Progress report for SW24-004
Project Information

Washington apple growers are experimenting with releasing natural enemies purchased from commercial insectaries. Lacewings releases, targeting aphids and mealybugs, are the most common. Aphid and mealybug organic control options are limited and damage from honeydew production alone can cause $400-$4,000/acre of yield loss. ≥25% of growers are conducting releases, spending $153/acre/year. The industry purchases $1M of natural enemies per year, with demand increasing annually, especially for releases by drone. Growers’ indicate that their biggest barrier to successful adoption is lack of best practice recommendations, not cost or perceived lack of efficacy. However, current recommendations are based on greenhouse use and are not well-matched to orchards, which greatly differ in climate, acreage, crop, and target pests. Therefore, this project addresses a need for scientifically-based recommendations on how to release lacewings in apple orchards. We will determine (1a) which release methods provide the most effective aphid and mealybug control by comparing life stages (releasing eggs or larvae), available species, and methods (sprinkled or hanging egg cards). We will compare the efficacy of the two commercially available species of lacewing, Chrysoperla rufilabris and Chrysoperla plorabunda (sold as “C. carnea”). We will conduct large plot tests of drone-released lacewings to determine (1b) if they are effective and to what extent release rates need to be increased for success compared to a ground release. For the most effective release method, we will (2) identify the release rate that best balances cost and pest control. Finally, we will (3) determine if and for how long residues of organic pesticides are harmful to lacewings, allowing for better integration of chemical and biocontrol tactics. To increase grower knowledge and adoption of release best practices, we will develop a “tailgate training kit” and a natural enemy releases webpage. The bilingual “Tailgate training kit” will be designed for managers to train crews, with a large flipchart and a plain language handout summary for in-field trainings on how to perform releases, and a pocket flipbook on identification and other key information for later reference. The natural enemy releases “how-to” webpage will host summaries of research findings, best practice recommendations, and four video tutorials on how to conduct releases (ground and drone), do quality control checks of lacewings received, and scout after release. A participatory research framework will be used to increase grower-to-grower information spread and adoption. Each grower-collaborator will host a field day, where they will discuss their experiences with releasing predators and where we will demonstrate release methods and scouting, in collaboration with insectary representatives. The grant team will conduct a half-day Natural Enemy Releases session, with a grower-collaborator panel discussion. At the end of the project, we will conduct a follow-up evaluation to capture producer adoption of new practices and the impacts on their farms. Increased adoption of and success with biocontrol releases will reduce grower expense on unsuccessful releases and decrease pesticide use. This will decrease harm to non-target organisms, pesticide environmental contamination, and farmer and community exposure to residues.
Research Objectives
1. Determine which method of releasing lacewings results in the greatest establishment and pest control
a. Compare species, life stages, and cards versus loose eggs
b. Compare drone to ground releases at orchard-scale
2. Determine which lacewing release rate is most effective for aphid control
3. Determine the effects of organic pesticides on insectary-reared lacewings
a. Determine acute toxicity of organic pesticides to lacewings
b. Determine the duration that field-aged residues remain harmful
Education Objectives
1. Increase grower knowledge and adoption of natural enemy release best management practices:
80% of participants in trainings will gain new knowledge and 50% will plan to try natural enemy releases on their farms or alter how they are currently doing releases.
Activity A. Create a toolkit for growers to train their workforce on lacewing releases
Output: Toolkit
Activity B. Create a web-based resource that acts as a “one-stop shop” for information on release best practices
Output: Website
Activity C. Increase grower adoption of successful lacewing releases using a participatory research framework
Outputs: Advisory meetings, Field days, Intensive Session
2. Disseminate research results to other agricultural professionals and the general scientific community
Activity A. Scholarly outputs
Outputs: journal articles, conference presentations
Activity B. Additional grower and other agricultural professional outreach
Outputs: Fruit Matters articles, grower meeting presentations
Cooperators
- - Producer
- - Producer
- - Producer
Research
1a. Compare species, life stages, and cards versus loose eggs (Years 1-2)
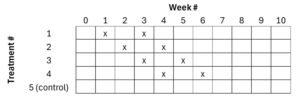
The study was conducted at three organic, commercial orchards in 2024. The experimental design was a randomized complete block design, with location in the orchard the blocking factor (pest pre-counts were too low to use). Treatments were applied once to five 0.15 or 0.25-acre replicates (plot size varied by location) per treatment, with ≥50 ft between plots. Releases occurred on 10 April (Rock Island), 19 April (Mattawa), and 26 April (Pateros). We noticed that the egg cards at the Pateros location appeared to have undergone a freeze event (dead eggs) and that initial recovery of larvae was lower than expected, so we conducted a second release at this location on 31 May. For the second release, we only re-released in the most promising treatments (#4 and #5). Treatments were monitored once weekly for six weeks, with Week 5 monitoring skipped at some locations. In Pateros, we monitored Treatments 4-6 for an extended period to observe the effects of the second release. Eggs were released at a rate of 100,000/acre and larvae at 20,000/acre. We attempted to hold eggs at room temperature prior to release to speed their rate of hatch, but this was logistically challenging as due to different release dates coordinated with the growers and different stages of maturity of the eggs when they arrived.
