Final Report for GNC05-047
Project Information
Surveys were conducted during 2004-2006 in Illinois to determine occurrence and distribution of viruses in pumpkin, squash, and gourd fields. CMV, PRSV, SqMV, TRSV, ToRSV, WMV, ZYMV, and unknown potyvirus(es) were detected as single and mixed infections. WMV was detected in more samples and SqMV was detected in samples from more counties than any other virus. ToRSV and TRSV were detected for the first time in Illinois in 2004 and 2005, respectively. Unknown potyvirus(es) are yet to be identified. General symptoms caused by viruses were mosaic and deformation of leaves and color-breaking and deformation of fruits.
Introduction:
Cucurbits are valuable crop in Illinois and other parts of the North Central region. There are approximately 20,000 acres of pumpkin and about 8,000 acres of other cucurbits (e.g., cucumber, gourd, melons, and squash) in Illinois (1). Diseases caused by viruses are serious threats to cucurbit industry in Illinois and other parts of the North Central region. Among the 32-reported viruses causing diseases on cucurbits (17), only five viruses including Cucumber mosaic virus (CMV), Watermelon mosaic virus (WMV), Papaya ringspot virus (PRSV), Squash mosaic virus (SqMV), and Zucchini yellow mosaic virus (ZYMV) (15), have been have been reported in Illinois. No previous study had been conducted to identify the viruses present in pumpkin and squash fields from all over the state of Illinois.
Different cucurbit viruses have been reported from different regions of the US. CMV (genus Cucumovirus, family Bromoviridae), has been reported from all over the US in squashes and watermelons (3,8,9,12,14). PRSV-W (3,10,12,14), WMV (3,8,10,12), and ZYMV (3,9,10,11,14) of the genus Potyvirus (family Potyviridae) have also been reported in squashes, pumpkins, and other cucurbit crops from many regions in the US. SqMV (genus Comovirus, family Comoviridae) has been detected in South Carolina and Texas (8,12). Tobacco ringspot virus (TRSV) and Tomato ringspot virus (ToRSV), in the genus Nepovirus of the family Comoviridae, have been reported in cucurbits in South Carolina, Texas, and Wisconsin (8,12,13), and in the northeastern US (17), respectively. Squash leaf curl virus (SLCV; genus Begomovirus, family Geminiviridae) has been reported on cucurbits from Arizona, California, and Texas (2,10).
Virus symptoms on cucurbits vary from mild mosaic or veinbanding to severe systemic mosaic and malformation of leaves and plant stunting (3). WMV can cause severe stunting and leaf distortion (8) and PRSV and ZYMV can induce severe leaf mosaic, plant stunting, and malformation of foliage with blisters and shoestring (3) on cucurbits. CMV, PRSV, SqMV, TRSV, and WMV infection in squash can induce systemic mottling with leaf malformation (16). In squash and pumpkins, SLCV induces leaf curl and mosaic symptoms (2). Virus infections in cucurbits produced unmarketable fruit due to deformation, color change, and cracking in fruits (12) depending on the stage of fruit development at infection (11,15).
The virus diseases in cucurbits can be caused by a single or mixed infection of two or more viruses that are capable of producing similar symptoms (3,12). CMV, PRSV, SLCV, WMV, and ZYMV can infect most cucurbit species and have low host specificity, whereas SqMV is found only on Cucurbita pepo and does not infect cucumber, melons or watermelons (2, 8,15). CMV has a wide host range and can infect more than 800 plant species (17).
Weed hosts are important sources of inoculum for CMV, PRSV, SqMV, WMV, and ZYMV on cucurbits (10,17). Transmission of cucurbit viruses in the fields is mostly by insect vectors. Bemicia tabaci transmit SLCV (2,10) whereas CMV, PRSV, WMV, and ZYMV are vectored by aphids in a non-persistent manner (10,11,16). SqMV is transmitted by seed, western striped cucumber beetle [Acalymma trivittatum (Mannerheim)], and spotted cucumber beetle [Diabrotica undecimpunctata howardi Barber] (10,17). The viruses ToRSV and TRSV are reported to be mostly transmitted by dagger nematode Xiphinema americanum (17). Most cucurbit viruses are also mechanically transmitted (17).
Detection of the viruses is done by utilizing enzyme linked immunosorbent assay (ELISA) procedures. Healthy plant is used as negative control (14). Double antibody sandwich enzyme linked immunosorbent assay (DAS-ELISA) method is used to test plant tissues for presence of several cucurbit viruses (3,12,14) SLCV antiserum has been used to detect SLCV in cucurbits (2). Detection of ToRSV and TRSV by reverse-transcriptase polymerase chain reaction (RT-PCR) is done by amplify conserved coat protein region of the viral RNA (6). A degenerate oligonucleotide primer, that amplifies 1.6 - 2.1 Kb fragment, has been reported as a general potyvirus primer in detection of potyvirus by RT-PCR (5).
Although this research was scheduled for 2005 and 2006, because of the importance of the overall results, some of the results related to studies in 2004 are also reported here.
Literature Cited
1. Babadoost, M., and Islam, S.Z. 2003. Fungicide seed treatment effects on seedling damping-off of pumpkin caused by Phytophthora capsici. Plant Dis. 87: 63-68.
