Differences in LC50values among populations were significant if 95% fiducial limits did not overlap. Resistance ratio (RR50) values were generated by dividing the respective LC50 value of the test population by the LC50 value of the susceptible population (T. picirostris populations from Alberta, Canada) (Haddi et al. 2018). Low, moderate, high, and very high resistance levels were determined if RR50 ranged between 2–10, 11–30, 31–100, and >100, respectively (Torres-Vila et al. 2002, Jiang et al. 2010). Topical assay data were analyzed using lme4 (Bates et al. 2015) and emmeans (Lenth 2023) packages in R (v4.3.1 R Core Team). The proportion of moribund T. picirostris adults was fitted to a binomial distribution and analyzed using generalized linear mixed models (GLMMs) for each unique formulation and region (i.e., Oregon or Canada) combination, with concentration treatment as fixed effects and random effects intercepts for the year of sampling, Oregon population identifier (i.e., OR 1–8), and replicate. Oregon population random effects were excluded from GLMMs with Canadian specimens. Differences between concentrations were calculated using estimated marginal means (EMMs), and the Tukey method was used for EMM comparisons with a significant level of α = 0.05. Assays with a singular response (i.e., proportion moribund = 1) were excluded from analysis (Brigade® 2EC with susceptible Canada populations and Malathion 8 Aquamul® assays for Oregon and Canadian populations). Descriptive statistics for topical assays will be presented as mean ± SE. Percent moribundity and probit moribundity data in surface contact and topical assays were visualized using the ggplot2 package in R (Wickham et al. 2016).
Large-plot field trials.
Year one trial (2022). Field efficacy experiments were conducted in commercial white clover fields in Linn County, Oregon, USA, during the 2022 and 2023 growing seasons. In 2022, a single trial was carried out in a third-year commercial white clover seed field (cultivar ‘Crusade’). The plot layout was organized in a randomized complete block design with eight treatments plus an untreated control, with three replications. Each plot size measured 8.8 m × 91.4 m. Two application timings were selected, including at first larval detection (BBCH 63-64) and the grower standard timing of crop phenology at 20% brown down, mid-late flowering stage (BBCH 67-69) (Meier, 1997). Treatments were applied using an ATV-mounted boom sprayer with TeeJet XR11002VS nozzles, calibrated to deliver a minimum of 15 gallons per acre at 20 psi. A nonionic surfactant was used in all treatments (0.25% v/v).
Year two trial (2023). Two field trials were located in second and third-year white clover seed fields, including cultivars ‘Marco Polo’ and ‘Mighty White Pro,’ respectively. Similar to the first-year trial, eight insecticide treatments and an untreated control were evaluated in second-year trials. The experimental design, plot size, and insecticide application equipment were the same as the first-year trial described above. Based on monitoring data and efficacy results from the first-year field trial, insecticide application timings were adjusted for second-year trials. Insecticide treatment timing of contact insecticides was at pre-bloom (BBCH 59-60) to control immigrating adult populations before egg-laying, and the timing of systemic insecticides was at full bloom (BBCH 65-66) to target egg and larval life stages.
Fig 4: Insecticide treatment sprayed during dusk.
Tychius picirostris sampling. Adult T. picirostris densities were assessed at 14, 21, and 28 days after insecticide treatment (DAT) using a standard 38 cm diameter heavy canvas sweep net. Twenty 90° sweep net samples were conducted across each experimental plot. Adult weevils from each plot were collected in labeled plastic bags, stored in a cooler, and transferred to the lab for counting. Larval abundance was assessed at 7, 14, 21, and 28 DAT by collecting 30 random inflorescence samples from each plot. Inflorescence samples were processed using a Berlese funnel with a 40-watt incandescent bulb and a glass collection jar filled with 70% ethanol solution. Extracted larvae in glass jars were counted after a 24 h extraction period.
Fig 5: Data collection from field efficacy trial.
Seed yield and economic returns. Plots were harvested for seed yield using commercial harvest equipment and weighed with a Parkan weigh wagon. Subsamples of harvested seed were collected and cleaned with a Clipper M-2B seed cleaner to determine the percent cleanout and calculate the total clean seed yield. To calculate economic returns across insecticide treatments, seed yield was priced at $6.63/kg (average market price of white clover seeds in 2022 and 2023). After consultations with multiple retailing companies, insecticide input costs were as follows: Brigade 2EC (0.47 kg ai/ha at $20.38/ha), Exirel (1.49 kg ai/ha at $247.24/ha), Harvanta 50 SL (1.19 kg ai/ha at $116.14/ha), Malathion 8 Aquamul (1.46 kg ai/ha at $59.43/ha), Steward EC (0.83 kg ai/ha at $86.79/ha), Vantacor (0.18 kg ai/ha at $101.31/ha). Total insecticide cost per hectare was calculated by summing insecticide cost per treatment with a standard application cost consistent across treatments ($20.00/ha).
