Final report for SW19-903
Project Information
Pests and diseases of pollinator-dependent crops can lead to damage or total crop loss, particularly if left untreated at or close to bloom. But bloom is also the time that beekeepers move honey bee colonies to pollinating these crops, leading to the prospect of the colonies being exposed to pesticides. The challenge of maintaining high crop yield without impact honey bee colony health is a challenge across the US and this prompted the Environmental Protection Agency to encourage state's to develop voluntary Managed Pollinator Protection Plans (MP3s) to better coordinate the activity of pesticide applicators and beekeepers. Although MP3s exist in most states, they can struggle to have impacts on pollinator health because they: (a) are based on best management practices (BMPs) may be too general to be meaningful to local growers and beekeepers; (b) are poorly articulated with extension activities, thereby being largely left to stakeholders to voluntarily adopt with little support; (c) lack metrics to measure progress towards reduced exposure of bees; and, (d) lack important tools that growers use to mitigate pesticide exposure around bees, particularly well-specified pesticide residual toxicity times. These shortcomings weaken the capacity of the MP3s to bring about improved bee stewardship.
Our proposal directly addresses these challenges by developing pilot Bee Protection Protocols for two high value seed crops in Oregon. The Protocols provide crop-specific guidance on how to manage pests in ways that minimize pesticide exposure to honey bees. The Protocols will be built around new data, generated through this project, on residual toxicity of pesticides used in these crops under different environmental conditions. Moreover, the Protocols will be shored up through extensive Extension and training activities that include evaluation methods that will track knowledge and adoption of the Protocols. Finally, through surveys of commercial beekeepers in Oregon we will be able to measure the impact of the Protocols towards increasing communication with growers and crop consultants and decreasing pesticide exposure to their bees.
- Residual Toxicity – Sources of Variation. We would determine the sources of variation in the current assay for residual toxicity and determine the protocol that most reflects field conditions by 2020. These results would be used by growers, crop consultants, beekeepers and regulators to identify the types of pesticides whose residual toxicity values would be vulnerable to change according to environmental conditions.
- Residual Toxicity Estimates. We would establish 3h, 6h, 8h, 1d and 5d residual toxicity values for all widely used fungicide and insecticide treatments applied at bloom, as well as common tank mixes by 2021. These values would enable growers, crop consultants, beekeepers and regulators in making pesticide selections that minimize impacts to honey bees.
- Bee Protection Protocols. We would expand on an existing vegetable seed protocol by 2021 and create a new protocol for the Oregon clover seed industry by 2020. These Protocols would increase levels of communication among growers, crop consultants and beekeepers around pesticide use during pollination and reduce pesticide exposure of bees.
- Extension and Training. We would deliver two 60-minute face-to-face trainings on the Bee Protection Protocols to the seed growers and beekeepers, and two day-long workshops covering advanced topics in bee stewardship on farms in 2019 and 2020. We would also provide two YouTube videos and four podcasts that show case growers who have achieved excellence in stewardship by 2021. Finally, we would produce two new Extension publications, revise an existing publication and produce two infographic postcards. This training would translate into reduced pesticide exposure of bees in vegetable and clover seed.
- Measuring Increased Communication and Reduced Pesticide Exposure. We would implement grower and beekeeper surveys to assess the effectiveness of the protocols to improve communication and reduce exposure.
Cooperators
- - Technical Advisor (Educator and Researcher)
- - Technical Advisor (Educator and Researcher)
- - Technical Advisor (Educator and Researcher)
- (Educator and Researcher)
- (Educator and Researcher)
- - Producer
- - Producer
- - Producer
- - Producer (Educator)
- - Producer (Educator)
- - Producer
Research
- Residual Toxicity – Sources of Variation. We would determine the sources of variation in the current assay for residual toxicity and determine the protocol that most reflects field conditions by 2020. These results would be used by growers, crop consultants, beekeepers and regulators to identify the types of pesticides whose residual toxicity values would be vulnerable to change according to environmental conditions.
- Residual Toxicity Estimates. We would establish 3h, 6h, 8h, 1d and 5d residual toxicity values for all widely used fungicide and insecticide treatments applied at bloom, as well as common tank mixes by 2021. These values would enable growers, crop consultants, beekeepers and regulators in making pesticide selections that minimize impacts to honey bees.
- Bee Protection Protocols. We would expand on an existing vegetable seed protocol by 2021 and create a new protocol for the Oregon clover seed industry by 2020. These Protocols would increase levels of communication among growers, crop consultants and beekeepers around pesticide use during pollination and reduce pesticide exposure of bees.
- Extension and Training. We would deliver two 60-minute face-to-face trainings on the Bee Protection Protocols to the seed growers and beekeepers, and two day-long workshops covering advanced topics in bee stewardship on farms in 2019 and 2020. We would also provide two YouTube videos and four podcasts that show case growers who have achieved excellence in stewardship by 2021. Finally, we would produce two new Extension publications, revise an existing publication and produce two infographic postcards. This training would translate into reduced pesticide exposure of bees in vegetable and clover seed.
- Measuring Increased Communication and Reduced Pesticide Exposure. We would implement grower and beekeeper surveys to assess the effectiveness of the protocols to improve communication and reduce exposure.
Objective 1: A key measure of the breakdown of pesticide residues to bees on flowers and leaf material is RT25, which is the time needed to reduce the activity of the test substance and bring bee mortality down to 25 percent (25%) in cage test exposures to field-weathered spray deposits. Pesticide registrants are required to estimate residual toxicity by calculating RT25 through protocols described in the following guidance document “Honey Bee Toxicity of Residues on Foliage” (OCSPP 850.3030).
