2016 Annual Report for GS14-131
Making Pest Management More Sustainable in Cucurbit Production
Summary
Squash bug is a major pest of squash and pumpkin in the southeastern U.S. Current control tactics involve the use of broad spectrum insecticide applications that are injurious to beneficial insects including pollinators and natural enemies. Based on a two-year survey in Virginia, we found that >45% of squash bug egg masses were parasitized in by the parasitic wasp, Gryon pennsylvanicum Ashmead. Because this parasitoid has such a high impact, we are currently evaluating the toxicity of various reduced risk insecticides to the parasitoid and testing their ability to control of squash bug.

Fig. 1. Gryon pennsylvanicum, the primary egg parasitoid of squash bug.
Objectives/Performance Targets
Objective 1: To survey the egg parasitoids of Anasa tristis throughout the Commonwealth of Virginia.
- Collections of squash bug egg masses
- The specimens collected will be taken to the Virginia Tech Vegetable Entomology Lab and reared in environmental chambers for identification to species where possible. Records of location, host plant, parasitism levels, and squash bug hatch rate will be made.
Objective 2: To assess the effects of narrow-spectrum insecticides on the eggs of Anasa tristis and its egg parasitoids.
- Squash bug egg masses collected will be utilized in insecticide screening assays for effects of specific narrow-spectrum insecticides. The controls will provide baseline data on the parasitism levels and squash bug hatch rates for each location. The assay will be structured as follows:
10 Squash Bug egg masses (averaged 18 eggs / mass in 2013) per treatment per bioassay
Six treatments:
Control = H2O
Flupyrafurone (Sivanto, Bayer Crop Science),
Pyrifluquinazon (Nichino America),
Flonicamid (Beleaf, FMC),
Sulfoxaflor (Closer, Dow),
Lambda-Cyhalothrin (Warrior II, Syngenta) – Pyrethroid
All treatment concentrations will be at maximum labeled rate.
Assays will be replicated as masses become available.
Accomplishments/Milestones
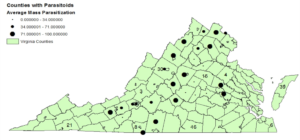
Objective 1: Egg masses were collected from the Eastern Shore to the mountains of Virginia. In 2014,
- Squash bug egg masses were collected from the Eastern Shore to the mountains of Virginia. In 2014, 26 of the 34 sampled counties had the egg parasitoid G. pennsylvanicum with 37% of the egg masses parasitized.
- Beltsville, Maryland averaged 55.7% parasitization only 48 hours after oviposition between 2013 and 2014.
- 89% of 9 egg masses collected in Tennessee
- 70% of 30 egg masses collected in South Carolina
- 10% of 10 egg masses collected in Kentucky
In 2015, 19 of the 25 sampled counties had the egg parasitoid G. pennsylvanicum with 46% of the egg masses parasitized. Data from these samples will be analyzed by date and region to explore temporal and spatial effects on Parasitization. Survey work in 2016 will focus on supplementing samples from previous seasons to create a more complete picture of the impact G. pennsylvanicum may be having on squash bugs across the Commonwealth of Virginia.
Objective 2: The 2015 field season permitted the completion of five separate bioassays, utilizing over 300 egg masses. Included in these bioassays were egg masses from 3 counties and 6 sites, all with known occurrence of, or visible parasitization, see figure 1. Data were analyzed using a chi square goodness of fit test. Controls from each of the nine trials served as a representative of parasitization levels for each site and were used as the “expected” values in our analysis. No significant differences in the rates of surviving parasitoids (Fig. 2) or squash bug nymphs (Fig. 3). The lack of difference between the untreated control hatch rates and the various treatments is likely due to the potential of penetration of the egg chorion by insecticides. Efforts in 2016 will focus on the susceptibility of adult egg parasitoid wasps to these compounds. We have some preliminary data from one bioassay (Fig 4).
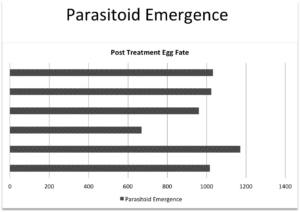
Fig. 2. Numbers of Gryon pennsylvanicum adults emerging from egg masses submerged in different insecticides.
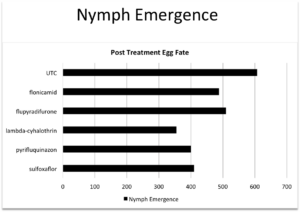
Fig. 3. Numbers of squash bug nymphs emerging from egg masses submerged in different insecticides.
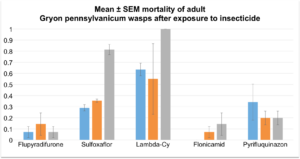
Fig. 4. Effects of different insecticides on adult Gryon pennsylvanicum.
Impacts and Contributions/Outcomes
After two years of surveying numerous counties in Virginia and surrounding states, we have shown that Gryon pennsylvanicum is a critical natural enemy of squash bug reducing the pest population by over 50%. We have made growers aware of this natural enemy and the need to conserve it in fields for more sustainable cucurbit production.
We’ve also identified a number of insecticides that appear to not negatively impact the parasitoid. To date, a number of outputs have been produced from thhis project including: five different contributions to the body of knowledge on cucurbit production, see cited publications and presentations below. Specifically these contributions include presentations to growers, Virginia Agricultural Council members, regional and National sections of the Entomological Society of America and an open access publication in the Journal of Integrated Pest Management. Future contributions will include cooperative extension publications as well as additional peer reviewed journal publications.
Doughty, H.B., Wilson, J.M., Kuhar, T.P., Schultz, P.B. 2016. The Squash Bug (Anasa tristis (DeGeer)): Biology and Management in Cucurbitaceous Crops. Jan. 2016 Journal of Integrated Pest Management Vol 7:1.
Wilson, J.M., Kuhar, T.P. Management of Squash Bug on Cucurbit Crop Proceedings of 2015 The Mid-Atlantic Fruit & Vegetable Convention Hershey, PA, pp. 219-220
Wilson, J.M., Anderson, T.D., Kuhar, T.P. Bees, Wasps, and Insecticides; Maintaining a Healthy Mix in Cucurbit Production Annual Meeting of the ESA Eastern Branch. Philadelphia, PA. Jan. 2016 Phd Poster Competition
Wilson, J.M., Anderson, T.D., Kuhar, T.P. The Impact of a Squash Bug Egg Parasitoid and its Sensitivity to Selective Insecticides Entomological Society of America Annual Meeting, Minneapolis, MN. Nov. 2015. Oral Presentation
Wilson, J.M. Improving Integrated Pest Management in Virginia Cucurbit Production Virginia Agricultural Council Farm Tour, Kentland Farm, Blacksburg, VA. Aug. 2015. Oral Presentation
Collaborators:
Graduate student
Virginia Tech
Dept. of Entomology (0319)
170 Drillfield Drive, Virginia Tech
Blacksburg, VA 24061-0319
Office Phone: 5402316129