Fig 6: White clover seed harvested using Parkan weigh wagon.
Small-plot field trials.
Year one trial (2022). Small-plot research trials were also conducted in commercial fields. In the first-year trial, five insecticide treatments and an untreated control were arranged in a randomized complete block design with four replicates. Individual plots were 9.1 m × 3.7 m and in a second year stand of cultivar ‘Louisiana S-1.’ Insecticide treatments of Malathion 8 Aquamul, Steward EC, and Vantacor were applied at BBCH 63-64 (first larval detection), and applications of Elevest and Brigade 2EC was made at the grower standard timing of crop phenology at BBCH growth stages 67-69 (20% brown down, mid-late flowering stage). Foliar insecticides were applied along with a nonionic surfactant (0.25% v/v) using a CO2 pressurized backpack sprayer equipped with a four-nozzle boom calibrated to deliver 20 gallons per acre through TeeJet XR11002VS nozzles at 20 psi.
Year two trial (2023). The second-year trial was expanded to 13 insecticide treatments and an untreated control and was arranged in a randomized complete block with four replicates. Individual plots were 18.3 m x 18.3 m in a second year stand at a commercial field planted with the cultivar ‘Synergy.’ Contact insecticide treatments were applied pre-bloom (BBCH 59-60) to control adult populations, and newer systemic formulations were applied at full bloom (BBCH 65-66), targeting egg and larval life stages (Tables 3.1 and 3.2). Brigade 2EC was also applied at full bloom to represent the ‘grower’s standard’ treatment. Treatments along with nonionic surfactant (0.25% v/v) were applied using an ATV-mounted boom sprayer with TeeJet XR11002VS nozzles, calibrated to deliver a minimum of 15 gallons per acre at 20 psi.
T. picirostris sampling. Sampling for adult and larval T. picirostris abundance, including laboratory processing of samples, followed the same methodology as large-plot field trials described above.
Larval scouting experiment. To validate the effectiveness of a do-it-yourself (DIY) funnel design for estimating larval densities in white clover during flowering, we compared larval counts using a DIY funnel and a standard Berlese funnel with inflorescence samples collected from commercial fields (n = 6 total fields) across two years in Linn County, Oregon. Fields were divided into four quadrants, and field sampling, extraction timing, and collection procedures were similar to Sabu et al. (2011). Floral heads (n = 200) were randomly collected from each quadrant using a 1 ft2 sampling grid >15 m from field borders. In the lab, 100 heads from each quadrant per field were placed into DIY and standard Berlese funnels following the methods of Guppy et al. (1975). The DIY funnel design consisted of an inverted 1-gallon plastic milk jug with the bottom removed and placed over 1-quart plastic jar. Wire mesh circles were affixed over the milk jug spout to retain plant material in the plastic jug. Standard Berlese funnels were commercially available models (Dondale et al. 1971, Smith 2012). Both funnel types were equipped with a 40-watt incandescent bulb above the sample to drive larvae downward into collecting jars filled with 70% ethanol. Larvae were counted after 24 h. The procedure was repeated three times at weekly intervals for each field during flowering following first larval detection.
Fig 7: Standard Berlese funnel. Fig 8: Do-it-yourself Berlese funnel.
Statistical analyses. All data analyses were conducted using the lme4 (Bates et al. 2015), emmeans (Lenth, 2023), and ggplot2 (Wickham et al. 2016) packages in R (v4.3.1 R Core Team). For large and small plot field trials, adult and larval T. picirostris abundance data were fitted to a negative binomial distribution and analyzed using generalized linear mixed models (GLMMs) with treatment and days after treatment (DAT) as fixed effects. Plot replicate was included as random intercepts and DAT as random slopes. Seed yield and economic return data were analyzed using linear mixed-effects models (LMMs) with seed yield (kg/ha) and economic returns (U.S. dollar/ha) as the response and treatment as fixed effects. Plot replicates were specified as random intercepts. Field site identifiers were also included as random effects intercept for T. picirostris abundance and seed yield analyses for second-year large plot field trials (i.e., large plot field trials in 2023 were analyzed together). Estimated marginal means from the GLMMs and LMMs were calculated and compared using Tukey’s HSD test at an α = 0.05 significance level to determine differences between treatments. Kenward-Rogers approximation was used to estimate degrees of freedom for seed yield and economic return models. To correlate insect abundance to associated seed yield loss, simple linear regression analysis was performed between seed yield and adult and larval T. picirostris abundance in first and second-year large plot field trials. Simple linear regression was also used to evaluate the relationship between larval counts from the DIY funnel compared to the standard Berlese funnel.
