Final Report for FNE13-782
Project Information
We have spent the last 4 years (two years with FNE11-721, and the last 2 years with FNE13-782) working to show that biostimulation, combined with strict sanitation protocols, roguing, and a 4-year rotation, can provide a management strategy to cope with Allium White Rot (AWR) in a garden or small farm setting.
Biostimulation is a method of awakening dormant AWR fungal bodies, called sclerotia, without providing them with the actual plant host needed to complete their life cycle. Thus the sclerotia die. We’ve been stimulating the fungus by applying simple materials prepared on-farm: garlic juice (in solution with water) and green garlic plant material (from the previous years crop, ground up with water and then frozen). Both of these preparations carry the chemical (the organosulphur compound allicin) needed to stimulate the sclerotia. The treated beds were planted to non-allium crops each year until 2015.
In 2015 the beds were all planted to alliums, with a random mix of plug-planted pearl onions and storage onions, and direct-seeded scallions. The three crops were harvested at appropriate times and sent to the University of Maine Insect and Plant Disease Diagnostic Lab. The results of these tests were very encouraging, showing very little AWR, no more than could be addressed with intense roguing in the field. In addition the assays of the pathogen present (performed at Nematodes, Inc. Selma, CA) showed an overall reduction in numbers of 78%. Both biostimulation treatments, garlic juice and green garlic plant pieces worked equally well.
I was asked to speak at various functions around New England: Common Ground Country Fair, the New England Vegetable and Fruit Conference, and NOFA events. Most recently (Jan. 2016) I presented a “Growing Great Garlic” class at NOFA Massachusetts Winter Conference and participated in a NEVF Conference program (Dec. 2015) about farm based research.
Introduction:
Whitehill Farm is a very small Certified Organic and diversified farm. We have been producing and selling garden vegetable and herb seedlings for local farmers and gardeners every year for about 25 years. Our own gardens provide vegetables for our own consumption, Farmers Market, and for an extensive line of value-added products.
A significant portion of our garden area has provided us with garlic both as a cash crop and for our value-added culinary herb products. When we “acquired” Allium White Rot, it quickly became established in a portion of the gardens and caused a significant financial loss.
Allium White Rot (AWR) has been present on our farm since 2004: we bought some seed garlic from out of state that year, and unknowingly also bought AWR. The problem soon became apparent. We destroyed the affected garlic crop, then isolated to a single 4’ x 10’ raised bed, and hoping to have dealt with the problem, planted that bed to perennial herbs. Three years later AWR showed up in a completely different area, and we suffered a tremendous loss, in excess of $2,000. We abandoned that area, grassed it over, and have since planted fruit trees there. In 2010 AWR again showed up, but this time in beds directly down hill and adjacent to the original affected bed. After a definitive diagnosis, I began the research that led to our SARE grants. It soon became apparent that biostimulation (see Methods section), already employed in other parts of the world to manage AWR, might prove to be a viable management tool here in New England.
Garlic is a high value cash crop for many farmers in New England. In recent years serious garlic diseases have arrived in New England, making garlic a far more challenging crop. At the top of the list of diseases, which also includes Nematode Bloat, AWR is very easy to acquire and spread, and is by far the most devastating. AWR can remain dormant in soil for up to 25 years and there are no methods for removing it that are approved for use in an Organic setting.
Our project, to test biostimulation, develop sanitation methods (this is of prime importance), and work within a 4-year rotation program in an Organic setting, should give farmers an effective tool for coping with/managing AWR.
My role at Whitehill Farm, as grower, direct marketer, and cook (!) has kept me very busy for 30 years. We’ve been Certified Organic since 1992, and have had our seedling business for 25 years.
 Winter Farmers Market table
Winter Farmers Market table
 Annual Seedling sale
Annual Seedling sale
Whitehill Farm is a very small operation. Our actual growing space is less than 1/4 acre, all in approximately 160 4' X 10' raised beds, 30 of which are under cover, in unheated polytunnels. We market Certified Organic vegetable and herb seedlings every spring, veggies during the summer months, and value added culinary herbs products year round. We also raise chickens for meat and eggs. We open our farm to the public for a seedling sale in June, for the Maine Open Farm Day every July, and occasionally to host classes.
