2015 Annual Report for LNC13-351
Neonatal Calf Diarrhea: Reducing Impacts and Antibiotic Use with Natural Therapies
Summary
We are very pleased with pleased with the progress and outcomes with the research components of our SARE project. We are on track to meet and exceed the stated objectives for the first two specific aims. In the first year of the project, we collected over 700 survey responses from conventional and organic dairy and calf producers in the Midwest. The results from the first specific aim have been useful to understand antimicrobial and non-antimicrobial treatment practices for both conventional and organic producers. The large sample size and representative nature have enabled precise estimates on prevention practices, antimicrobial use, and attitudes on non-antimicrobial alternative therapies for calf diarrhea. These results have been presented at multiple conferences, and a manuscript is under review. The results will from the first specific aim will be additionally presented at the American Association of Bovine Practitioners (AABP) conference, and at the American Dairy Science Association Conference (ADSA). Secondly, we have completed a large field trials on calf diarrhea that tested the effects of garlic extract and lactoferrin as treatments for calf diarrhea on organic farms. Importantly, the results demonstrate significant reductions in mortality when calves are treated with lactoferrin. These results will result in the promotion and availability to organic producers a practical and efficacious tool for the treatment of calf diarrhea. We are excited to begin the 3rd component of our project in its third year, which will deliver the results of the first two aims within a strong extension component (2016).
Objectives/Performance Targets
The short-term outcomes stated in the original proposal included the following: 1) representative survey data from 300 calf producers across three NCR states about the knowledge, skill or resource limitations associated with implementing prevention practices, judicious antimicrobial use, and alternative therapies; 2) efficacy data on two promising alternative therapies for calf diarrhea; 3) improved knowledge and skills on 20 farms on optimal calving and calf management practices, responsible antimicrobial use, and evidence-based treatment protocols for calf diarrhea. For the first two full years of the project, we have met or exceeded the first two objectives. Details on the results are below.
Accomplishments/Milestones
Objective 1