Article published in Journal of Economic Entomology. DOI: 10.1093/jee/toae012
First year field efficacy trial data published in 2022 Seed Production Research Report: https://cropandsoil.oregonstate.edu/sites/agscid7/files/crop-soil/tiwari_clover_seed_weevil.pdf
Log-Probit Assays.
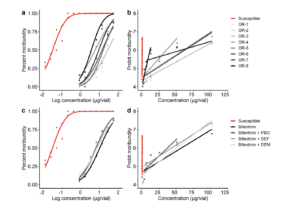
Surface Contact Toxicity Assays. The highest levels of resistance were observed with bifenthrin assays; resistance ratios (RR50) in Oregon adult populations ranged from 178.00 to 725.67 compared to susceptible Canadian field populations. Significant differences in LC50 values were also observed among T. picirostrispopulations in both years, ranging from 5.34 to 21.77 µg vial-1 (0.12 to 0.48 µg/cm2 ) and 8.96 to 14.37 µg vial-1 (0.20 to 0.32 µg/cm2) in 2022 and 2023, respectively (Fig. 1a and b); the LC50 value range for bifenthrin relates to 0.098 to 0.43 times the formulated product’s (Brigade® 2EC) highest label rate. Bifenthrin resistance for all Oregon populations was categorized as ‘very high’ compared to the susceptible population (Fig. 1a and b). In both years, low to high RR50 values were observed, with malathion assays ranging from 9.20 to 32.80 across evaluated populations. Significant differences in LC50 values were observed among Oregon populations in 2023 only, with values ranging from 1.57 to 1.64 µg vial-1 (0.035 to 0.036 µg/cm2) and 0.39 to 1.05 µg vial-1 (0.009 to 0.023 µg/cm2) in 2022 and 2023, respectively. The malathion LC50 value range relates to 0.001 to 0.002 times the formulated product’s (Malathion 8 Aquamul®) highest label rate.
Oral Toxicity Assays. Oregon and susceptible Canadian adult T. picirostris populations used in surface contact assays were also evaluated using oral toxicity assays with chlorantraniliprole. Differences in LC50 values compared to the susceptible population were detected in five of the eight Oregon populations, albeit RR50 values were low for all Oregon populations (i.e., RR50 < 4.59), suggesting minimal resistance in adult T. picirostris populations. In 2022 and 2023, LC50 values for Oregon populations ranged from 0.89 to 1.62 µg vial-1 (0.02 to 0.04 µg/cm2) and 2.42 to 4.96 µg vial-1 (0.05 to 0.11 µg/cm2), respectively. The chlorantraniliprole LC50 values ranged from 0.96 to 4.96 μg vial-1 across sample years, relating to 0.05 to 0.10 times the label rate of formulated chlorantraniliprole.
Synergistic Assays. Reductions in bifenthrin LC50 values were observed across the three synergists evaluated, albeit synergism was site-specific (Table 2.2, Fig. 2.2c and d). Synergistic effects were greater in the four Oregon populations with higher bifenthrin resistance (LC50 >10 µg vial-1) (LC50 >0.22 µg/cm2) with an average synergistic ratio (SR50) of 3.35, 2.84, 3.39 for the mixed-function oxidase inhibitor (PBO), esterase inhibitor (DEF), and GST inhibitor (DEM), respectively (Fig. 1c and d). The SR50 values for Oregon populations with lower bifenthrin resistance (LC50 <10 µg vial-1) (LC50 <0.22 µg/cm2) averaged 1.10, 0.92, and 2.41 for PBO, DEF, and DEM, respectively. The GST inhibitor had the greatest synergistic effects across T. picirostris populations with varying levels of bifenthrin resistance. Synergism was also observed with PBO and DEF in populations with very high resistance to bifenthrin. Minimal synergistic effects were observed with oxidase and esterase inhibitors for populations with less pronounced resistance.
Figure 1. Percent moribundity (a) and probit moribundity (b) in surface contact assays with technical grade bifenthrin for T. picirostris susceptible and field-collected Oregon populations and cumulative percent moribundity (c) and probit moribundity (d) across Oregon populations compared to the susceptible strain and the addition of one of three synergists, including piperonyl butoxide (PBO), S,S,S-tributyl phosphotrithioate (DEF) and dimethyl maleate (DEM).