2. Cohen, S., Duffus, J.E., Larsen, R.C., Liu, H.Y., and Flock, R.A. 1983. Purification, serology and vector relationships of Squash leaf curl virus, a whitefly-transmitted geminivirus. Phytopathology 73: 1669-1673.
3. Davis, R.F., and Muzuki, M.K. 1987. Detection of cucurbit viruses in New Jersey. Plant Dis. 71: 40-44.
4. Freitag, J.H. 1956. Beetle transmission, host range, and properties of Squash mosaic virus. Phytopathology 46: 73-81.
5. Gibbs, A., and Mackenzie, A. 1997. A primer pair for amplifying part of the genome of all potyvirids by RT-PCR. J. Gen. Virol. 63: 9-16.
6. Hughes, P.L., and Scott, S.W. 2003. First report of Tomato ringspot virus in Butterfly Bush (Buddleia davidii). Plant Dis. 87: 102.
7. Jossey, S., and Babadoost, M. 2006. First Report of Tobacco ringspot virus in Pumpkin (Cucurbita pepo) in Illinois. Plant Dis. 90: 1361.
8. McLean, D.M., and Meyer, H.M. 1961. A survey of cucurbit viruses in the lower Rio Grande Valley of Texas: Preliminary report. Plant Dis. Rep. 45: 137-139.
9. McLeod, P.J., Scott, H.A., and Morelock, T.E. 1986. Zucchini yellow mosaic virus: a new severe cucurbit disease. Arkansas Farm Res. 35: 2.
10. Nameth, S.T., Dodds, J.A., Paulus, A.O., and Laemmlen, F.F. 1986. Cucurbit viruses of California: An ever changing problem. Plant Dis. 70: 8-11.
11. Provvidenti, R., Gonsalves, D., and Humaydan, H.S. 1984. Occurrence of Zucchini yellow mosaic virus in cucurbit for Connecticut, New York, Florida, and California. Plant Dis. 68: 443-446.
12. Sammons, B., Barnett, O.W., Davis, R.F., and Mizuki, M.K. 1989. A survey of viruses infecting yellow summer squash in South Carolina. Plant Dis. 73: 401-404.
13. Sinclair J.B. and Walker J.C. 1956. A survey of ring spot on cucumber in Wisconsin. Plant Dis. Rep. 40: 19-20.
14. Ullman, D.E., Cho, J.J., and German, T.L. 1991. Occurrence and distribution of cucurbit viruses in the Hawaiian Islands. Plant Dis. 75: 367-370.
15. Walters, S.A., Kindhart, J.D., Hobbs, H.A., and Eastburn, D.M. 2003. Viruses associated with cucurbit production in southern Illinois. HortScience 38: 65-66.
16. Webb, R.E. 1971. Watermelon mosaic virus 1 and 2 in squash on the Atlantic seaboard. Plant Dis. Rep. 55: 132-135.
17. Zitter, T.A., Hopkins, D.L., and Thomas, C.E. 1996. Compendium of Cucurbit Diseases. APS Press, St. Paul, MN.
The over-all goal of this two-year project was to identify viruses associated with cucurbit crops mainly pumpkin and squash in Illinois. The specific objectives were: 1) to determine the occurrence and distribution of viral diseases in pumpkin and squash in Illinois using serological detection methods; 2) to determine symptoms associated with each virus; 3) to test the “Koch’s Postulates” for all the viruses identified in pumpkin and squash; and 4) to use molecular diagnostic methods to confirm the identification of viruses detected for the first time in Illinois.
Research
Surveys were conducted in 2004, 2005, and 2006 growing seasons to determine viruses associated with pumpkin and squash crops in Illinois. In 2004, 16 jack-o-lantern pumpkin samples and one squash sample were collected from 11 random counties in August and September. Sample collection in 2005 and 2006 was carried out after dividing the 102 counties in the state into four regions namely the northeast, southeast, southwest, and northwest (Fig. 1A).
In 2005, 85 jack-o-lantern pumpkin, 12 processing pumpkin, 37 squash, and six gourd samples were collected from 54 counties from June through October (Fig. 3). The counties included Boone, Champaign, Cook, Dekalb, Dupage, Iroquois, Kane, Kankakee, Kendal, McHenry, McLean, Piatt, and Will in the northeast. The counties included from southeastern region were Clay, Cumberland, Douglas, Edgar, Fayette, Gallatin, Jasper, Jefferson, Lawrence, Macon, Marion, Moultrie, Wayne, and White. In the southwest, samples were collected from Adams, Brown, Cass, Calhoun, Jackson, Johnson, Jersey, Macoupin, Madison, Pulaski, Sangamon, Scott, St. Clair, and Union counties. In the northwest, the counties included in the sample collection were Bureau, Henderson, Jo Daviess, Mason, Marshall, McDonough, Mercer, Ogle, Peoria, Rock Island, Tazewell, Warren, and Winnebago.