Our originally proposed approach to investigating the variability of RT25 values had to be altered owing to poor clover and alfalfa establishment, which was compound by covid-19-related disruptions. Since we were unable to directly compare residual toxicity values among different crops, we took an alternative approach of comparing residual toxicity values of the most widely used bloom-time insecticide, bifenthrin (using the formulated product Brigade 2EC), across multiple crops and across four different years. An additional reason for selecting bifenthrin was that it was excluded by EPA in its publication of RT25 values owing to concerns over variability in test results provided by registrants. The combination of being the most common bloom insecticide spray across all bee-pollinated seed crops in western Oregon and EPA concerns of variability in residual toxicity test results rendered it an ideal candidate insecticide for our experiment.
We treated plots (ranging from 1m2 - 40m2 in size, with at least a 1m2 buffer among plots) of radish, red clover or white clover in Oregon's Willamette Valley at either the pre-bloom or full bloom phase with the highest label rate of Brigade 2EC (6.4 oz/acre) in the months of June-July in 2019-2021. In all trials untreated controls involved spraying plots with water. Treatments were replicated across 3-4 plots and randomly assigned to plots. All treatments were applied using a CO2 pressurized backpack sprayer equipped with either a two or four-nozzle boom calibrated to deliver 20 GPA through TeeJet XR11002VS nozzles at 25 psi. Residues of leaves and/or flowers were harvested at 3, 6, 8, 24 and/or 96h, packed on ice packs and returned immediately to the lab where they were placed in cages with approximately 25 adult honey bees incubated at 30°C for 24h, after which the number of dead bees are tallied (as outlined EPA guidance document OCSPP 850.3030). Honey bees used in the cages were collected from frames of open brood the day before and fed continuously with a 50% sucrose solution using a gravity feeder.
We hypothesized that if environmental or crop factors were a significant source of variation, that RT25 values would vary by crop and across years. Since the number of hours associated with RT25 is not as important to growers as whether or not the RT25 value spans the course of an evening (i.e., whether a spray at bloom time applied in the evening will dissipate over the course of an evening ~ 8h) we investigated whether the extended residual toxicity determination (i.e., a RT25 value of greater than 8 hours; Office of Chemical Safety and Pollution Prevention [OCSPP], 2012) varied across crop or year.
In, addition, following discussion with our Advisory Committee we addressed a second hypothesis, namely whether the extended residual toxicity for insecticides sprayed at bloom varied between leaves (the test material specified in OCSPP 850.3030) and flowers (i.e., the part of the plant bees are most likely to contact during bloom). If there was extended residual toxicity for an insecticide on leaves but not flowers, this might suggest these products may be safer to bees than previously expected. We conducted these experiments on using replicated plots (three replicates per treatment - plot size 40m2) in a commercial white clover field in 2020 and 2021. We evaluated RT25 on flowers versus leaves for an insecticide treatment applied at dusk for the control of white clover weevil (Tychius picirostris). We compared the highest label rates for Malathion 57% EC (malathion - 20 oz/acre), Warrior II (lambda cyhalothrin - 3.8 oz/acre) and Brigade 2EC (bifenthrin - 6.4 oz/acre). We also included a number of insecticides being considered for white clover weevil, namely, in 2021 Avaunt eVo (active ingredient indoxacarb - 6 oz/acre), Besiege (lambda cyhalothrin + chlorantraniliprole - 10 oz/acre), Exirel (cyantraniliprole - 20.5 oz/acre) and Harvanta 50SL (cyclapryn - 16.4 oz/acre). Based on the results from 2022, we looked more closely at products containing the active ingredient lambda cyhalothrin, Warrior II and Besiege (lambda cyhalothrin + chlorantraniliprole). We also evaluated a third product that contained chlorantraniliprole but a different pyrethroid than lambda cyhalothrin, namely Elevest (bifenthrin + chlorantraniliprole - 9.6 oz/acre). Finally, we consider aproduct only containing chlorantraniliprole without a pyrethroid (Vantacor - chlorantraniliprole - 20.5 oz/acre). We hypothesized that there should be no difference between the percent mortality of bees exposed to residues of leaves versus flowers allowed to weather to products for the same period.
Objective 2: We estimated RT25 for major insecticides used in vegetable seed and clover seed crops. These were bifenthin (Brigade 2EC - 6.4 oz/acre) and cyantraniliprole (Exirel - 20.5 oz/acre) for radish seed (2019, 2020), malathion, lambda cyhalothrin and bifenthrin for white clover seed (2021, 2022 - see rates listed in Objective 1) and malathion (Malathion 57% EC - 20 oz/acre), bifenthrin (Brigade 2EC - 6.4 oz/acre), afidopyropen (Sefina - 6 oz/acre), flonicamid (Beleaf 50SG - 2.8 oz/acre) and flupyradifurone (Sivanto Prime - 10.5 oz/acre) for red clover seed (2021 and 2022). Plot, spray application and residual toxicity cage assays were as described in Objective 1.
Treated foliage were typically harvested at 3, 6, 24 and 96 h post-application. In addition, after recognizing that many of the newer insecticide chemistries had little to no residual toxicity, we investigated whether the application of these products in the evening during full bloom resulted in the insecticides being expressed in the nectar and pollen. This work was encouraged by our Advisory Committee and went beyond the experiments that were originally proposed. We used three methods to ascertain insecticide levels in flowers. First, in white clover (2021) we placed four honey bee colonies adjacent to the plots and installed pollen traps to capture all incoming pollen. Pollen was trapped the day before treatment, 24h after treatment and 48h after treatment, frozen at -20C before being shipped to for pesticide analysis (Synergistic Pesticide, Portland, Oregon). Pesticide clean up used the quick, easy, cheap, effective, rugged, and safe (QuEChERS) procedure and dispersive solid phase extraction (dSPE) and pesticides were detected using an LC-MS/MS method that was validated using standard solutions spiked into control samples, sample blanks, and Quality Control (QC) samples. Since honey bees are less common in red clover fields, we analyzed foraging bumble bees for insecticide residues. This involved either grinding up the bodies of bumble bees found foraging on treated plots for chemical analysis (2021) or analyzing the bodies and nectar they collected separately (2022). To expel the nectar from foraging bumble bees in our 2022 experiments, we applied pressure to the abdomens of cooled bees returned from the field, which forced them to regurgitate nectar from their crops.