Our first objective was to use a cross-sectional survey to identify strategies to improve implementation of prevention practices, improve judicious antimicrobial use, and assess the use of non-antimicrobial alternative therapies by calf producers. A survey was designed with three sections that focused on each of the three components of the objective. The survey was pilot tested on a group of 10 calf producers to gather critical feedback from farmers, determine time requirements, and identify potentially confusing or misleading questions. Next, we selected a representative study population by obtaining lists of grade A dairy producers from the Departments of Agriculture in Ohio and Michigan, the list of certified organic dairy or calf producers from the USDA National Organic Program website, and a list of calf raisers belonging to the Dairy Calf and Heifer Association (DCHA). From a total of 3,967 grade A Dairy producers in Ohio or Michigan, 1,200 were randomly selected using a random number generator. Because of the low number of organic producers (321) and calf raisers (136), all certified organic calf or milk producers were selected for participation. In total, 1,657 producers were selected and mailed a survey. To thank producers for participation and incentivize The survey response rate was exceptional. The original goal of 300 producers was surpassed by a wide margin, and a total of 632 (45%) of dairy producers returned the survey, including 173 organic and 459 non-organic dairy producers. Additionally, we received survey results from 37 (33%) of heifer raisers that were mailed the survey. Figure 1 provides an example of the type of data available from the manuscript. Organic and conventional producers were provided with scenario-based descriptions of calf diarrhea that described either mild, moderate, or severe. Producers were provided with options for the treatment of diarrhea, which resulted in a clear picture when different antimicrobial alternatives are implemented by both organic and conventional producers. The data generated from this survey has resulted in presentation at one conference and the submission of one manuscript. The results from the survey will additionally presented at the American Association of Bovine Practitioners in September, and the Joint Annual Meeting – American Dairy Science Association Conference this coming summer. We expect the results to be critical to improve awareness among calf professionals and veterinarians on the critical needs of organic calf raisers, and to design additional research projects to address critical research gaps within organic calf production systems, especially the lack of data to support the efficacy of organic therapies for common livestock diseases.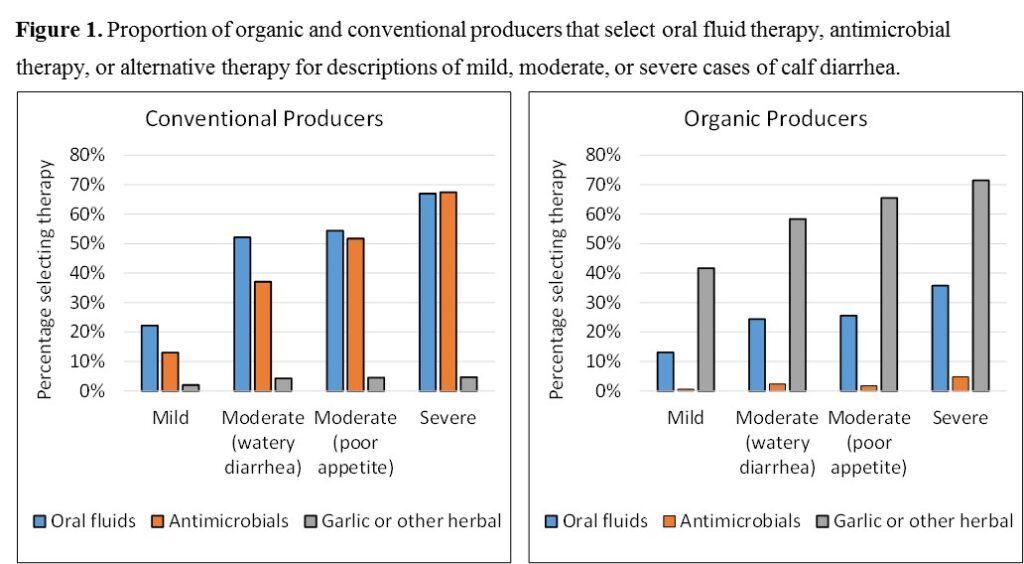
Objective 2