In 2006, 85 jack-o-lantern pumpkin, 16 processing pumpkin, 51 squash, and 18 gourd samples were collected from 47 counties from July through September (Fig. 4). The counties from northeast were Boone, Champaign, Cook, Dekalb, Iroquois, Kane, Kankakee, LaSalle, Livingston, McHenry, and Will. In the southeast, the counties included in the survey were Clay, Douglas, Edgar, Gallatin, Jefferson, Macon, Massac, and Moultrie. In the southwest, samples were collected from Adams, Cass, Calhoun, Jackson, Johnson, Madison, Randolph, St. Clair, Union, Williamson, and Washington counties. In the northwest, the counties included in this study were Bureau, Carroll, Henderson, Henry, Knox, Mason, Marshall, Ogle, Peoria, Rock Island, Putnam, Stark, Stephenson, Tazewell, Winnebago, Whiteside, and Woodford. In 2006, samples were also collected monthly from selected commercial fields to determine the viruses present during the various growth stages of jack-o-lantern pumpkin, processing pumpkin, and squash. The samples of jack-o-lantern pumpkins and squashes were collected from one field in each of Kankakee county (in northern Illinois), Champaign county (in central Illinois), and Gallatin county (in southern Illinois). Processing pumpkin samples were collected from three fields in Tazewell county in central Illinois. The samples were collected during the months of June, July, August, and September.
Samples from symptomatic pumpkin, squash, and gourd plants were collected from 10 different spots within each field by walking diagonally in the field. At each spot, at least two leaves exhibiting virus symptom and at least one six-inch vine were collected. If no symptoms were evident in the plants at the time of sample collection, the newest leaves on the plant were collected from ten locations at random. The samples collected from the field were placed in plastic bags which were labeled. The samples were transported to the laboratory, while kept at the coolest possible conditions, on the same day or shipped overnight after cooling at 4oC for at least two hours. Upon reaching the laboratory, the samples were immediately placed at 4oC and were assayed for the viruses or inoculated onto healthy plants for symptom development within a week. The samples collected were also stored in plastic bags at -20oC or -80oC for later studies. The symptoms observed on the leaves, vines, and fruits; the cultivars of the crop; and the distribution of the infected plants in the field were recorded for each sample collected. Further information on the symptoms exhibited was also recorded in the laboratory.
The freshly collected samples were tested for CMV, PRSV, SqMV, TRSV, ToRSV, WMV, and ZYMV utilizing DAS-ELISA kits from Agdia Inc. (Elkhart, IN). The potyviruses were also detected using indirect enzyme-linked immunosorbent assay general potyvirus group kits from Agdia Inc. In 2005 and 2006, 40 and 48 randomly selected samples, respectively, were tested for the presence of Squash leaf curl virus (SLCV) utilizing triple-antibody sandwich enzyme-linked immunosorbent assay kits from Neogen Europe (Scotland, UK). The ELISA’s were conducted as the virus detection kit instructions. The ELISA reaction was measured using spectrophotometer (Multiskan Ascent, Labsystems, Helsinki, Finland) at 405 nm and also confirmed visually. Samples were considered positive for virus in ELISA if the absorbance reading at 405 nm was equal to or more than 3x the absorbance of the negative control. Positive controls (Agdia Inc.) for each virus were included in every ELISA. Negative controls consisting of one well with extract from healthy pumpkin or squash tissue and one well with ELISA extraction buffer were also included in every ELISA.
TRSV and ToRSV, which were detected for the first time in pumpkin and squash plants in Illinois, were also identified by reverse transcription-polymerase chain reactions (RT-PCR). Primers for TRSV and ToRSV were synthesized by identifying conserved regions in the coat protein gene among different strains from the Genbank database (SDSC Biology Workbench) using the multiple sequence alignment program ClustalW. Primers were designed using the program Primer3 for this conserved sequence in the SDSC Biology Workbench, a web based tool. The primers used for TRSV were forward primer CTTGCGGCCCAAATCTATAA and reverse primer ACTTGTGCCCAGGAGAGCTA to produce a product of 348bp. The PCR was done by initial denaturing of DNA at 94°C for 30 s followed by 35 cycles of 94°C for 30 s, 58°C for 45 s, and 72°C for 1 min, with a final extension period of DNA at 72°C for 10 min. The PCR product and HyperladerV ladder (Bioline USA Inc., Randolph, MA) were loaded on a 3.5% agarose gel and stained with ethidium bromide. A positive control consisting of a TRSV-infected plant sample and a negative control consisting of a healthy pumpkin or squash sample were also included in all RT-PCR. The primers for detection of ToRSV were forward primer CATGGGCGCATTAAAAAGAT and reverse primer AAGTGGTTGCACTGTTGACG to produce a product of about 609bp The PCR was done by initial denaturing of DNA at 94°C for 30 s followed by 35 cycles of 94°C for 30 s, 57 °C for 45 s, and 72°C for 1 min with a final extension period of DNA at 72°C for 10 min. The PCR product and TrackIt 100bp DNA ladder (Invitrogen, Carlsbad, CA) were loaded on a 1% agarose gel and stained with ethidium bromide. A positive control consisting of a lyophilized ToRSV infected plant sample and a negative control consisting of a healthy pumpkin or squash sample were also included in all RT-PCR. The RT-PCR was conducted on total RNA extracted from 50-100 mg of symptomatic leaf using TRIzol Reagent (Invitrogen) and c-DNA was produced by reverse transcription with M-MLV Reverse Transcriptase (Invitrogen) using the reverse primers, and RNase inhibitor RNaseOUT (Invitrogen) following the instructions provided by the manufacturer.