We did not work with tank mixes as originally proposed as it became clear that growers of these crops do not tank mix insecticides with fungicides. None of the fungicides were tested because none of the products were acutely toxic to bees.
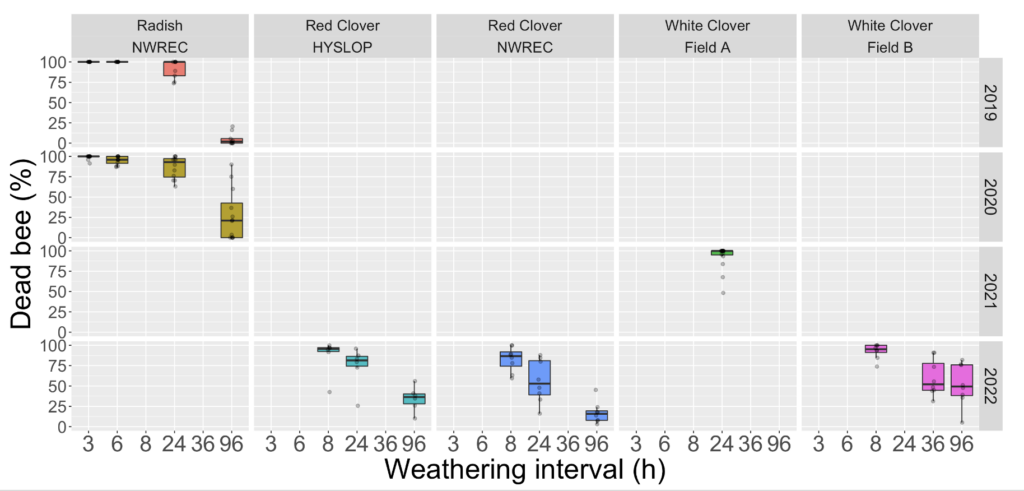
Objective 1 and 2. We evaluated the bifenthrin product Brigade 2EC in six trials across three different crops and four separate years. In all cases the RT25 value exceeded 8h, indicating that the product exhibited extended residual toxicity, meaning in every case bees exposed to treated leaf material weathered for less than 8h resulted in greater than 25% mortality (Figure 1). In fact, despite being applied to different crops and on different years and under different weathering conditions the RT25 for all situations exceeded 24h. This suggests that when it comes to the practices of vegetable and clover seed growers in Western Oregon, that variability associated with different crops or weathering conditions did not effect the extended residual toxicity of the pesticide. In turn, it confirmed that the insecticide should not be used at bloom time (which surveys from 2019 indicated was the most common practice used by white clover seed growers in managing white clover weevil during bloom - with 82% of respondents indicating this was their first choice of managing this pest).
Fig 1. Box and whisker plot of the mortality of honey bees exposed to leaf material from plots treated with the insecticide bifenthrin harvested at different field weathering intervals, across four different years, three different seed crops (radish, red clover and white clover) and four different fields (NWREC, Hyslop, Field A and Field B). The horizontal line signifies the median mortality, boundaries the 25 and 75th percentiles and whiskers the most extreme data point that is no more than 1.5 times the length of the box. Mortality among bees exposed to untreated plots sprayed with water in all trials (not shown) ranged from 1-10%.
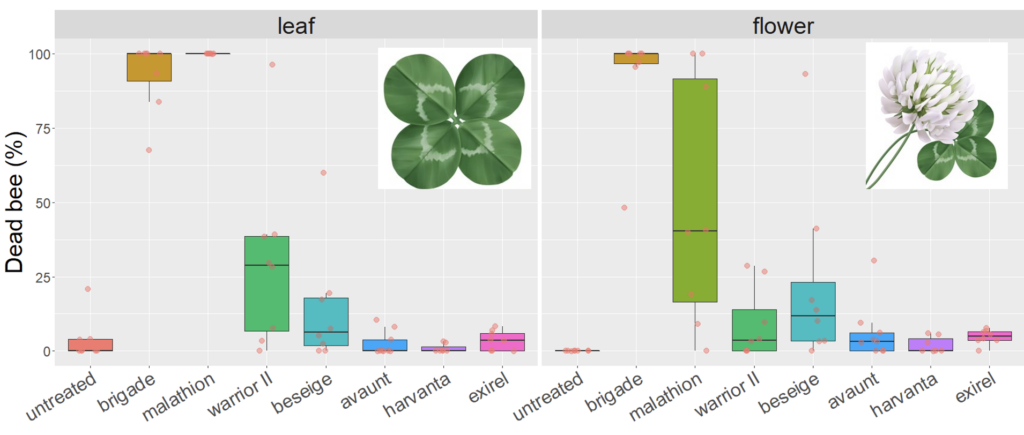
We expected the residual toxicity on leaves to match that on flowers in our white clover trials. While we observed very similar mortality of bees on leaf and flower in our first trial in 2020, there was a pronounced difference for Malathion 57% EC with complete mortality of bees exposed to leaf material weathered for 8h, compared to 40% on flowers (Figure 2). A less pronounced difference was observed for Warrior II (containing the active ingredient lambda cyhalothrin), where, again, mortality was higher for leaves compared to flowers. Other products were below 25% mortality after 8h weathering, regardless of tissue (i.e., leaf or flower), including a second product containing lambda cyhalothrin (Besiege).
Fig 2. Box and whisker plot of the mortality of honey bees exposed to leaf material from plots 8h after plots of white clover were treated with seven different insecticides or a water-sprayed control (2020). The horizontal line signifies the median mortality, boundaries the 25 and 75th percentiles and whiskers the most extreme data point that is no more than 1.5 times the length of the box.