Treatment List:
- C. rufilabris eggs sprinkled
- C. rufilabris egg cards
- C. rufilabris larvae sprinkled
- C. plorabunda eggs sprinkled
- C. plorabunda egg cards
- No-release control
Sampling occurred on 9-12 trees in plot centers. We counted the number of infested leaves (green apple aphid, rosy apple aphid) or distinct colonies (woolly apple aphid) on 36 shoots/plot (Markó et al. 2013, Orpet et al. 2019). Mealybugs on beat trays were counted at all locations, and were also sampled with burlap traps (Grasswitz & Burts 1995) and 1-foot shoot samples (5 per plot) at the Pateros orchard, which had high mealybug pressure. We used beat tray samples (12/plot) to count released lacewings and ants and sticky cards (2/plot) for adult lacewings. Lacewings collected from beat trays were immediately placed in molecular grade ethanol and frozen.
While it is known that lacewing egg cards cannot be used in orchards with high ant populations, whether predation by other insects or cannibalism is a significant issue with the cards has not been investigated. In 2024, we used preliminary trials in the lab to fine-tune our use of field cameras and determine the best methods for filming at night. We acquired small, battery-powered infrared lights and five additional field cameras, in addition to upgrading our camera lenses to better visualize small arthropods. In 2025, using the improved camera equipment, we will monitor C. rufilabris egg cards in orchards for predation events and in the lab for cannibalism events (Brinno TLC300). We will record ten egg cards per trial for one week post-release. We will record any predators visiting the cards and visit length. Ten egg cards per lacewing species will be similarly monitored in the lab to determine if cannibalism is common.
Molecular methods. All collected lacewings (taps, sticky cards) will be sequenced using COI primers (Palomares‑Pérez et al. 2019), which we have determined can distinguish released from resident lacewings. We will also collect lacewing larvae directly from each shipping container prior to release. This provides us with a point of comparison for lacewings collected in field – the genetics of captured lacewings are compared to the genetics of the “from the bottle” lacewings. In the analysis, individuals clustering (Geneious Prime, Geneious Tree Builder) with the “from the bottle” lacewings are related and therefore from the release treatment. As of January 2025, all lacewing larvae have been surface sterilized and undergone DNA extraction, with PCR to follow on combined 2024-2025 samples.
Lacewing larvae collected from the field will also undergo molecular gut content analysis (Krey et al. 2020, Cooper et al. 2022) to determine what pests are consumed by released lacewings and whether they consume resident natural enemies. Because we have determined that our three aphid pest species do not amplify well with our usual molecular gut content protocol, we will also screen each sample using primers that we have determined amplify RAA/GAA (Chapman et al. 2010) and WAA (Orpet et al. 2019). We are also using this project as an opportunity to develop a multiplex PCR for simultaneously identifying prey consumption of multiple apple aphid pests.
Originally, we planned to repeat this trial in only one of the study orchards in 2025 and instead move to performing multi-site trials of our release rate objective (Obj. 2). After consultation with our grower collaborators, insectary industry representatives, release practitioners, and the limited prior literature of lacewing releases in other crops, and considering our results from this year (see below) and prior work, we have determined that it is more essential to focus on determining the best timing for lacewing releases. Therefore, we are submitting a project modification request to alter Obj. 2.