Results from the first objective gave a clear picture of the frequency and perception of efficacy of antimicrobial alternatives among organic producers. Although alternatives to antimicrobials are frequently used to treat calf diarrhea on organic operations, there is little data confirming their effectiveness. In the second year of the project we conducted a blinded, randomized field trial lactoferrin and garlic extract for the treatment of calf diarrhea. Large field trials of diarrhea therapeutics are uncommon due to the large number of clinical cases of diarrhea required to test the hypothesis. We originally aimed to enroll a total of 600 diarrheic calves, and randomize the calves to receive one of 3 treatments: allicin, lactoferrin, or a placebo. This past summer, we collaborated with a large organic dairy farm and enrolled a total of 633 calves with diarrhea. Students working on the project clinically assessed calves enrolled in the project each day for 10 consec-
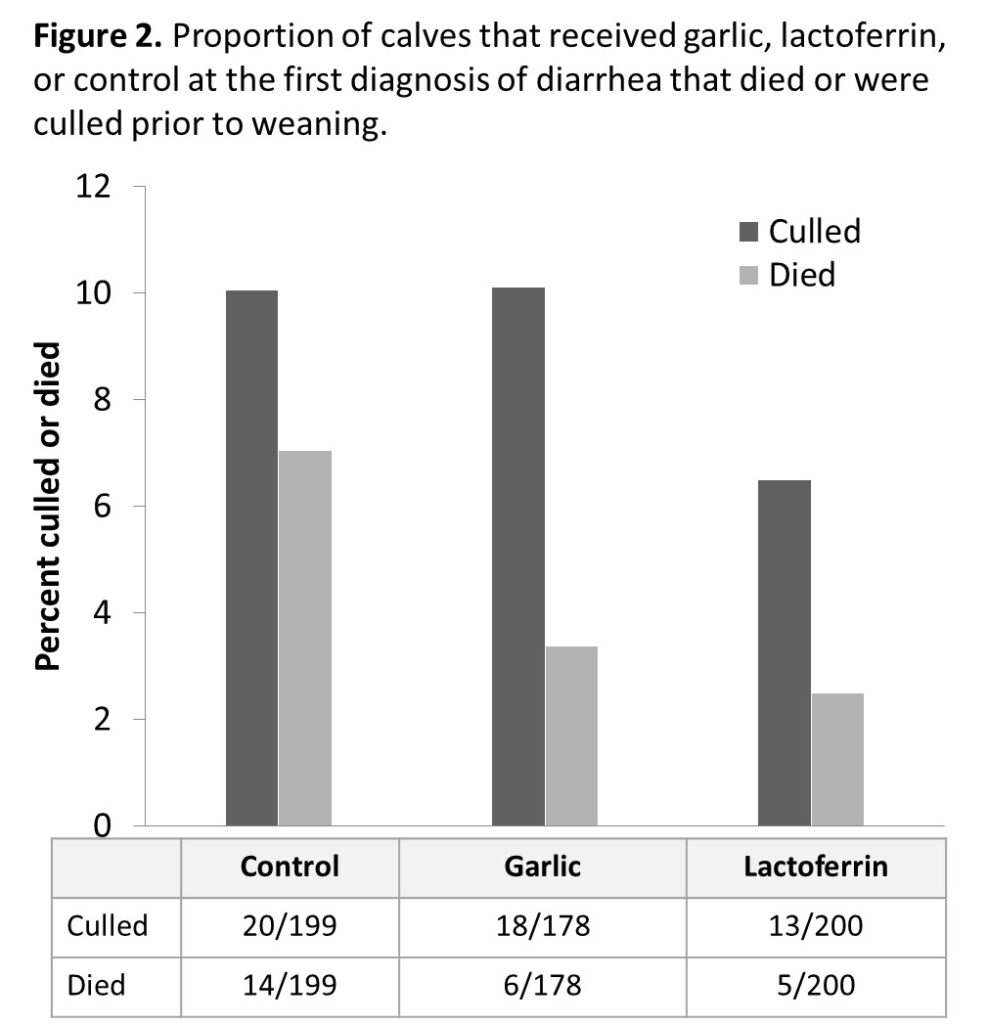
utive days, and additional treatments, deaths, and culls were recorded through the pre-weaning period. Importantly, calves enrolled in the lactoferrin treatment group had a significantly lower mortality rate. Lactoferrin significantly (p < 0.05) reduced the risk of death and culling in the preweaning period. In total, 7.5% (15/198) of calves in the control group died compared to only 3% (8/201) of calves treated with lactoferrin.
Objective 3
Our third objective, planned for the third year of the project, is to develop and assess a comprehensive, research-based extension programming for dairy producers on best management practices, judicious antimicrobial use, and treatment protocols for calf diarrhea. The content of this objective will be based in part on the results of our first two objectives. Regardless of the outcomes, we expect the extension program to directly address the needs of organic and conventional calf raisers in the North Central Region.
Impacts and Contributions/Outcomes
So far, the original objectives of the proposal have been met or exceeded. For objective 1, we obtained survey data from twice the planned number of study participants, and we are confident in the representative nature of the data. We expect a minimum of two scientific publications based on the results of the survey, and the results will be presented at the American Association of Bovine Practitioners (AABP) in the Fall of 2016. We completed the large calf trial on non-antimicrobial alternatives in the Summer of 2015(Objective 2), exceeded the targeted enrollment for the trial, and have good estimates of the efficacy of the proposed therapies. These data are expected to lead to the promotion availability of an efficacious and novel product for the treatment of calf diarrhea. Additionally, we expect both scientific and lay publications on the efficacy of these non-antimicrobial therapies that will be directly useful to organic and conventional producers in the North Central Region.
Collaborators:
Professor
The Ohio State University
601 Vernon Tharp St
Columbus, OH 43210
Office Phone: 6142926661
Assistant Professor
The Ohio State University
1920 Coffey Rd
Columbus, OH 43210
Office Phone: 6142929453