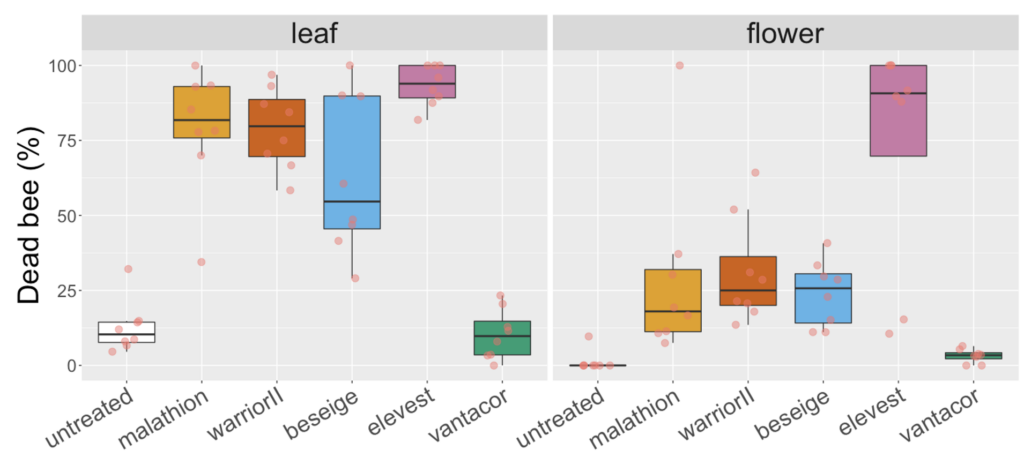
A variation on the white clover experiment in 2021 yield more pronounced differences between malathion and lambda cyhalothrin products on leaves versus flowers. While all three products containing one of these ingredients had mortality above 50% when bees were exposed to treated leaf that had been weathered for 8h, mortality was at or below 25% when exposed to flower material (Figure 3). A similar effect was not observed for products containing a different pyrethoid than lambda cyhalothrin namely, Elevest, which is analogous to Besiege in that it contains chlorantraniliprole, but bifenthrin instead of lambda cyhalothrin. There was also similar mortality for bees exposed to leaf and flower material treated with a product only containing chlorantraniliprole (i.e., Vantacor). The results across the two years suggest the residual time on flowers may be shorter than on leaf material, which is the tissue specified by EPA's OCSPP 850.3030, and that these differences appear to be consistent for Malathion 57% EC and, potentially, for products containing the ingredient lambda cyhalothrin.
Fig 3. Box and whisker plot of the mortality of honey bees exposed to leaf material from plots 8h after plots of white clover were treated with hive different insecticides or a water-sprayed control (2021). The horizontal line signifies the median mortality, boundaries the 25 and 75th percentiles and whiskers the most extreme data point that is no more than 1.5 times the length of the box.
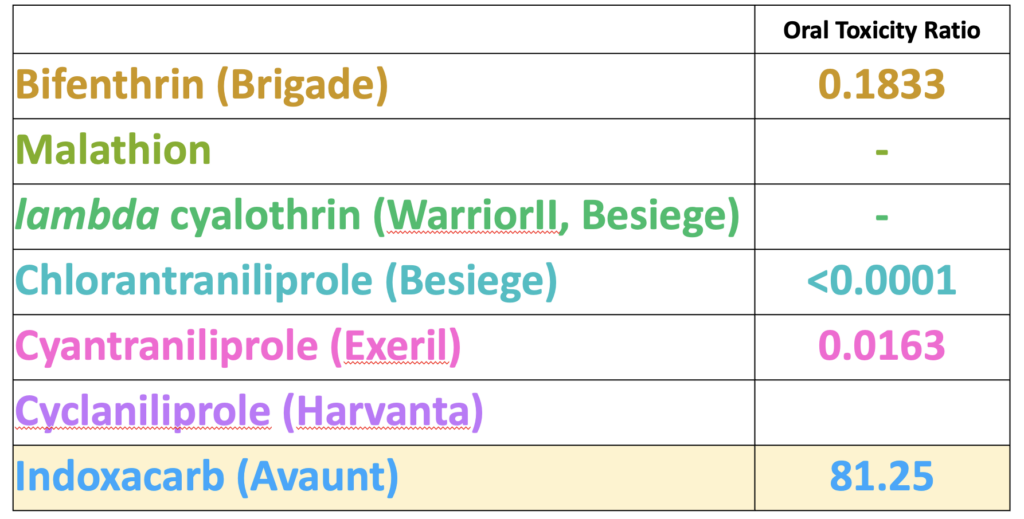
As a number of the insecticides evaluated on white clover in 2020 demonstrated less than 25% toxicity to bees after weathering overnight (Figure 2) we were interested to see if the insecticide was present in pollen collected by bees foraging on the treatment plots. Only one of the insecticides was detected in the pre-treatment period (collected from 0-24h prior to treatments), namely bifenthrin, which is widely used by growers and likely applied to adjacent fields prior to our experiment beginning (Table 1). The day after treatment (24h), we detected pollen containing all the insecticide active ingredients, with the exception of lambda cyhalothrin and cyclaniliprole, with malathion falling below detection limits by 48h. When comparing levels in pollen against the oral LD50 values for honey bees available through EPA's ECOTOX database, we discovered that levels of indoxicarb at 48h were 81 times that the LD50, whereas other residues were well below LD50 values (Table 2). These results suggest may be an unsuitable product to control white clover weevil during bloom from the perspective of bee health, whereas products such as Exeril and Harvanta appear to show promise.