1b. Compare drone to ground releases at orchard-scale (Years 1-3)
This trial was conducted in five ‘Granny Smith’ blocks, which were divided into thirds, resulting in three ~4-acre plots per block. Each block third received a treatment: (1) drone release, (2) ground release, or (3) no release. The first release occurred on 10 April, C. rufilabris eggs were released at 20,000/acre. We noticed that the eggs were still very immature (green) when released and subsequently, there was no recovery of released lacewings. Therefore, we conducted a second release on 10 May, this time of 20,000 larvae/acre. The drone pilot (Weaver) also indicated that we should try a different flight pattern; our first release was directly over the orchard rows, the second release was perpendicular. We conducted sampling at six fixed “stops” even distributed across the block. At each stop, we collected four tap samples (mealybugs, lacewings, ants), counted aphid colonies on six shoots, and replaced one sticky card.
2. Determine which lacewing release timing is most effective for aphid control (Years 2-3)
Originally, this objective sought to determine the ideal rate of release rate for aphid control.
This objective has been modified to test release timing, which we have concluded is likely the most important factor for release success. This trial will occur in all three trial orchards in 2025-2026. Plot set up and sampling will be as described in Obj. 1. Here, the treatments will consist of two sequential releases of 20,000 C. rufilabris larvae per acre, spaced two weeks apart. This method of sequential release was recommended by multiple insectary contacts and Teah Smith (Zirkle, grower collaborator). Each of four timing treatments will be initiated one week after the previous and compared to a no-release control. This will determine the ideal timing for treatment of orchards with lacewing larvae, which have been the most reliable method we have tested. The first release at each location will correspond to ~500 degree days (apple bloom model, Washington State University Decision Aid System), which we have determined through our previous work is the minimum for good larvae survival rates and typically avoids lime sulfur thinning sprays.
3a. Determine acute toxicity of organic pesticides to lacewings (Year 1)
Treatment List:
- Dipel
- Cyd-X HP
- Entrust SC
- Serenade Opti
- Sonata
- Blossom Protect (+Buffer)
- Previsto
- Cueva
- Instill-O
- Rex lime sulfur
- Problad Verde
- Double Nickel LC
- OSO 5%SC
- Regalia
- Kaligreen
- OxiDate 5.0
- 440 Superior Spray Oil
- Control - water
We tested eggs of C. plorabunda and C. rufilabris via two exposure methods: direct application and on residues. In the direct application, treatments were applied as 2 mL of solution (highest labelled rate) using a Potter Spray Tower, then eggs were individually transferred to single wells in a 96-well plate. In the residue test, treatments were pipetted into the wells of each plate, allowed to sit briefly, then pipetted back up. Plates were then placed in a fume hood until residues dried. This resulted in ~96 replicates per treatment (occasionally, wells were unintentionally not loaded). We monitored egg hatch daily for five days.
Through preliminary testing, we determined that original proposed assay design observed lacewing larvae for a too short of a time period (48 h) to detect the effects of some organic pesticides. Therefore, we modified this assay design to observe the lacewings for a much longer duration (to adulthood, 3-4 weeks). This required preliminary testing of arenas and feeding methods to provide adequate humidity to allow for molting, without resulting in high quantities of mold.
In Feb-April 2025, we will the test the effects of direct contact+residues application on C. rufilabris larvae by placing them individually in plastic portion cups and spraying them with 2 mL of pesticide solution (highest labelled rate) using a Potter Spray Tower (n=30 lacewings/treatment). Lacewings will be monitored daily for mortality until pupation, with food (Ephestia eggs) and water (a small moistened piece of cotton) replaced every 2-3 days. We will also monitor pupation, adult eclosion rates, and time to development.
In separate assays using the same treatment methods, we will also monitor effects of 24 h exposure on movement and predation. Up to 10 surviving larvae from each treatment will be monitored using motion tracking software (Ethovision XT14) for 30 minutes, measuring distance travelled and time spent moving. Then, we will provide the larvae with 20 pea aphids each (from colony) and record aphids consumed in 24 h.
3b. Determine the duration that field-aged residues remain harmful (Years 2-3)
In the USDA-ARS research orchard, individual apple trees will be sprayed with pesticide solution (highest labelled rate) or water to drip using a backpack sprayer. Trees will be treated at different times so that 3, 7, and 14 day-old residues of each pesticide are simultaneously available as needed. We will make 3.8 cm leaf disk arenas, on which a C. rufilabris larvae or egg (both species) will be placed (30 individuals per treatment×residue-age combination). Larval mortality and egg hatch will be assessed as described above.