To test “Koch’s Postulates”, fresh foliage samples exhibiting viral infection were collected from the various locations and tested for viruses by ELISA. Samples that tested positive for viruses were inoculated onto pumpkin (C. pepo ‘Howden’ and C. moschata ‘Dickinson’) and squash (C. pepo ‘Fortune’ and ‘Grey Zucchini’). The samples were ground in cold 0.025M potassium phosphate buffer, pH 7.1, with 0.01M sodium sulfite and spread with cheese cloth onto 2-3 leaf-stage seedlings, which were dusted with Carborundum prior to the inoculation. The inoculated plants were then rinsed with water and maintained in a greenhouse for 4 weeks at day temperatures ranging from 23oC to 25oC, night temperatures ranging from 21oC to 23oC, and a photoperiod from 6 a.m. to 9 p.m., to allow symptom development. The presence of a virus was verified in symptomatic plants by ELISA.
Viruses detected in the pumpkin, squash, and gourd fields surveyed in 2004, 2005, and 2006, were CMV, PRSV, SqMV, TRSV, ToRSV, WMV, ZYMV, and unknown potyvirus(es). ToRSV was detected only in one jack-o-lantern pumpkin sample in 2004 and TRSV was detected in jack-o-lantern pumpkin and processing pumpkin samples only in 2005. Processing pumpkin samples were generally free of viral infection and only single virus infections of PRSV, unknown potyvirus(es), and TRSV were detected in one sample each, collected in 2005. Most of the viruses detected during the surveys produced similar symptoms on pumpkin, squash, and gourd plants as the same virus produced a wide range of symptoms on different cultivars. Common symptoms observed on the infected plants were light and dark green mosaic, veinbanding and veinclearing, sometimes accompanied with puckering or deformation of pumpkin and squash leaves. Severe symptoms on the leaves were fernleaf and shoestring with reduction of fruit size, deformed fruit, and color breaking of fruit.
Cucumber mosaic virus (CMV). The occurrence of CMV in pumpkin and squash fields was mostly confined to the northeastern region of the state. In 2004, CMV was detected in one jack-o-lantern pumpkin sample from McHenry county as a single-virus infection (Table 1, Fig. 1B). In 2005, CMV was identified in five samples collected from Cook, Kane, McHenry, and Will counties in the northeastern region and Madison county from the southwestern region (Table 2, Fig. 2A). CMV was detected as a single-virus infection in two samples; mixed infection of two viruses (CMV and WMV) in 2 samples; and mixed infection of four viruses (CMV, PRSV, SqMV, and ZYMV) in one sample (Table 2). In 2006, CMV was detected in five samples from Kane and McHenry counties (northwestern region); and Madison county (southwestern region) (Table 3, Fig. 2B). Mixed infections of CMV with WMV were identified in three samples and CMV with WMV and SqMV were detected in two samples (Table 3).
The most common symptoms observed on leaves of squash and pumpkin infected with CMV was mosaic of green and light green or yellow with curling. Severe symptoms caused by CMV included puckering of leaves with mosaic followed by fernleaf. The fruits exhibited galls, distortion, and reduction of size. CMV symptoms were observed in isolated spots in early- and mid-August to all through the field by late-August or September.
Papaya ringspot virus (PRSV). In 2004, PRSV was detected in a single jack-o-lantern pumpkin sample collected from Champaign county, as a mixed infection with ZYMV (Table 1, Fig. 1B). In 2005, PRSV was identified in 15 samples, from McHenry, Dekalb, and Kankakee counties in the northeastern region; Wayne county in the southeastern region; Cass and Sangamon counties in the southwestern region; and Tazewell, Peoria, and Rock Island counties, in the northwestern region (Fig. 2A). PRSV was detected as a single virus infection in six samples; two-virus mixed infection of PRSV with WMV in two samples; and two-virus mixed infection of PRSV with SqMV in four samples. Three-virus mixed infection of PRSV with WMV and SqMV was detected in two samples and a four-virus mixed infection of PRSV with CMV, SqMV, and ZYMV was detected in one sample (Table 2). In 2006, PRSV was identified in seven samples tested from Champaign and Iroquois counties (northeastern region); Moultrie county (southeastern region); and Whiteside county (northwestern region) (Table 3, Fig. 2B). Single-virus infection of PRSV was detected in two samples; two-virus mixed infection of PRSV with SqMV was recorded in two samples; three-virus mixed infection of PRSV with WMV and SqMV was detected in one sample; and three-virus mixed infection of PRSV with WMV and ZYMV was detected in two samples (Table 3). The low incidence of PRSV suggests that it does not pose a threat to pumpkin and squash production in Illinois.
Severe mosaic of green and dark green with leaf deformation and blistering were noticed on squash and pumpkin leaves infected with PRSV. Pumpkin leaves also exhibited veinbanding and veinclearing on leaves. The fruits on PRSV-infected plants showed color breaking.