We have worked with Dave Fuller and Dr. Steve Johnson, of the University of Maine Cooperative Extension, and Dr. Fred Crowe, Professor Emeritus, University of Oregon since 2010. They helped get our research started and in developing our project plans.
Dr. Steven Johnson, Crops Specialist, University of Maine Cooperative Extension, and David Fuller, University of Maine Agriculture and Non-Timber Forest Products Extension Professional, have done work on a SARE project (LNE11-306) to study producing certified disease free seed garlic via tissue culture.. They were both involved in helping design my project and have been monitoring it along the way.
Dr. Fred Crowe, Professor Emeritus, Department of Botany and Plant Pathology, Oregon State University, has done extensive work with biostimulation with farmers in Oregon, New York State, and Canada. Dr. Crowe is currently working with farmers as an independent consultant. He has provided information, inspiration, and nuts-and-bolts assistance with planning the biostimulants and methods of application.
Our Organic advisor along the way has been Eric Sideman, MOFGA’s (Maine Organic Farmers and Gardeners Association) “Extension Agent”. Eric has been invaluable as a trouble-shooter and sounding board.
Our objective was to show that biostimulation, along with strict sanitation protocols and the recommended roguing of any diseased plants, can prove to be a low-cost viable way of gaining control/management of AWR. We were able to sustain our plan – with only a couple minor adjustments – for the entire four years. Every spring we took samples from the beds and sent them off for an assay of the amount of pathogen present. Then we applied the biostimulants. Through 2014 we then planted the beds to non-allium crops. In 2015 we planted and harvested a test planting of alliums. The results of that planting and the subsequent lab tests for the presence of AWR were very positive.
Cooperators
Research
Background and summary of our 2011 (FNE11-721) grant process and the progress we made: Early May 2011 - We chose 6 AWR affected beds and constructed dividers. The resulting sections are numbered (the Bed numbers and section numbers correspond to the numbers on the lab test sheets) and with the help of Rick Kersbergen, (Crop Specialist, UMaine Cooperative Extension) we designed a randomized assignment of biostimulants and controls.
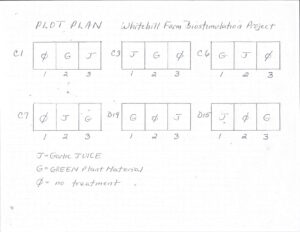
Plot plan showing bed section and randomized treatments
 Installing dividers in our raised beds
Installing dividers in our raised beds
June 2011 – We took the first soil samples for baseline AWR assessment, when the soil had warmed. We dug into each section, in at least 6 different places, taking soil at all depths up to 10”. We thoroughly mixed those samples from each bed section, and then packaged a half gallon sample from each section to send to the lab. All 18 samples went to be tested at Nematodes, Inc., in Selma, CA, a lab that specializes in testing for Allium White Rot.
 Digging for soil samples
Digging for soil samples
 Mixing soil samples for testing
Mixing soil samples for testing
June 2011 - We then prepared and applied the first treatments. We made fresh garlic juice (using a juicer) from purchased fresh organic garlic and applied the juice diluted in water – per Dr. Crowe’s suggestion, “with a strong garlic odor”, a quarter cup per 4-gallon sprayer load. We sprayed the water/garlic mix and simultaneously cultivated until the soil was evenly wet at least 6-8” deep. Incorporating the garlic juice and water quickly is important as the garlic juice is very volatile. It took 1 ½ sprayer fills per bed.