Cost Comparison. In Obj. 1-2, we will also perform a cost comparison for any treatment found to decrease pest abundance relative to the control. Cost estimates will include lacewing purchase, shipping, and labor/drone pilot fees (estimate provided by the grower). Cost will be scaled relative to pest control (percent reduction/dollar spent).
Statistical analysis: Obj. 1-2. For arthropod counts, statistical differences were compared using a generalized linear mixed model (GLMM), with treatment as a fixed effect and replicate and date (repeated measures, with spatial power covariance structure) as a random effects, specifying the negative binomial distribution (count data). For video data, Wilcoxon rank-sum tests will be used to compare number and length of visits between natural enemy groups.
Statistical analysis: Obj. 3. We analyzed data using GLMMs, specifying the most appropriate distribution based on data visualization/testing.
Citations
Chapman, E. G. et al. 2010. Molecular Ecology Resources 10: 1023-1033.
Cooper, W. R. et al. 2022. Ann. Entomol. Soc. Am. 115: 275-284.
Grasswitz, T. R. & E. C. Burts. 1995. Entomophaga 40: 105-117.
Krey, K. L. et al. 2020. Environ Entomol 49: 1300-1306.
Markó, V. et al. 2013. Biocontrol Sci. Technol. 23: 126-145.
Orpet, R. J. et al. 2019. Biol. Control 132: 189-198.
Palomares‑Pérez, M. et al. 2019. Mol. Biol. Rep. 46: 6577–6583.
1a. Compare species, life stages, and cards versus loose eggs
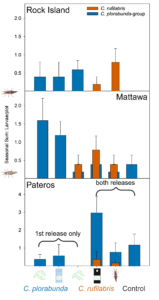
We are currently conducting the molecular work to determine which of the C. plorabunda-group larvae collected from our trials are released versus resident individuals. Comparing the number of C. plorabunda-group larvae in treatments where they were not released as a preliminary method for separating released versus resident indicates that in Pateros, most of the captured C. plorabunda-group are from releases (Fig. 1). In Mattawa, we estimate that ~0.5 larvae/plot were resident and that the rest of the C. plorabunda-group were from releases. In Rock Island, the same number of C. plorabunda-group larvae were found in a treatment where they were not released (C. rufilabris eggs) as the two C. plorabunda release treatments; it is therefore possible that all of the individuals found in C. plorabunda release plots were actually resident lacewings.
Because the egg/egg card treatments were held for variable amounts of time after shipment, each trial differed in the relative recovery rate of these treatments relative to the C. rufilabris larvae treatment (Fig. 1). When the egg treatments could be held until substantial hatching was observed (Mattawa trial), recovery of loose eggs was the same as recovery of larvae. However, when releases needed to be conducted soon after receiving the shipment (Rock Island), more larvae were recovered from the larvae release treatment compared to the loose egg and egg card treatments. Across all of our prior projects, larvae and egg card releases have had the highest recovery rates (Table 1), potentially indicating better survival and delivery to the target. This is supported by recently completed gut content work from our previous studies (2021-2023), where larvae from egg treatments were less likely to test positive for woolly apple aphid DNA (25%) compared to those collected from egg card (73%) or larvae (55%) treatments (Schmidt-Jeffris et al., in review). For consistency between trials in future work, we will only use C. rufilabris larvae. However, we will also begin to investigate the best methods for growers to order eggs and convert them to equivalent rates of larvae, to reduce cost.
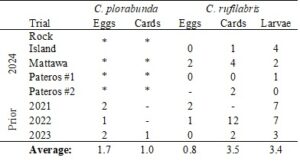
We did not observe any differences in pest counts between treatments in the Rock Island or Mattawa trials. Initially, it appeared that spring temperatures were warming up faster than average, so we conducted mid-April releases at these locations. However, the weather returned to cooler, wetter conditions during and post-release. We speculate that releases were conducted too early, which is supported by lower lacewing larvae recovery rates in this trial compared to previous years. Additionally, at the Rock Island location, a spinosad application for thrips control made on 25 April was associated with reduced counts of both resident and released lacewings in the following weeks (data not shown).