Topical Assays.
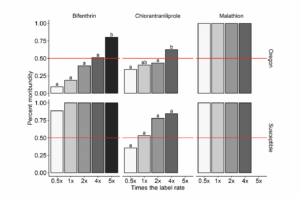
Oregon T. picirostris populations were highly resistant to formulated bifenthrin (Brigade® 2EC) in topical assays (Fig. 2). Significant increases in moribundity were only observed at the highest concentration of 5 times the label rate (230 ppm), averaging 91 ± 2 and 70 ± 6 percent of moribund adults in 2022 and 2023, respectively (χ2 = 8.70; df = 4; P < 0.0001). Moreover, the LC50 value for Oregon populations to formulated bifenthrin was 2.7 times the label rate (95% FL = 1.66 to 5.90). Low efficacy was observed with chlorantraniliprole assays with a similar response in moribundity across the concentrations evaluated for both Oregon (χ2 = 4.71; df = 3; P = 0.003) and susceptible populations (χ2 = 0.98; df = 3; P = 0.40). Although differences in moribundity were observed across concentrations for Oregon populations, the highest concentration evaluated (4 times the label rate; 1877 ppm) was the same as the label rate (469 ppm) (Fig. 2). The proportion of moribund adults equaled one (100 percent moribundity) in bifenthrin assays with the susceptible population, and for both Oregon and susceptible populations in malathion (Malathion 8 Aquamul®) assays across all concentrations tested.
Figure 2. Moribundity response in susceptible and field-collected Oregon T. picirostris populations exposed to formulated bifenthrin (Brigade® 2EC), malathion (Malathion 8 Aquamul®), and chlorantraniliprole (Vantacor®) in topical assays. Rows represent the populations evaluated (i.e., Oregon or susceptible field populations), and columns indicate the MoA of the formulated product evaluated. The red line indicates 50 percent moribundity.
Large-plot field trials.
Tychius picirostris abundance. In the first-year trial, we detected differences in T. picirostris adult abundance across treatments (treatment main effect: χ2 = 22.75; df = 8; P = 0.0037) and sample dates (DAT main effect: χ2 = 48.22; df= 2; P < 0.0001). Plots treated with Brigade 2EC and Exirel + Brigade 2EC had greater adult populations, with means of 19.78 ± 4.73 and 17.56 ± 3.71 per 20 sweeps, respectively, compared to 7.44 ± 2.46 adults in the Harvanta 50 SL + Brigade 2EC treatment. No other pairwise differences between treatments were significant. For T. picirostris larval abundance, there were no differences observed among treatments in the first-year trial (treatment main effect: χ2= 9.21; df = 8; P = 0.325).
Seed yield. There were no differences in seed yield between insecticide treatments and the untreated control in first-year (F = 1.73; df = 8, 16; P = 0.166) large plot field trials. Seed yield ranged from 603.55 kg/ha in the untreated control to 738.59 kg/ha in the Steward + Brigade 2EC treatment in the first-year trial.
Economic returns. Similar to seed yield, differences in economic returns across insecticide treatments were not observed in large plot trials in 2022 (F = 1.35; df = 8, 16; P = 0.287). In 2022, the untreated control had a total economic return of $4,002.05 ± 437.66 (US dollars/ha). Plots treated with Steward EC + Brigade 2EC generated $767.88 ± 376.77 in net returns above the untreated control, and Harvanta 50 SL + Brigade 2EC and Exirel + Brigade 2EC treatments had net returns of $565.13 ± 490.30 and $433.08 ± 383.75 above the untreated control, respectively.
Small-plot field trials.
Tychius picirostris abundance. Differences were detected in T. picirostris adults across insecticide treatments in the first-year small plot trial (treatment main effect: χ2 = 19.17; df = 5; P = 0.002). Treatment differences were not observed for larval abundance (treatment main effect: χ2 = 8.25; df = 5; P = 0.143) in year one. Adult counts ranged from 42.08 ± 4.26 in the untreated control to 61.67 ± 6.10 in the Elevest treatment. Larval abundance was low overall, ranging from 1.08 ± 0.50 larvae in the Elevest treatment to 3.58 ± 1.08 larvae in the untreated control.