 Juicing out fresh garlic
Juicing out fresh garlic
 Garlic juice frozen for later use
Garlic juice frozen for later use
 Spraying juice with agitating sprayer and digging it in thoroughly
Spraying juice with agitating sprayer and digging it in thoroughly
We also dug in thawed green garlic plant material (prepped and frozen in July of 2010), mixing it thoroughly into the soil to a depth of about 6-8”. The green garlic material was spread on the surface, about ½ inch deep, and dug in thoroughly using a fork to make sure it was incorporated at least 6-8”. The approximate volume of green material applied to each section was 2 ¼ gallons. Photos of grinding it up and putting it in the boxes NEW photo of storing it in the freezer Photos of applying green material
 Healthy garlic tops to process into material
Healthy garlic tops to process into material
 Healthy tops cut into little bits for the Cuisinart
Healthy tops cut into little bits for the Cuisinart
 Green material prep in the Cuisinart
Green material prep in the Cuisinart
 Green material prepped and stored - frozen - for the next years applications
Green material prepped and stored - frozen - for the next years applications
year  Spreading green material
Spreading green material
 Forking in green material
Forking in green material
In the initial proposal we planned to apply a second round of garlic juice and green material later in the season. This did not happen as it proved not practical – in fact nearly impossible - to try cultivating 6-8" deep while other crops were maturing in the beds. Since we are using hand tools we had to develop a method to do the applications that was fast and effective. We found that loosening the soil first, with a pitchfork, and then cultivating with a claw while the juice is being sprayed works very well. Similarly, we loosened the soil and then thoroughly dug in the ground up green material, incorporating it to 6-8”. We then planted the beds to non-allium crops, following our rotation plans for the year.
 Sample non-allium crop. We planted lettuce, tomatoes, corn, squash, spinach, soybeans, etc. in the beds.
Sample non-allium crop. We planted lettuce, tomatoes, corn, squash, spinach, soybeans, etc. in the beds.
Springs of 2012 and 2013 We repeated the entire process of taking samples for testing, doing the biostimulant applications, and planting non-allium crops in all the beds. All the other aspects of the growing process were the same as our other gardens: mulching, fertilizing, dealing with any insect pests, harvesting, and cleaning up at the end of the season.
Plan of work for the 2013 grant:
In 2014 and 2015 we continued this process: sampling and sending the samples out for testing at Nematodes, Inc., applying treatments, and planting the beds. In 2014 the crops planted were again non-alliums.
The plantings in 2015 were designed to be a test of the entire process. We planted three types of onions: Pearl onions (short days to maturity), planted as plugs; Storage onions (longer days to maturity), planted as plugs; and Scallions, direct seeded as if it were a succession planting. As each crop matured and was harvested we delivered samples to the University of Maine Insect and Plant Disease Lab to be tested for the presence of AWR.
 Test planting in 2015, storage onions and scallions
Test planting in 2015, storage onions and scallions
 Prepping pearl onions to send to the Disease Lab
Prepping pearl onions to send to the Disease Lab
For all 5 years we have been following and streamlining our sanitation protocol. No garden activity takes place without cleaning materials – we carry a bucket set up with soapy water and a brush to every task. We clean tools, hands, wheelbarrows, boots - everything that touches soil - before moving on to any next task. Although this sounds tedious and onerous, we’ve gotten to the point where it is “just the thing we do”. We have a discreet set of tools for our new garlic area (see below), which simplifies the cleaning needs. At the end of the day, or when the cleaning bucket needs it, we dump the soiled water into a 4’ deep sinkhole dug for this purpose. Then any further rinsing of tools, equipment, or buckets is done right into the hole.
 Sanitation bucket with filthy water, ready to be dumped
Sanitation bucket with filthy water, ready to be dumped
 Below grade "Dump" hole
Below grade "Dump" hole
As we have both returning and new help every year, sanitation protocol training continues to be very important. Because of the nature of AWR, a complete understanding of the dangers of moving the pathogen cannot be stressed enough. We review the necessary sanitation measures, every time, before setting foot or tool in any garden area.
 Sanitation fall cleanup
Sanitation fall cleanup
To ensure a sufficient garlic crop for our value-added farm products we’ve been building new beds every year. By adding 5-6 beds every year we’ve been able to keep pace with our rotation and begin rebuilding our seed crop. The new beds are up hill from the contaminated gardens, are filled with Certified Organic raised bed mix (a mix of loam and composted cow manure) that we are bringing in, and as part of the sanitation protocol have their own discreet set of tools.