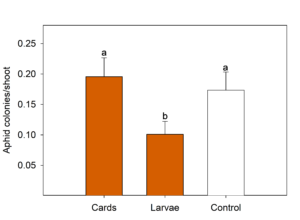
In Pateros, seasonal means of both aphids (Fig. 2) and mealybugs (Fig. 3) differed between treatments in the four-week period after the second release. For this analysis, we only compared the two treatments that were released twice (C. rufilabris egg cards and larvae) and the control. C. rufilabris released as larvae had fewer aphid colonies per shoot than the egg card or the control treatments (Fig. 2).
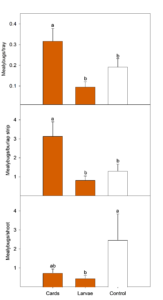
There were also significantly fewer mealybugs per shoot in the larvae release treatment (Fig. 3); this was also observed as a numerical trend via the other two sampling methods (burlap, beat tray). Although there were numerically fewer mealybugs/shoot in the egg card treatment compared to the control, there were statistically more mealybugs in the beat tray and burlap strip samples. Of the three trials, this location had by far the most ants. We speculate that the egg cards may have attracted ants into the release plots and they may have then tended the mealybugs, causing the mealybug populations to increase; the week following the second release, ants were over twice as abundant in the egg card plots compared to the control. Based on this trial, it appears that appropriately-timed releases of lacewing larvae can decrease both aphid and mealybug populations in orchards, but that in some situations, egg cards may increase pest populations.
These results, combined with our past trial work, have led to us focusing on testing releases of lacewing larvae only. Unfortunately, C. plorabunda is not sold as larvae from any commercial supplier. Therefore, our future work on this project will exclusively test C. rufilabris larvae releases.
1b. Compare drone to ground releases at orchard-scale
After the initial (10 April) release of C. rufilabris eggs resulted in no recovery of larvae, we conducted a second release on 10 May.
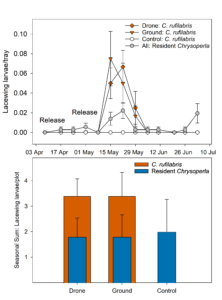
Poor recovery after the first release (Fig. 4) was likely due to a combination of releasing very immature eggs and using a relatively low release rate (20,000 eggs/acre). Following the second release, recovery of larvae was substantial and equal between both treatments (Fig. 4). This demonstrates that drone delivery of lacewing larvae, flying perpendicular to the orchard rows, is as effective as hand releases.
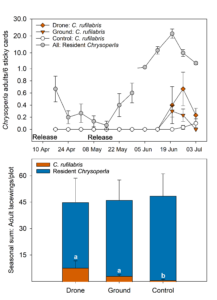
Results of this trial and previous release studies indicate that released lacewings can be found in the orchard for 3-4 weeks post release before they pupate (Fig. 4.). Because of the large plot sizes of this trial, we were also able to analyze the number of adults observed in each treatment (they were unable to move between plots as quickly). Adults were found in the orchard six weeks after the larvae were released (Fig. 5). In the first week C. rufilabris adults were observed, there were none in the control plots, but small numbers of them were captured the following two weeks as they moved out of release plots. There were no differences in the total number of adult C. rufilabris captured in drone versus ground release treatments, although both of these treatments were greater than the control (Fig. 5). We also found no differences between treatments in counts of resident lacewing larvae or adults (Fig. 5), indicating that our released C. rufilabris did not affect resident lacewings.
No differences in mealybug or aphid abundance were observed between treatments in this trial. This further emphasizes the importance of determining the proper timing of release for success. One additional challenge with this trial was variation in pest pressure between sections of each orchard block used. Because the treatments were randomly assigned to each section within a block, the control plot was placed in the center in 3 out of 5 replicates; pest pressure was higher closer to the outer edges of the block. In 2025, we will attempt to account for this by using this year’s pest counts to better randomize the location of each plot within the orchard.