Potyvirus genus. In 2005, unknown potyvirus(es), serologically distinct from PRSV, WMV, and ZYMV, were detected in three samples. Infected samples had been collected from Kane county in the northeastern region, and Marshall and Tazewell counties in the northwestern region (Table 2, Fig. 2A). Further ELISA of the samples failed to show positive reaction for the three potyviruses (PRSV, WMV, and ZYMV) included in this study. In 2006, potyvirus assays detected serologically-distinct potyvirus(es) from WMV, PRSV, and ZYMV in eight samples from Kankakee and Will counties in the northeastern region; Jackson, Randolf, and Williamson counties in the southwestern region; and Henderson county in the northwestern region (Table 3, Fig. 2B). Infection of the unknown potyvirus(es) alone was detected in three samples; and mixed infection of the potyvirus(es) with SqMV was detected in five samples (Table 3). The results of this test suggest the possibility of different potyvirus(es) in the samples.
The general symptoms associated with the unidentified potyvirus(es) were mild mosaic, veinbanding, fernleaf and reduction of leaf size. Plants infected with TRSV exhibited mild yellow mosaic, crinkling, and thickening of leaves. Some plants also showed general yellowing of the leaves. The symptoms produced by this unknown potyvirus(es) were similar to the symptoms produced by the other viruses infecting pumpkin and squash. Potyviruses Clover yellow vein virus, Watermelon mosaic virus-Morocco and Zucchini yellow fleck virus have also been reported to infect cucurbits (17). However, we have not been able to confirm the presence of this virus using RT-PCR. Further investigation is required to identify the unknown potyvirus(es).
Squash mosaic virus (SqMV). In 2004, SqMV was detected in only one jack-o-lantern pumpkin sample tested, which was as a single-virus infection from Dekalb county (Table 1, Fig. 1B). In 2005, 57 samples representing 34 counties (63% counties surveyed) throughout the state were found infected with SqMV (Table 2, Fig. 3A). Single-virus infection of SqMV was detected in 27 samples. Two-virus mixed infection of SqMV with WMV was detected in 21 samples; mixed infection of SqMV with PRSV was detected in four samples; and mixed infection of SqMV with TRSV was detected in two samples. Three-virus mixed infection of SqMV, WMV, and PRSV was detected in two samples and four-virus mixed infection of SqMV, PRSV, ZYMV, and CMV was detected in one sample. In 2006, SqMV was detected in 81 samples collected from 38 counties (81% counties surveyed) throughout the state (Table 3, Fig. 3B). Single-virus infection of SqMV was identified in twenty samples. Mixed virus infection of SqMV with CMV was detected in one sample; SqMV with unidentified potyvirus(es) was determined in five samples; SqMV with PRSV was detected in two samples; and SqMV with WMV was detected in 50 samples (29% of samples tested). Three-virus mixed infection of SqMV, ZYMV, and WMV was detected in two samples and mixed infection of SqMV, CMV, and WMV was determined in one sample. SqMV was detected in more counties than any of the other viruses identified in pumpkin and squash fields in 2005 and 2006. The possible reasons for high incidence of SqMV in Illinois could be (i) its introduction to the fields by undetected infected-seeds (8) and/or (ii) its spread by cucumber beetles from overwintering weeds and within a field and between neighboring crops or fields (4,10,12). Cucumber beetles were prevalent in pumpkin and squash fields in Illinois during sample collection.
Mosaic of light green and dark green with thickening of leaves and crinkling or slight puckering were associated with SqMV on pumpkin and squash. In severely infected plants, shoestring and fernleaf symptoms were observed on jack-o-lantern pumpkin. SqMV also caused veinbanding, ringspot, blistering, and deformation on leaves. Color breaking on fruit was observed in squash plants infected with SqMV. The symptoms associated with single infection of SqMV were mostly present in isolated plants in the fields, although SqMV-infection was noticed throughout the fields in Cook, Gallatin, Marshal, Scott, and Winnebago counties in 2005, and in Macon, Marshal, and Randolph counties in 2006.
Tobacco ringspot virus (TRSV). TRSV was detected in 2005 for the first time in Illinois (7). The virus was detected in four pumpkin samples from Kankakee and Piatt counties in the northeastern region; Douglas county in the southwestern region; and Tazewell county in the northwestern region (Table 2, Fig. 2A). Single-virus infection of TRSV was detected in two samples and mixture infection of TRSV with SqMV was recorded in two other samples (Table 2). TRSV was not detected in the samples tested in 2004 and 2006. Plants infected with TRSV exhibited mild yellow mosaic, crinkling, and thickening of leaves. Some plants also showed general yellowing of the leaves.
Tomato ringspot virus (ToRSV). ToRSV was detected by ELISA as a mixed infection with WMV in only one sample a (jack-o-lantern pumpkin) collected from St. Clair county (southwestern region) in 2004 (Table 1, Fig. 1B). The presence of this virus however could not be confirmed by RT-PCR, as the sample failed to produce the expected PCR product. In 2005 and 2006, ToRSV was not detected in any sample assayed.