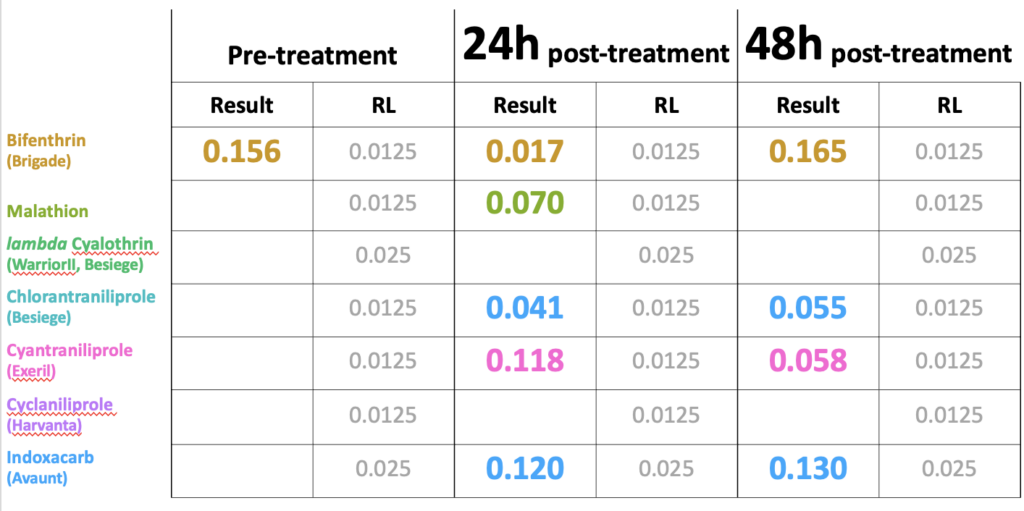
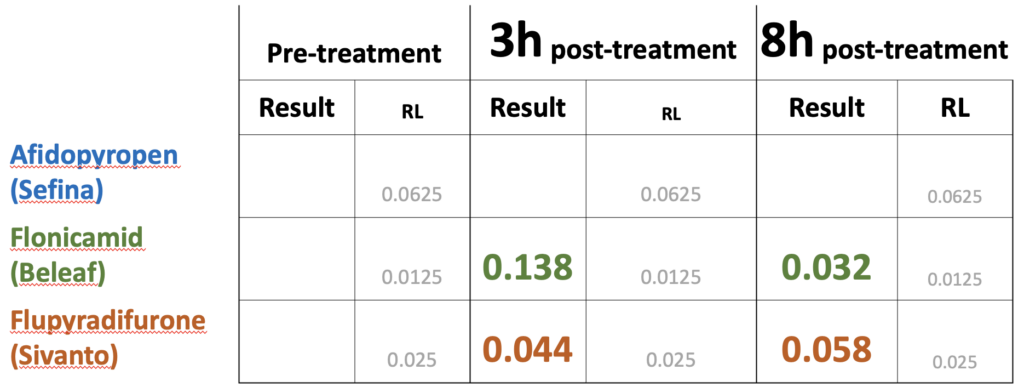
Table 1. Concentrations of insecticide residues (ppm) found in trapped pollen samples from colonies directly adjacent to plots treated with seven insecticide products (2020, see Figure 2). The concentrations ('Results') were compared to detection levels ('RL') for three time intervals: pre-treatment (0-24h before treatment), 24h post-treatment (0-24h after treatment) and 48h post-treatment (24-48h post-treatment).
Table 2. The ratio of insecticide concentration detected in pollen relative to the oral LD50 of reported by EPA ECOTOX database. Pollen was trapped from colonies directly adjacent to plots treated with seven insecticide products (2020, see Figure 2).
Two years of experiments on red clover (2020 and 2021) revealed that all products identified by our Advisory Committee for aphid control during bloom had RT25 values less than 8h (data not shown). We did not find evidence that these products translocated into nectar that bees were feeding on. We detected residues of these insecticides on bumble bees foraging on the treated plots at 3 and 8h after treatment for two of three insecticides (Table 3). These levels were well below the oral LD50 values to honey bees (Table 4). A repeat of the experiment in 2020 yielded similar findings. In this experiment the nectar contained in bumble bees was forcefully regurgitated after collection, allowing us to determine whether insecticide residues were more concentrated in nectar as compared to the bodies of the bumble bees. We found no evidence of concentration (Table 5).
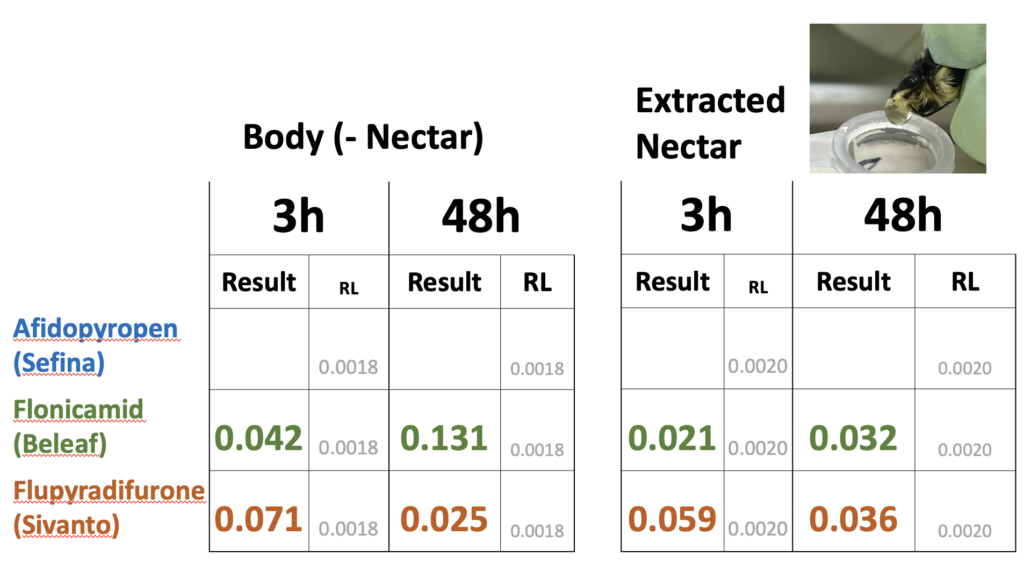
Table 3. Concentrations of insecticide residues (ppm) found in whole foraging bumble bees foraging on plots treated with three insecticides in red clover (2020). The concentrations ('Results') were compared to detection levels ('RL') for three time intervals: pre-treatment (immediately prior to treatment), 3h post-treatment and 8h post-treatment. Bees were caught equally from all treated plots and pooled for analysis.