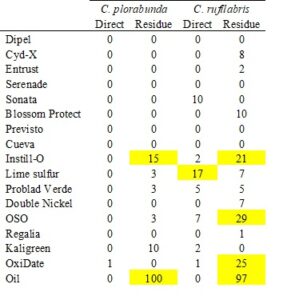
3a. Determine acute toxicity of organic pesticides to lacewings
Previous studies have shown that lacewing eggs are much less sensitive to pesticides than larvae. Our results provide an initial indication of this; there were very few pesticides that differed from the control, and of these, the effects were typically moderate (Table 2). Egg hatch was slightly higher for C. plorabunda (93%) compared to C. rufilabris (87%) (averaging treatments that did not differ from the control). Oil residues (but not direct spray) resulted in near complete prevention of egg hatch in both species. The oil residue never dried, so eggs were essentially smothered. This effect would likely be much less extreme in the field and warrants further testing with larvae and residues on leaves. Instill-O residue caused a 15% and 21% reduction in egg hatch compared to the control in C. plorabunda and C. rufilabris, respectively. Lime sulfur was the only product to reduce egg hatch as a direct spray, and only in C. plorabunda. Additionally, OSO and OxiDate residues reduced egg hatch in C. plorabunda. Our planned work testing effects of these pesticides on larvae will likely be more informative regarding organic pesticide compatibility with releases. However, this provides initial indication that of these two insectary populations, C. rufilabris may be more sensitive than C. plorabunda. Additionally, Instill-O and oil residues appeared to be consistently harmful to the eggs of both species.
Research outcomes
This project has only completed one year of research, but based on these results and previous studies, we currently have the following recommendations:
- When immediately released upon receipt, loose lacewing eggs have low in-field survival in apple orchards and are not likely to result in substantial pest control. Growers should avoid using this method to save labor and purchase costs.
- However, releases of lacewing larvae, properly timed, can decrease aphid and mealybug pressure. Therefore, growers interested in using lacewings for biological control in apples should release larvae.
- However, the price difference between eggs and larvae may make this economically unviable for smaller operations. Therefore, we also recommend that future research focus on the best methods for growers that order loose eggs to consistently hatch them into the correct quantities of larvae prior to release.
Education and Outreach
Participation summary:
Planned work:
Objective 1. Increase grower knowledge and adoption of natural enemy release best management practices.
Goal: 80% of participants in trainings will gain new knowledge and 50% will plan to try natural enemy releases on their farms or alter how they are currently doing releases.
Obj. 1. Activity A. Create a toolkit for growers to train their workforce on lacewing releases
Orchard workforce training is primarily performed during monthly meetings and in-house trainings provided by company personnel. In a recent survey, “training for trainers” and training in Spanish were identified as key: “As an industry, we must do a better job training the trainers. Then trainers must communicate the same message to our employees” (Galvin et al. 2017).
Output: Tail-gate kit. We will create a ‘Tail-gate training kit’ which will facilitate managers training crews who will be deploying natural enemy releases and related activities (e.g., pesticide applications). The training kit will include:
- Illustrated 24×36 inch laminated plain language flipchart (10 pages)
- Plain language handout in English and Spanish
- Pocket flipbook identifying key natural enemies
The training kit will allow managers to train new workers, quickly facilitating distribution of accurate information. Trainers will be able to put the flipchart on the truck tailgate, use flipchart illustrations to review information, and provide employees with an easily reproduced handout. The handout will be illustrated and use plain language. Spanish translation on the reverse side will allow for easy bilingual application. Flipbook and handout content will include:
- suggested release rates and timings
- information on application methods
- how to assess quality of lacewings when they arrive
- proper storage of lacewings prior to release
- identification and scouting techniques
- pesticide compatibility information
A more informed workforce will have greater success and decrease application mistakes, such as using an incompatible pesticide too soon after a release.
The printed resources will be distributed to >90 orchards, reaching ~500 workers. The toolkit will be developed in Year 2 and finalized, printed, and distributed in Year 3. Distribution will occur during the intensive sessions (Obj. 1C.2) and at North Central Washington Tree Fruit Days (January 2027, ~200 in-person attendees annually).
Obj. 1. Activity B. Create a web-based resource that acts as a “one-stop shop” for information on release best practices
Audience. Washington orchardists and consultants and the general public accessing the WSU Tree Fruit website, including Fruit Matters listserv subscribers (2,222 individuals).
Output: Webpage. A dedicated webpage on natural enemy release best practices will be created and added to the WSU Tree Fruit website (https://treefruit.wsu.edu/). This website is the go-to resource for orchardists in Washington and receives 47,000 views per month; it includes the annual Crop Protection Guide, Orchard Pest Management (a digitized reference book), Spanish language resources, and a video resource library. While Orchard Pest Management includes sections on each major orchard natural enemy group (https://treefruit.wsu.edu/crop-protection/opm/beneficials/), information on releases is minimal and outdated.