Watermelon mosaic virus (WMV). WMV was the most prevalent virus throughout the state as it was detected in 161 of 327 (49%) pumpkin, squash, and gourd samples tested during the surveys over the three years. In 2004, WMV was detected in eight jack-o-lantern pumpkin samples tested. Infected samples were collected from Adam, Cook, Madison, and St. Clair counties (Fig. 1B). Single infection of WMV was detected in six samples. Mixed infection of WMV with ToRSV was detected in one sample and WMV with ZYMV in another sample (Table 1). In 2005, WMV was detected in 64 samples representing 28 counties (51% counties surveyed) throughout the state (Table 2, Fig. 4A). WMV was identified as a single-virus infection in 36 samples. Two-virus mixed infection of WMV and SqMV was detected in 21 samples; mixed infection of WMV and PRSV in two samples; and mixed infection of WMV and CMV in two samples. Mixed infection of three viruses of WMV with PRSV and SqMV was detected in two samples and WMV with ZYMV and SqMV was detected in one sample. In 2006, WMV was detected in 89 samples from 37 counties (79% counties surveyed) throughout the state (Fig. 4B). Single-virus infection was detected in 30 samples (Table 3). Mixed infection of two viruses WMV and SqMV were identified in 50 samples (29 % of samples tested); WMV and CMV were detected in two samples; WMV and PRSV were detected in one sample; and WMV and ZYMV were detected in two samples. Three-virus infection of WMV with PRSV and ZYMV was detected in one sample; mixed infection of WMV with SqMV and ZYMV in two samples; and mixed infection of WMV with SqMV and CMV was detected in one sample (Table 3). The results of this study are similar to reports from southern Illinois, where WMV was determined to be the most prevalent virus in jack-o-lantern pumpkin and squash fields (15).
WMV-infection induced light and dark green mosaic with or without puckering and deformation on pumpkin and squash leaves. Severe symptoms of WMV infection on leaves included fernleaf symptom and veinbanding or veinclearing on jack-o-lantern pumpkin. Deformation and galls were commonly evident on pumpkin fruits. Symptoms caused by WMV were generally noticed throughout the field during the growing seasons, indicating its rapid spread in the fields.
Zucchini yellow mosaic virus (ZYMV). ZYMV was detected in three jack-o-lantern pumpkin samples in 2004, which were collected from Adams, Champaign, and Iroquois counties (Fig. 1B). Single virus infection of ZYMV was detected in one sample. Two-virus mixed infection of ZYMV with PRSV and ZYMV with WMV was detected in one sample each (Table 1). In 2005, ZYMV was detected in five samples collected from Champaign and McHenry counties in the northeastern region and Adams county in the southwestern region (Table 2, Fig. 2A). Single virus infection of ZYMV was recorded in three samples. Three-virus mixed infection of ZYMV, SqMV, and WMV was identified in one sample; and four-virus mixed infection of ZYMV, CMV, SqMV, and PRSV was detected from another sample (Table 2). In 2006, ZYMV was detected in six samples collected from Champaign and Kankakee counties in the northeastern region and Clay county in the southeastern region (Fig. 2B). Single-virus infection of ZYMV was detected in one sample. Mixed infection of ZYMV with WMV was detected in two samples; three-virus infection of ZYMV with WMV and SqMV was detected in two samples; and three-virus infection of ZYMV with PRSV and WMV was detected in one sample (Table 3). The results of this study showed that the overall incidence of ZYMV was lower than expected.
ZYMV-infection in pumpkin or squash plants was usually associated with severe symptom of green and dark green mosaic and upward curling of leaves. Veinbanding, fernleaf, and shoestrings were also associated with ZYMV-infection in squash and pumpkin. Squash plants infected with ZYMV produced fruits with color breaking symptoms. The symptoms caused by ZYMV in the field were usually present throughout the field.
Squash leaf curl virus (SLCV). None of the samples tested (40 samples in 2005 and 48 samples in 2006) was found positive for SLCV, indicating that SLCV did not exist in pumpkin or squash fields in Illinois. SLCV has been reported from Arizona, California, and Texas on squash and pumpkin (2,10). It appears that this virus does not occur in Illinois and perhaps its geographic distribution is limited to the warmer areas of the south and southwestern US.
All the viruses detected during the surveys were present alone and as in mixed infections with other viruses in the fields. Earlier in the growing seasons (July and early August), mainly single virus infections were detected. Mixed infections were more common from the second week of August until the end of the growing season in October, when two to four viruses were detected in the same plant or in the same field.
Monthly sample collection. In Kankakee county (northern Illinois), the infection by the unknown potyvirus(es) was detected in jack-o-lantern pumpkin samples in the first week of August. In September, WMV was detected in the jack-o-lantern pumpkin samples from Kankakee. Similarly, single-virus infection of WMV was detected in squash samples in August in Kankakee county. Two-virus infection of the unknown potyvirus(es) with SqMV was detected in September in this county. In Champaign county (central Illinois), three-virus mixed infection of WMV, PRSV, and SqMV was detected from jack-o-lantern pumpkin and squash fields, in September. The jack-o-lantern pumpkin and squash samples from Gallatin county (southern Illinois) had single-virus infection with SqMV in August, whereas jack-o-lantern pumpkin samples collected in September from Gallatin county showed two-virus mixed infection of WMV with SqMV. None of the processing pumpkin samples collected in July, August, or September, tested positive for the viruses included in this study.