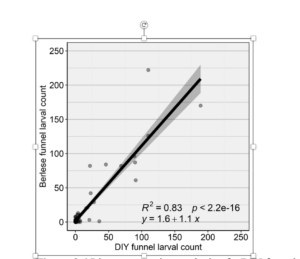
Larval scouting experiment. Larval abundance assessments estimated with DIY and standard Berlese funnel scouting methods were strongly correlated in commercial fields sampled across 2022 (n = 3) and 2023 (n = 3) (R2 = 0.83, P < 0.0001) (Figure 5). Mean larvae extracted per 100 floral heads averaged 13.61 ± 3.78 for DIY funnels and 16.54 ± 4.56 for standard Berlese funnels.
Figure 3. Linear regression analysis of a DIY funnel compared to standard Berlese funnel for estimating T. picirostrislarval abundance in commercial white clover seed fields in 2022 (n = 3 fields) and 2023 (n = 3 fields).
Research outcomes
This study is the first documentation of CSW insecticide resistance development. Based on this research finding, we recommend stopping further reliance on pyrethroid-based insecticides in the CSW management program.
Our findings confirmed the use of bifenthrin should be discontinued. After confirming high insecticide resistance, white clover growers are advised to stop using bifenthrin insecticides and incorporate two-application timing management strategies to target adults and larval stages with contact and systemic insecticides, respectively. We recommend applying a contact insecticide at pre-bloom (Malathion) and systemic insecticide (Vantacor) at full-bloom for season-long protection and to prevent seed yield loss. Other insecticide resistance management (IRM) strategies include following economic threshold levels, following recommended label rates, spray volume, and adjuvants as per the manufacturer’s recommended guidelines to improve coverage. We demonstrated the potential of new modes of action to include in a spray rotation program and to develop a sustainable IRM program for this pest. I supported a Specialty Local Needs application for Steward (indoxacarb), which the ODA is currently reviewing. I also got funding approved by the IR-4 program to evaluate indoxacarb efficacy during the 2024 growing season. The impact of the recent research and Extension work was shown in the difference between respondents past management and their plans for future management. Past management has relied heavily on bifenthrin products, but nearly two-thirds (64.2%) of respondents plan to stop using bifenthrin for clover seed weevil control completely. All of the remaining respondents planned to adjust their management by increasing the use of other products, and none of the respondents planned to continue using bifenthrin as before.
Education and Outreach
Participation summary:
1)Improving current knowledge of clover seed growers and crop advisors on CSW biology and behavior to better time insecticide applications. We provided informal consultations, and oral presentations during grower and extension events to enhance grower/ other industry partners' knowledge about the insect life cycle and develop a management plan to incorporate new mode(s) of action. Elevated resistance to bifenthrin was identified, and we reported the research findings via grower meeting talks and popular press articles for industry-wide dissemination.
2) Facilitating the incorporation of insecticide resistance management strategies in clover seed production systems through training and
workshops.
In collaborating with the Oregon Clover Commission, results were presented at four grower meetings and one OSU Extension-led workshop. Two extension seed production reports were made available during the project duration. These provide growers with yearly progress on the project. One peer-reviewed article was published in the Journal of Economic Entomology in 2024, and the second is in the review.
3) Gather input from growers and local crop advisors about their perceptions of alternative methods included in the integrated management plan (e.g.,
host plant resistance, cultural control, and biocontrol). One online questionnaire was developed and distributed to collect baseline data about growers' current practices and perceptions of alternative methods of the integrated management plan (IPM). Data from this survey was disseminated during a coffee hour presentation.
All our extension events and grower meetings were well attended (>50 audiences representing growers, crop advisors, and other stakeholders at all events). Project evaluations were done during the Oregon clover growers annual meeting on Feb 1, 2023 and Jan 31, 2024. The results are attached and a positive feedback was received from the audience who attended this event.
WSARE project evaluation year 1
Baseline data from the industry on spray records for CSW management and insecticide use patterns revealed that over-reliance on bifenthrin-based products, low spray volumes, and the poor timing of insecticide application to be an industry-wide issue that may have caused poor control in the first place leading to resistance problem. We discussed these survey results with our audience during a coffee hour presenation and recommendation were made to incoporate insecticide resistance management strageies including targetting weak links in insect life cycle (selective chemistries for larval control), and rotating mode (s) of action in CSW management plans.
Education and Outreach Outcomes
Our research and educational activities effectively engaged the growers and other stakeholders from the white clover seed industry. The insecticide trial was performed at two grower cooperators' field sites. Eight different grower cooperators were involved in recruiting fields for CSW collections and insecticide resistance screening. All producers indicated that they would continuously participate in this study and will likely use some aspects of this project. Overall positive feedback was received during the project evaluation survey (n=23)
Adoption of new chemistries, Using diverse mode(s) of action, Networking, and peer-to-peer teaching among producers,