Lacewings will be the first section added, developed using information obtained from this project. This will allow for the creation of a modular webpage, where sections are added as future research creates recommendations for other species, or new information is learned about lacewing releases. The grant team will use their recent newsletter article (Schmidt-Jeffris 2023) on the topic as the starting point for creating more in-depth webpages.
The webpage will include at least four instructional videos recorded by the grant team on how to conduct releases (ground and drone), do quality control checks, and scout. Videos and photos of lacewings will also be provided to help with identification. Downloadable versions of Obj. 1A materials will also be available on this website. Previously written Orchard Pest Management articles will be updated with links to the natural enemy releases page to provide viewers with the latest information on best practices for releasing a particular natural enemy.
Videos and articles posted to the website will also be pushed out through the Fruit Matters listserv and the Association for Natural Biocontrol Producers newsletter. The webpage will be created in Year 1 and updated at least once annually, with the complete lacewing release section finished in Year 3.
Obj. 1. Activity C. Increase grower adoption of successful lacewing releases using a participatory research framework
Output 1: Advisory meetings. Twice-annual advisory meetings will be conducted with producer-collaborators. The first meeting will be held shortly after receiving the project funding decision and then each following year in February (to plan for next year) and November (to discuss project results). During this meeting the project team will ask for feedback on planned research and Extension activities, including written materials and phrasing of survey questions. Feedback will help avoid pitfalls, increase relevance, and refine Extension outputs.
Output 2: Field days. The producer collaborator orchardists are located in each of our three major apple growing regions of the state (Brewster, Wenatchee, Yakima). This will allow us to conduct three on-farm field days (Year 3), reaching over 180 orchardists geographically distributed throughout the region. At the field days, the producer collaborators will describe how they have released natural enemies on their farms, including the benefits, challenges, and practical solutions. They will also discuss their experiences specific to the lacewing release studies. The sites of the experiments will be used to provide participants hands-on training scouting for and natural enemies, including the released lacewings. Insectary and other industry representatives will be invited to each field day to conduct demonstrations, facilitating discussions between Washington apple growers and the commercial insectary industry. Invitations to attend to conduct demonstrations will be sent to individual industry contacts.
Field days will be conducted in mid-to late June after growers have completed bloom activities. Field days will be marketed to growers via the WSU Irrigated Ag listserv (2,222 recipients), the Washington Tree Fruit Association, local distributors (GS Long, Wilbur Ellis, Chamberlin, etc.), stakeholder organizations (North Central Washington Fieldmen’s Association, Okanogan Horticultural Association) and local fruit warehouses.
Output 3: Intensive session. The producer collaborators will also share their experiences with their peers at a half-day Natural Enemy Releases session at Tree Fruit Days at the Convention Center in Wenatchee, WA (Year 2). Tree Fruit Days is a WSU Extension event that reaches a large audience annually (~250 in-person, ~200 Zoom) and session recordings are available online (WSU YouTube, ~100 views/presentation). The session will include four to six presentations detailing recommendations for natural enemy releases and a panel with producer-collaborators to share their experiences including the most successful release methods, benefits and challenges. Marketing will be conducted via mailings, listservs and local grower groups (see field days).
Objective 2. Disseminate research results to other agricultural professionals and the general scientific community
Obj. 2. Activity A. Scholarly outputs
Output 1: Journal articles. We will submit four peer-reviewed publications summarizing study results (Obj. 1a, 1b, 2, 3). Our journal targets are Biological Control, Journal of Pest Science, Journal of Economic Entomology, and Pest Management Science, respectively. Obj. 1a results will be submitted in Year 3, the other three papers will be submitted 1-2 years after project completion.
Output 2: Conference presentations. Schmidt-Jeffris attends the national and Pacific Branch meetings of the Entomological Society of America annually and she and the grant-funded project manager will present research updates at these meetings. A minimum of eight presentations on project research will be given at these meetings (Year 1: one, Year 2: three, Year 3: four) to anticipated audience of 20-50 researchers per talk.
Obj. 2. Activity B. Additional grower and other agricultural professional outreach
Output 1: Fruit Matters articles. Each March, the grant team will publish an article in Fruit Matters newsletter. The article will update growers on the prior year’s findings, including resulting recommendation updates. It will also provide an additional opportunity to highlight the releases webpage (Obj. 1B). The publication timing will target 1-2 months before growers usually start their release program for the season.