The presence of TRSV, detected by ELISA, was confirmed by reverse transcription-polymerase chain reactions (RT-PCR), which produced a product of 348bp. This is the first time that TRSV was identified in pumpkins in Illinois. The presence of ToRSV could not be verified using RT-PCR due to the failure of the sample to produce a product of 609bp, although the positive control produced the required PCR product of 609bp.
Testing for pathogenicity. Among the six viruses (CMV, PRSV, SqMV, TRSV, WMV, and ZYMV) tested for “Koch Postulates”, only five viruses CMV, PRSV, SqMV, WMV, and ZYMV caused symptoms in the inoculated plants, and were subsequently detected in the inoculated plants using ELISA. TRSV failed to produce symptoms and was not detected by ELISA in any of the inoculated plants. Symptoms on CMV-inoculated plants were noticed by the third week after inoculation on C. moschata ‘Dickinson’ and C. pepo ‘Howden’ and ‘Grey Zucchini’. The symptoms were yellow and green mosaic with downward curling of leaves and severe reduction in leaf size and stunting of the plant. PRSV symptoms were noticed on C. moschata ‘Dickinson’ and C. pepo ‘Howden’ and ‘Grey Zucchini’ three weeks after inoculation. The symptoms varied from very mild mosaic on C. moschata ‘Dickinson’ to a moderate mosaic on C. pepo ‘Howden’ and ‘Grey Zucchini’. Plants of all the cultivar inoculated with SqMV showed symptoms within two weeks after inoculation. Symptoms induced by SqMV included mild mosaic on C. moschata ‘Dickinson’ to moderate mosaic on C. pepo ‘Howden’ and ‘Grey Zucchini’ and severe symptoms of blistering and fernleaf on C. pepo ‘Fortune’. WMV produced symptoms on leaves of inoculated plants in all four cultivars inoculated, beginning two weeks after inoculation. Symptoms included mild mosaic on C. moschata ‘Dickinson’ to severe fernleaf symptom on C. pepo ‘Howden’. Symptoms were noticed on all cultivars inoculated with ZYMV within two weeks after inoculation. ZYMV induced severe symptoms of mosaic, blistering, and veinbanding on leaves of C. moschata ‘Dickinson’ and mosaic, upward cupping, and shoestrings on leaves of C. pepo ‘Howden’, ‘Fortune’, and ‘Grey Zucchini’.
![]() Table 1. Incidence of viruses in pumpkin, squash, and gourd fields in Illinois in 2004.
Table 1. Incidence of viruses in pumpkin, squash, and gourd fields in Illinois in 2004.![]() Table 2. Incidence of viruses in pumpkin, squash, and gourd fields in Illinois in 2005.
Table 2. Incidence of viruses in pumpkin, squash, and gourd fields in Illinois in 2005.![]() Table 3. Incidence of viruses in pumpkin, squash, and gourd fields in Illinois in 2006.
Table 3. Incidence of viruses in pumpkin, squash, and gourd fields in Illinois in 2006.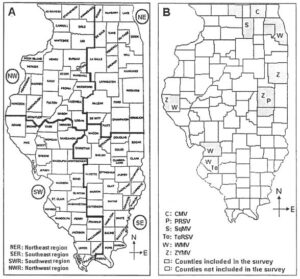
Fig. 1. A. Counties in Illinois where Cucumber mosaic virus (CMV), Papaya ringspot virus (PRSV), Squash mosaic virus (SqMV), Tobacco ringspot virus (ToRSV), Watermelon mosaic virus (WMV), and Zucchini yellow mosaic virus (ZYMV) were detected in jack-o-lantern pumpkin samples in 2004. B. Four-region divisions of Illinois for surveying pumpkin, squash, and gourd fields for incidence of viruses in 2005 and 2006.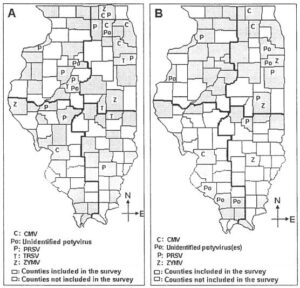
Fig. 2. Counties in Illinois where Cucumber mosaic virus (CMV), unidentified potyvirus(es), Papaya ringspot virus (PRSV), Tobacco ringspot virus (TRSV), and Zucchini yellow mosaic virus (ZYMV) were detected in pumpkin, squash, and gourd samples A. in 2005. B. in 2006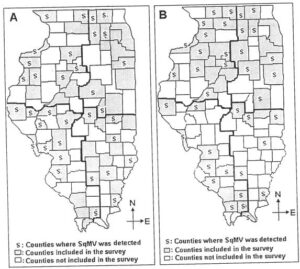
Fig. 3. Counties in Illinois where Squash mosaic virus (SqMV) was detected in pumpkin, squash, and gourd samples A. in 2005 B. in 2006.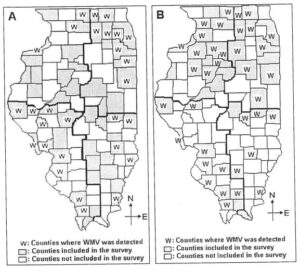
Fig. 4. Counties in Illinois where Watermelon mosaic virus (WMV) was detected in pumpkin, squash, and gourd samples A. in 2005 B. in 2006.