Table 4. The ratio of insecticide concentration detected in whole foraging bumble bees 24h after treatment (2020) relative to the oral LD50 of reported by EPA ECOTOX database. Bees were caught equally from all treated plots and pooled for analysis.
Table 5. Concentrations of insecticide residues (ppm) found in whole foraging bumble bees where nectar had been removed ('Body (-Nectar)') compared to nectar ('Extract Nectar') at two periods after treatment. No detectable residues detected in bumble bees collected immediately prior to treatment. The concentrations ('Results') were compared to detection levels ('RL') for two time intervals: 3h post-treatment and 48h post-treatment. Bees were caught equally from all treated plots and pooled for analysis.
Similar to studies in white clover, we confirmed RT25 values for cyantrailiprole had an RT25 value for radish seed that was less than 3h (Figure 4). This is in contrast to the current insecticide standard (bifenthrin) which has consistent extended residual toxicity (see Objective 1) remains toxic to bees >24h after treatment. This result for cyantrailiprole was consistent across two years, suggesting little environmental variation in residual toxicity of this product. In contrast to bifenthrin, cyantrailiprole demonstrated very little residual toxicity relative to leaves harvested from plots that were not treated with insecticide. The finding with cyantrailiprole was notable, as EPA has indicated this product has a long residual toxicity (in the Directions for Use on Exeril, the cyantrailiprole product labelled for radish seed, indicate that the product cannot be applied until "flowering complete").
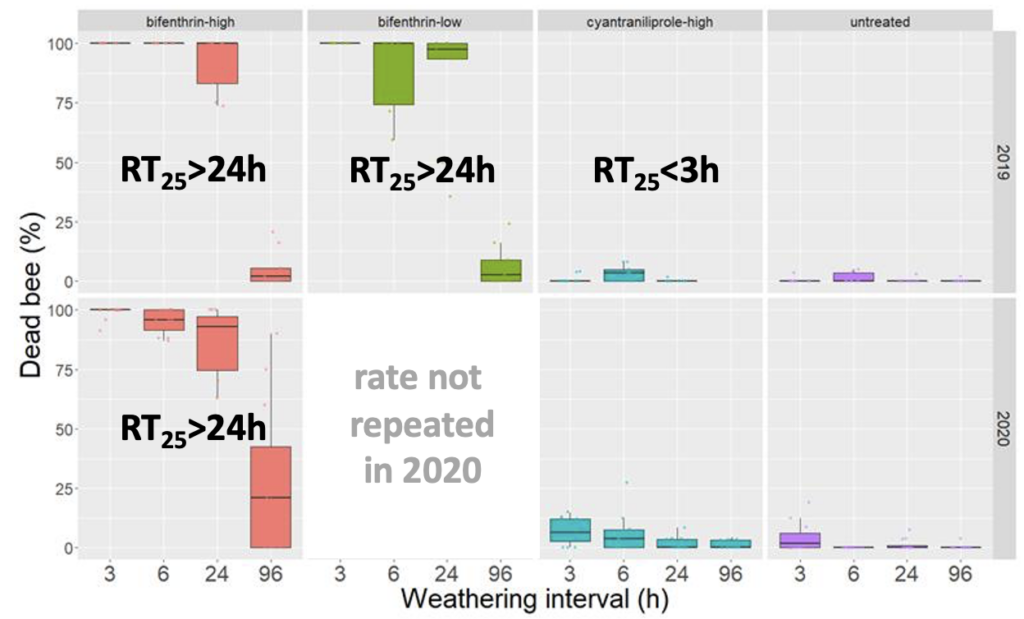
Fig 4. Box and whisker plot of the mortality of honey bees exposed to radish leaf material from plots treated with the insecticide bifenthrin (high rate - 6.4 oz/acre low rate - 2.1 oz/acre) or cyantrailiprole harvested at four weathering intervals across two years (2019 and 2020). The horizontal line signifies the median mortality, boundaries the 25 and 75th percentiles and whiskers the most extreme data point that is no more than 1.5 times the length of the box. Estimated RT25 values indicated on graphs.
Note research findings from Objectives 1 and 2 were presented to peers four times, two of which were invited. One presentation was part of a national webinar organized by the Environmental Protection Agency highlighting agricultural stewardship efforts to protect bees.
Melathopoulos, A. P., Anderson, N. P., Buckland, K., Lightle, D. M., Entomological Society of America Annual Meeting, Vancouver, British Columbia, Canada " Oregon's seed producer bee protection protocols: Dispatches from the trenches," (November 4, 2022). Invited
Melathopoulos, A. P., Anderson, N. P., Buckland, K., Lightle, D. M., Entomological Society of America Annual Meeting, Denver, Colorado, "Does persistence of toxic insecticide residues on field-weathered foliage correspond to the actual dissipation of toxic residues to honey bees (Apis mellifera L.) in the field?," (November 2, 2021).
Melathopoulos, A. P., Anderson, N. P., Buckland, K., Pacific Branch of Entomology Society of America Annual Meeting (virtual), "Getting Real: Increased Adoption of Bee-Friendly Farming through Industry-Led Bee Protection Protocols in Oregon. Symposium: Supporting Pollinators and Pollination in the PNW," Virtual. Regional, Invited. (April 6, 2021).