The overall results of our study are very positive, although it is very clear from the assays (Nematodes, Inc.) that there is still a definite presence of Allium White Rot in our beds. Overall the assays indicate that there IS a reduction of the pathogen, approximately 78%. Please consult Tables C and D for a complete outline of the assay results.
Clearly the pathogen is not gone. The assays also indicate that there is little difference in the efficacy of the two biostimulants that we tried. Early on there was variability in the assays that we thought at first might be attributable to volatility of the garlic juice treatment. And then variability showed up in the green garlic material sections AND in the control sections as well.
When sampling soil and searching for the AWR sclerotia, which are about the size of a poppy seed, there is a needle-in-the-haystack aspect to the whole process. Compared to a standard soil test situation where most of the soil in a field will have similar characteristics, it is possible to simply dig in the wrong place while sampling for AWR!
To further complicate the situation, Dr. Crowe assured us that there will also be between 10 and 15% natural degradation of the dormant pathogen per year.
A definite conclusion is that BOTH biostimulants (garlic juice and green garlic material) work. For someone to replicate our biostimulant treatments choosing one that is more practical for the farm situation would be the right choice. For example, farms using larger equipment to incorporate biostimulation treatments can use conventional equipment found on their farm (bulk tank sprayers, plows, rotovators, etc.). Please consult Tables A and B to see the lab results/conclusions at the end of our first grant FNE11-721. Clearly we knocked down a good percentage of the pathogen in the first two years.
TABLES SUMMARY
Tables A through D chart the information found through the assays (Nematodes, Inc.)
Tables A and B were submitted in the final report of our first grant, FNE11-721. These tables show a significant loss of viable sclerotia -91%- over the first two years. Clearly we knocked down a good percentage of the pathogen in those first two years.
Table D charts the actual numbers of sclerotia found per year, compared to the percentage that actually germinated in the lab. This demonstrates another aspect of this process, that natural degradation cannot be anticipated or charted.
Table C shows a better total picture, including the inevitable ups and downs.
In 2013, ’14, and ’15 sclerotia were found in some beds that recorded a zero count the previous year. According to Dr. Crowe this amply demonstrates the “needle-in-a-haystack” aspect of sampling. This table also shows the total number of sclerotia lost, by any means. Many could have been killed by biostimulants and others through natural degradation.
Significantly, over the four years we’ve demonstrated an overall drop in found sclerotia of 78%.
SUCCESS WITH THE 2015 PLANTING OF ALLIUMS
The real and impressive success is shown by the results of our 2015 planting of alliums in all the beds. We planted plugs of Pearl onions knowing they would be harvested quickly. The screening for AWR showed NO fungal mycelium or sclerotia. The plugs went in on approximately June 5 and were harvested on July 25th.
We planted plugs of Storage onions knowing they would be vulnerable as they would be in the soil far longer. It is impressive that only one sample showed AWR infection. The bed, D14-1, was treated with green garlic material. The plugs were planted approximately June 5 and were harvested on September 16th.
We planted scallions, direct seeded, as if this were a succession crop. In my conversations with Dr. Crowe he has repeatedly mentioned that as well as a quick crop, a scallion crop can also provide biostimulation. We were gratified that there was NO fungal infection in this crop which made this an additional success. The scallions were direct seeded approximately on July 15th and were harvested on October 24th.
ADDITIONAL CONSIDERATIONS
Because we depend on our garlic production for value-added products and seed, as well as for using and selling garlic all winter for fresh eating, it is important to use every possible bit of the crop. It is possible to have a good – and careful - harvest from beds that show spot infection. That harvest goes directly into eat-only status to prevent spreading AWR by planting infected cloves. The next step is roguing/removing the affected plants and soil.
Part of a successful control/management of AWR must include roguing out any infected plants. Due to the slope on our property, all the beds downhill from the originally infected bed are vulnerable. And because beds are now coming up in our rotation for the first time since we started the biostimulation project, we are discovering spot infections every year. In 2015 two beds had individual AWR infected garlics. We removed them, along with the adjacent surrounding plants and the soil to a depth of about 10 inches. We replaced the soil with clean Certified Organic raised bed mix. We will biostimulate these beds for the next four years with confidence that they will be clean the next time they come up for alliums in our rotation.