Educational & Outreach Activities
Participation Summary:
1. Jossey, S., and Babadoost, M. 2006. First Report of Tobacco ringspot virus in Pumpkin (Cucurbita pepo) in Illinois. Plant Dis. 90: 1361.
2. Jossey, S., and Babadoost, M. 2006. Detection and identification of viruses affecting squash and pumpkin in Illinois. Phytopathology 96:S57, In the Abstracts of papers of joint meeting of APS, CPS, and MSA. July 29-August 2, 2006, Quebec City, Canada.
3. Jossey, S. 2006. Incidence of viruses in pumpkin and squash in Illinois. Masters thesis, Department of Crop Sciences, University of Illinois, Urbana-Champaign.
4. Jossey, S., and Babadoost, M. 2006. Occurrence and distribution of pumpkin and squash viruses in Illinois. Plant Dis. 90: (in review).
5. Jossey, S, and Babadoost, M.. 2006. Detection and identification of viruses affecting pumpkin and squash in Illinois. Pages …(in press) 10th Annual Illinois Fruit and Vegetable Research Report.
Outreach
1. Poster presentation (Detection and identification of viruses affecting squash and pumpkin in Illinois) in 2006, in Quebec City, Canada, during July 29-August 2, in the annual meetings of the American Phytopathological Society (APS), Canadian Phytopathological Society (CPS), and Mycological Society of America (MSA) with more than 2,000 participants from all over the world.
2. Oral presentation (Viral diseases of pumpkins and squashes in Illinois) on the Illinois Pumpkin Field Day on September 8, 2006, in Champaign, IL. More than 100 growers, extension educators, and agribusiness personnel from Illinois and other states of the North Central region participated in the meeting.
3. Oral presentation (Review of vegetable diseases in 2006) in Schererville, Indiana, on January 3, 2007, in annual meeting of vegetable growers of Indiana and Illinois, with more than 100 participants.
4. Oral presentation (Management of pumpkin diseases) in Springfield, Illinois, on January 11, 2007, in Pumpkin Workshop, with more than 110 participants from Illinois, Iowa, Missouri, and Indiana.
5. Oral presentation (Viral diseases of pumpkins and squashes in Illinois) in Leamington, Ontario, Canada, March 1, 2007, in the annual meeting of research/extension specialists of Great Lake states including the North Central region.
6. Based on findings of this research, Abbot and Cobb Inc. together with the University of Illinois initiates an extensive screening and breeding program on squashes in 2006, which have very promising results.
Project Outcomes
Illinois grows about 20,000 acres of pumpkin and 8,000 acres of other cucurbits (cucumbers, melons, and squashes). More than 90% of commercial processing pumpkins are produced and processed in Illinois. Also, more than 100,000 acres of cucurbits are produced in the North Central region annually. Viral diseases occur in cucurbit field in Illinois, as well as in the North Central Region, every year causing up to 100% crop losses. The identity and distribution of the viruses infecting in the North Central region was not precisely known. This research determined the occurrence and distribution of viruses in pumpkin, squash, and gourd fields in Illinois, which likely represent the North Central region. With the findings of this study, the researchers initiate the necessary program to develop effective methods for management for the threatening viral diseases in Illinois, the North Central region, and likely in the nation.
Economic Analysis
This research focused on determining the occurrence and distribution of viruses affecting pumpkin and squash fields in Illinois, which also would apply to the North Central region. In short-term, the economic impact of this research may not be noticed. But, the long-term economic impact of this research in cucurbit industry is expected to be considerable. As an example is 100% crops losses in cucurbit fields in northeastern Illinois in 2003, which was due to large extend to occurrence of viral diseases. Average farm-gate value of cucurbit crops in Illinois is estimated to be $2,500 per acre. Thus, the value of crop losses in 10,000 acres of jack-o-lantern pumpkins in Illinois only could be as high as $25,000,000. Findings of this research would facilitate developing of effective methods for managing viral diseases in cucurbits. In deed, accurate identification of pathogens is the most important step in developing effective methods for management of plant diseases.
Farmer Adoption
Adaptation of the results of this research by growers is already underway. One of the main findings of this research was wide occurrence of SqMV (Squash mosaic virus) in Illinois. SqMV is a seed-borne pathogen. The growers intend to make sure that their seeds come from reliable sources and are SqMV-free. Another finding of this research is that occurrence of CMV, PRSV, and ZYMV were less important than originally thought.
Areas needing additional study
Additional studies are needed to: 1) identify unknown potyvirus(s), 2) determine genetic and pathogenic variations among isolates of the identified major viruses (especially SqMV and WMV), 3) study epidemiology of squash mosaic and watermelon mosaic diseases, and 4) initiate serious programs for developing effective measures for management of viral diseases in cucurbit crops.