Melathopoulos, A. P., Agricultural Stewardship and Best Management Practices to Reduce Pollinator Risk, Environmental Protection Agency (EPA) Webinars on Pollinator Health and Habitat (webinar), “The Oregon Bee Project: Case study of best management practices in a specialty crop state,” (August 18, 2020). Invited
Objective 3. The Clover Seed Bee Protection Protocol was developed and the Vegetable Seed Bee Protection Protocol was revised following meetings involving the Extension and Research Team, the Advisory Committee, representatives from the Oregon Clover Commission, Specialty Seed Growers of Western Oregon and Oregon State Beekeepers Association and selected crop consultants. The Clover Seed Bee Protection Protocol was developed through two meetings in January 2020. The revision of the Vegetable Seed Bee Protection Protocol was completed through a single meeting in February 2020. We used electronic clickers at the annual meetings of the Oregon Clover Commission and Specialty Seed Growers of Western Oregon in winter 2020 to evaluate the standard industry practices in order to help inform the Protocols. Card form summaries of the Protocols were provided to growers in 2020, with a more extensive guide published in 2022 (Vegetable Seed) and 2023 (Clover Seed).
Melathopoulos, A., Buckland, K. and Sagili, R. (2022). Bee Protection Protocol for Western Specialty Seed Crops. EM 9353, Corvallis, OR: OSU Extension. https://extension.oregonstate.edu/pub/em-9353
Melathopoulos, A., Sagili, R. and Anderson, N. (2023). Bee Protection Protocol for Clover Seed Crops. EM XXXX, Corvallis, OR: OSU Extension. in press.
Objective 4. A total of five trainings were provided to growers on the Bee Protection Protocols, either during the development of the protocols or after they were released. These trainings are listed below:
- Oregon Clover Commission Meeting, Wilsonville, OR, February 2, 2022, (237 participants)
- Specialty Seed Growers of Western Oregon Meeting (webinar), January 20, 2022, (23)
- Specialty Seed Growers of Western Oregon (webinar), January 19, 2021, (18)
- Oregon Clover Commission, Wilsonville, OR, February 5, 2020, (152)
- Oregon Clover Commission Annual Meeting, Wilsonville, OR, February 6, 2019, (125)
We also developed a public facing a You-Tube video on the clover seed Bee Protection Protocol distributed over 7,500 seed packs to promote the Bee Protection Protocol (Figure 5).

Fig 5. Seed packs distributed to the public to promote the Bee Protection Protocols developed during the course of the grant. Since 2020, over 7,500 seed packs have been distributed.
Through the grant we also developed over 25 podcast episodes dedicated to bee stewardship and pesticide safety, including on beekeeper grower communication systems, accessing NRCS programs to create bee habitat, certifying crops as bee friendly and adjuvants and bee health, . Finally, we developed an advanced 5 module self-paced training program in bee stewardship (Figure 6) which is now available online through Oregon State University Extension: https://extension.oregonstate.edu/bee-steward
Fig. 6. A self-paced online training on bee stewardship was developed to provide growers additional training on how to manage habitat on farm headland to support bee populations.
Research Outcomes
The research suggest the relatively robust character of RT25 values for the key insecticide used in Western Oregon vegetable and clover seed cropping systems at bloom (bifenthrin) under a range of different weathering conditions. This finding challenges assertions from EPA that the RT25 for bifenthrin is too variable to list in its public-facing database. Regardless of the crop or year, we found that bifenthrin always exhibits extended residual toxicity (i.e., RT25 >8h), and as such, cannot be used by growers during bloom without resulting in toxic residues on leaf material. Since most white clover seed growers reply on bifenthrin to control white clover weevil at bloom, our findings suggest an urgent need to register a less toxic and less residual products to control this pest to avoid poisonings of pollinating bees. Fortunately, a number of new products evaluated in our white clover trials in 2020 and 2021 suggest insecticides with fewer negative effects to bees are available. The only new active ingredient the showed a cause for concern was the product Avaunt (active ingredient indoxicarb). While the product had a relatively short residual toxicity, it appeared in pollen at 81 times the LD50 up to 48h after treatment. Fortunately, none of the other newer products for white clover, nor products registered for the control of aphids in red clover, appeared at elevated levels in nectar or pollen. Nevertheless, future research should look more closely at new insecticides with <8h RT25 values to confirm that the pesticide is not being expressed in nectar or pollen.
While our work on white clover suggests bifenthrin also remains toxic on flowers after 8h of weather, we observed a different pattern for two widely used insecticides, namely malathion and lambda cyhalothrin. Both products appeared to have extended residual toxicity on leaf material, but not on flower material. Differences in residual toxicity between leaf and flower has not been previously reported. Since we did not observe a similar pattern for bifenthrin, it is possible that lower residual times on flowers compared to leaf may be specific to active ingredients. This observation is potentially significant for growers, since EPA does not require RT25 data generated from flowers, only leaf material. Consequently, there may be products that dissipate over the course of an evening on flowers that are currently labelled as having extended residual toxicity to bees. These products may ultimately be less damaging to colony health, particularly when the only option to growers at bloom are products with extended residual toxicity. More research should be conducted to validate our finding and see if it is specific to malathion and lambda cyhalothrin or extends to other insecticides.
The research for associated with this project has been presented to peers four times, two of which were invited. One presentation was part of a national webinar organized by the Environmental Protection Agency highlighting agricultural stewardship efforts to protect bees.
Melathopoulos, A. P., Anderson, N. P., Buckland, K., Lightle, D. M., Entomological Society of America Annual Meeting, Vancouver, British Columbia, Canada " Oregon's seed producer bee protection protocols: Dispatches from the trenches," (November 4, 2022). Invited
Melathopoulos, A. P., Anderson, N. P., Buckland, K., Lightle, D. M., Entomological Society of America Annual Meeting, Denver, Colorado, "Does persistence of toxic insecticide residues on field-weathered foliage correspond to the actual dissipation of toxic residues to honey bees (Apis mellifera L.) in the field?," (November 2, 2021).