Output 2: Grower meeting presentations. Schmidt-Jeffris is invited to speak at ~8 grower meetings per year (40-500 attendees/meeting) and is typically requested to cover natural enemy release and pesticide compatibility information. Annually, Schmidt-Jeffris will overview research results and recommendation updates at a minimum of two grower meetings (beyond Obj. 1 Activities); G.S. Long and Wilbur Ellis (consulting companies) each host large meetings for their organic growers where Schmidt-Jeffris has previously presented.
Completed activities:
Obj. 1A. Tailgate kit
- Began taking photographs to be used for the kit
Obj. 1B. Web resource
- Updated "Lacewings" page on WSU's Orchard Pest Management website (https://treefruit.wsu.edu/crop-protection/opm/lacewings/) to provide more accurate information on species biology, releases, and update taxonomic names used.
- A ‘Lacewing and Aphid Scouting Video’ was designed, story board created, filmed, and published at https://treefruit.wsu.edu/videos/lacewing-and-aphid-scouting/
- "Assessing Lacewing Orders" video was designed, story board created, and filmed. Scripting and editing is in progress.
- Took additional photos to be used for the website, including project activities and lacewing identification
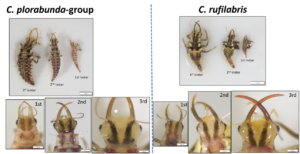
Obj. 1C. Participatory research: Advisory meetings, field days, and intensive session
- Conducted Year 1 advisory meeting (March 28, 2024). Schmidt-Jeffris presented lacewing release research from prior projects and outlined needed next steps. The grower advisory team provided feedback on the project goals and sampling methods, which were adopted into the research plan. The advisory team also provided feedback and suggestions for improvement on the education plan and confirmed their interest in future activities (Obj. 1 Activity C).
- Year 2 advisory meeting scheduled for March 7, 2025
- Regular one-on-ones with advisory team members throughout the growing season regarding research progress
- Consultations with other industry professionals performing beneficial releases regarding research goals and project plans
Obj. 2A. Scholarly outputs
- Data analysis completed for field data collected from Year 1 research
- Conference presentation by Schmidt-Jeffris: Droning on about lacewings: Can we make releases work in apple orchards? Entomological Society of America Annual Meeting. 10-13 November 2024, Phoenix, AZ
Obj. 2B. Additional grower outreach
-
Grower meeting talk by Schmidt-Jeffris: Lacewing releases: Increasing your odds for success. North Central Washington Tree Fruit Days – Apple Day. 23 January 2025, Wenatchee, WA
-
Grower meeting talk by Schmidt-Jeffris: Lacewing release updates. G.S. Long Field Staff Meeting. 22 January 2025, Yakima, WA
-
Grower meeting talk by Schmidt-Jeffris: Challenges in deploying and scouting for natural enemies. G.S. Long Organic Grower’s Meeting. 8 January 2025, Yakima, WA
- Grower meeting talk by Erica Moretti (Schmidt-Jeffris technician): Research-based best practices for releasing natural enemies in tree fruit. Northwest Horticulture Expo. 9 December 2024, Yakima, WA
Citations
Galvin, K. et al. 2017. Emerging issues and concerns in the Washington State tree fruit industry spring 2016 results from a survey of orchard growers. University of Washington Department of Environmental and Occupational Health Sciences, Seattle, WA

Obj. 1B. Create a web-based resource that acts as a “one-stop shop” for information on release best practices
- "Lacewing and Aphid Scouting" video has received 136 views on YouTube (https://treefruit.wsu.edu/videos/lacewing-and-aphid-scouting/)
Obj. 2A. Scholarly outputs
- ~30 Entomological Society of America attendees informed on lacewing releases research
Obj. 2B. Additional grower outreach
-
~600 grower meeting attendees learned about lacewing releases at grower meetings held during the reporting period
- At the North Central Washington Tree Fruit Days – Apple Day talk, 100% of online attendees responding to a poll indicated that they learned new information about lacewings during the talk.
- At the G.S. Long Organic Grower’s Meeting, attendees were asked to rate the talk for Content, Clarity, and Relevance on a 1-5 scale (5 highest) and the average scores were 4.5, 4.6, and 4.6, respectively. The qualitative comments captured generally indicated that the audience appreciated that the research and its implications were clearly explained.
Education and Outreach Outcomes
- Pest and natural enemy scouting
- How to successfully release beneficial insects
Lacewing biology