 Individual AWR infected garlic plants
Individual AWR infected garlic plants
 Digging out soil after removing infected plants
Digging out soil after removing infected plants
 Roguing hole completed and ready to be filled with new soil
Roguing hole completed and ready to be filled with new soil
The biostimulation technique vs. AWR has been used for many years in the United Kingdom, both in industry and for home gardeners (http://www.growyourown.info/page173.html). Fred Crowe is promoting biostimulation in Oregon, Washington, and Canada when it is a good fit for a particular farm situation. I firmly believe that this technique IS worthy of farmer adoption and continued research.
Our contribution shows that this technique can be adopted by a gardener and equally effectively by a small farm AND will yield positive results.
Education & outreach activities and participation summary
Participation summary:
I am taking, and will continue to take, every opportunity to open or continue the discussion of the challenges to growing alliums, and specifically garlic, in New England. It is important that every farmer and gardener who grows garlic knows how to recognize garlic diseases, and especially Allium White Rot. Every grower needs to know how not to SPREAD the problem on their own property or to other farms, and needs to be comfortable asking for help from the University of Maine plant disease labs when needed (or other local lab/University Extension). Of prime importance is understanding how to avoid the problem in the first place.
We have participated in the following events, spreading information about the importance of understanding garlic diseases, and the methods we've employed to limit and reduce the presence AWR on our farm:
-Open sessions at Whitehill Farm when we are open to the public (our own spring seedling sale, Open Farm Day, classes held here
-University of Maine at Farmington classes working in collaboration with Dr. Grace Eason, Associate Professor of Science and Science Education, with her focus on sustainability.
-University of Maine at Farming Gold LEAF program Senior life-long learning program
-Participating in MOFGA public events - Common Ground Fair (annually), Farm and Homestead Day (annually)
-Speaking at agricultural and trade events: Most recently at the New England Vegetable and Fruit Conference, December 2015; and at the NOFA Massachusetts Winter Conference, January 2016.
-When the Call For Papers arrives I will submit a proposal to make a presentation about our biostimulation research at the 2017 IFOAM World Congress.
2014 2015 handout THE JOY OF THE STINKING ROSE
Project Outcomes
Potential Contributions
Biostimulation, while not a silver bullet, is a method with merit: it is simple, can be prepared on-farm, can be combined with careful sanitation practices, and can make a huge impact on the presence of Allium White Rot.
It is our hope that our stress on the importance of sanitation will be heard by many gardeners and farmers. Preventing an infestation with AWR is the first priority. Second, if you DO have AWR on your property it is essential to understand how NOT to spread it. We have shown that sanitation is key and can make it possible to work towards healthy crops.
Biostimulation is definitely a viable option for combating AWR. What is needed is continued efforts to produce or inspect garlic seed to ensure there is no disease infestation.
Future Recommendations
We DID answer the question we set out to study – we asked if biostimulation could be a viable management tool for AWR - we showed that it is a viable tool and we found some additional positive results along the way.
We have also learned that sanitation is an essential key to making biostimulation work as it is essential not to continue spreading the disease while simultaneously trying to manage it! We have also learned that this is “easier said than done” Only by sticking to “the rules” long enough for our protocol to become routine, and continuing these practices, will we be able to prevent spreading and eventually knock AWR down.
We will absolutely continue using biostimulation to combat AWR. We can prepare the products here on the farm, for very little cost, and doing the spring applications has become routine. Because AWR is still moving around and showing up in our rotation, we feel it is essential to continue roguing and treating beds. This way we can knock the numbers down consistently we will have an advantage!
There is still much to be learned about allium diseases. With climate change affecting and/or causing diseases to move to different areas of the country, there are, and will continue to be new challenges to growing garlic (and other alliums) here in New England.
Research needs to continue to find ways of providing disease-free planting stock for producers. This may be simply an improved testing and certifying program such as is in place for potatoes. It may also become possible to do laboratory production of clean planting stock
Study needs to continue on the importance of sanitation in the farming community. Every aspect of the process can be improved upon to prevent the spread of unwanted problems: weeds, insects, as well as diseases.