Melathopoulos, A. P., Anderson, N. P., Buckland, K., Pacific Branch of Entomology Society of America Annual Meeting (virtual), "Getting Real: Increased Adoption of Bee-Friendly Farming through Industry-Led Bee Protection Protocols in Oregon. Symposium: Supporting Pollinators and Pollination in the PNW," Virtual. Regional, Invited. (April 6, 2021).
Melathopoulos, A. P., Agricultural Stewardship and Best Management Practices to Reduce Pollinator Risk, Environmental Protection Agency (EPA) Webinars on Pollinator Health and Habitat (webinar), “The Oregon Bee Project: Case study of best management practices in a specialty crop state,” (August 18, 2020). Invited
Education and Outreach
Participation Summary:
We surveyed growers from both the clover and vegetable seed industries at their annual meetings in 2022 using electronic clicker questions. Questions focused key features of the Bee Protection Protocols that growers planned to adopt, in addition to questions included in the Western SARE survey and evaluation tool.
1. Survey results from Oregon Clover Commission Meeting, Wilsonville, OR, February 2, 2022, (237 participants)
When growers were asked "how many people do you estimate you will share some aspect of this project within the next 12 months?" clover seed growers indicated, on average, would share the project with 3.9 additional growers. Moreover, as survey results below indicate, all growers indicated that the project "improved average awareness" of bee health and the large majority indicated the project had changed their attitudes to bee health and that they were likely to "adopt one or more of the practices" outlined in the Bee Protection Protocol.
| Question | n | Yes | No | N/A |
| This project improved my average awareness of the topics covered | 61 | 97 | 0 | 3 |
| This project modified my options and/or attitudes | 76 | 71 | 13 | 16 |
| In the next year I am likely to adopt one or more of the practices shown | 91 | 66 | 8 | 26 |
When asked specifically, which practices the growers adopted from the project the majority indicated they were now applying their bloom sprays in the evening. Notably, few growers were encouraged to develop contracts with their beekeepers.
| "Bee Protection Protocol practice I adopted on my farm because of this project" | n | % respondents |
| I apply bloom sprays in the evening | 38 | 51% |
| I pay closer attention to colony strength | 32 | 43% |
| I think more about colony placement | 26 | 35% |
| I time my clean-up spray at least 4 days before bees arrive | 26 | 35% |
| I check in with my beekeeper at least 10 days before I need bees | 19 | 25% |
| I am more mindful of my beekeeper's access to fields | 19 | 25% |
| I call my beekeeper around 50% bloom to discuss when to move bees out | 16 | 21% |
| I use a pollination contract | 11 | 15% |
2. Survey results from Specialty Seed Growers of Western Oregon Meeting (webinar), January 20, 2022, (23)
When growers were asked "how many people do you estimate you will share some aspect of this project within the next 12 months?" growers indicated, on average, would share the project with 6.3 additional growers. All responding growers indicated that the project improved their awareness of bee health issues, modified their opinions and/or attitudes and that they were "likely to adopt one or more" practices outlined in the Bee Protection Protocol.
| Question | n | Yes | No | N/A |
| This project improved my average awareness of the topics covered | 10 | 10 | 0 | 0 |
| This project modified my options and/or attitudes | 10 | 10 | 0 | 0 |
| In the next year I am likely to adopt one or more of the practices shown | 10 | 10 | 0 | 0 |
When asked specifically, which practices the growers adopted from the project, most practices had been adopted by the growers, although, like the clover seed growers, few were encouraged to develop contracts with their beekeepers.
| "Bee Protection Protocol practice I adopted on my farm because of this project" | n | % respondents |
| I pay closer attention to colony strength | 10 | 100% |
| I time my clean-up sprays at least 4 days before the bees arrive | 9 | 90% |
| I think more about colony placement | 8 | 80% |
| I apply bloom sprays in the evening | 7 | 70% |
| I check in with my beekeeper at least 10 days before I need bees | 6 | 60% |
| I am more mindful of beekeeper access to colonies | 5 | 50% |
| I call my beekeeper at around 50% bloom and discuss the move out date | 4 | 40% |
| Use a pollination contract | 1 | 10% |
Education and Outreach Outcomes
Our findings suggest that an industry-led and Extension-supported development of bee stewardship guidelines can result in high levels of industry adoption of bee-friendly farming practices. Notably, practices that were less likely to be adopted, such as using pollination contracts or communicating with the beekeeper at full bloom, involve beekeeper-grower communication during pollination. From discussions with the Advisory Committee, we believe that communication during pollination remains an area that is difficult for beekeepers and growers to allocate adequate time. Future iterations of the Bee Protection Protocols, therefore, should focus on beekeepers and growers identifying irritants during regular winter meetings (i.e., outside of the busy season and to anticipate emerging problems before they become acute). Of great concern is that white clover seed growers lack control options for of white clover weevil that do not have extended residual toxicity. Consequently, growers may need to apply insecticides in the evening that remain toxic to honey bees the following morning if weevil pressure exceeds action thresholds during bloom. The next iteration of the Bee Protection Protocol for clover seed, should involve a more concerted discussion between growers and beekeepers to ensure beekeepers have sufficient time to remove their colonies if a treatment needs to be applied.
- apply bloom sprays in the evening
- ensure colonies meet minimum strength guidelines for pollination
- place colonies away from high traffic areas, spray drift or pooling irrigation water
- apply a broad-spectrum insecticide clean-up spray at least 4 days before bees arrive
- growers should check in with their beekeeper at least 10 days before they need bees delivered
- do not block beekeeper access to colonies with equipment or locked gates
- call beekeeper around 50% bloom to discuss when to move bees out
- growers should use a pollination contract to ensure expectations for pollination are clearly laid out with the beekeeper
More growers apply their pesticide sprays in the evening during bloom.
More growers are checking the colonies delivered to ensure they are of adequate strength for pollination.
More growers are selecting locations for beekeepers to place their colonies that away from high traffic areas, spray drift or pooling irrigation water.