Final report for GNC17-238
Project Information
Understanding the biology of beneficial insects in agricultural landscapes for the development of conservation biological control schemes is an understudied area of research that is of vast importance. Hover flies (family Syrphidae) uniquely provide multiple beneficial services for growers during different life stages. Adults are important pollinators of several crops while many larvae are voracious predators of soft-bodied insects like aphids. While most research focuses on attracting adult hover flies and other natural enemies to agricultural landscapes during the growing season, little work has explored methods to retain populations over the winter, which is critical for controlling early aphid outbreaks in the spring. This project is exploring hover fly winter survival strategies and developing methods for predicting and enhancing their prevalence in the landscape during the spring. This knowledge is of substantial interest to growers as it reduces wasted resources spent seeking control measures during the growing season. Using a combination of field observations and lab experiments, three objectives were addressed to investigate two key aspects of hover fly winter behavior: migration and diapause. Objective one was to use stable isotope analysis to determine if hover flies migrate south in the fall to avoid winter temperatures. Objective two was to use a series of laboratory experiments to determine the lower temperature survival thresholds of three common hover fly species, allowing predictions as to their ability to survive winter conditions. Finally, the third objective was to determine whether diapausing hover flies and other beneficial arthropods preferentially overwinter within cultivated fields or managed field margins. The results of these three objectives are presented below.
Introduction
Hover flies (Family Syrphidae) play multiple key roles in agricultural systems, serving as important pollinators as adults (Orford et al. 2015) and voracious predators as larvae, controlling aphids on a variety of crops (Bugg et al. 2008). They are among the most important predators of aphids feeding on Michigan winter wheat (Safarzoda et al. 2014), lettuce (Nelson et al. 2012), and soybean (Noma and Brewer 2008), consuming as many as 20 aphids in 15 minutes (Rotherey 1983). Current conservation biological control schemes that utilize hover flies focus on attracting adults using flowers (Colley and Luna 2000), weedstrips (Frank 1999), aphid pheromones (Verheggen et al. 2008), and hedgerows (Wratten et al. 2003). However, these studies often fail to discuss retaining populations over the winter, which is critical to ensuring eggs are laid early in the spring when aphid populations are low and more easily controlled (Safarzoda et al. 2014). Understanding the winter strategies of hover flies is key to retaining them in the landscape, providing efficient and cost-effective pest control.
In response to onset of winter, many insects seek shelter in dirt and dead vegetation where they enter a state of dormancy known as diapause (Hondelmann and Poehling 2007) and remain until conditions become favorable in the spring. Many aspects of this strategy in hover flies, however, are poorly understood. It is not known which life stages enter diapause, where in agricultural landscapes they do this, what cues cause them to emerge, and what temperatures ensure survival, all of which are important for predicting abundances in the spring. It is possible, for example, that if winter temperatures are less harsh, more individuals will survive and thus provide better aphid control in the spring. Additionally, if hover flies prefer to diapause within fields, agricultural practices such as tilling could be timed to allow for higher survival. As an alternative to diapause, some hover flies may migrate in response to winter cues, which could significantly affect the source of hover flies on a year-to-year basis.
A subset of individuals for some hover fly populations may migrate in response to decreasing temperatures rather than enter diapause immediately (Ouin et al. 2011). Although migrations of hover flies were observed in Europe (Aubert et al. 1976), little is known about the migrations of North American hover flies and the consequences for pest suppression. Migrations may provide an opportunity for farmers to boost hover fly populations before winter by attracting migrating individuals using fall-flowering plants and providing overwintering habitat. This is of particular benefit to farmers as migrating adult individuals likely emerge earlier than individuals that diapause as pupae or larvae, making them important for stopping aphid outbreaks early. No studies to date, however, have investigated the mass migrations of North American hover flies, although field observations suggest some hover flies exhibit this behavior (Skevington 2016). Knowledge of this unique winter survival strategy, therefore, is critical in designing conservation biocontrol schemes.
Objectives
The purpose of this project is to investigate two key strategies that hover flies use to survive the winter, which are important for controlling pest populations in the spring: migration and diapause. The three major objectives (see also Figure 1) are as follows:
- Objective 1: understand the fall migration dynamics of hover flies occupying agricultural landscapes
- Objective 2: determine the lower winter temperature survival thresholds for common hover fly species occupying local farms
- Objective 3: investigate where hover flies and other beneficial arthropods overwinter in agricultural landscape
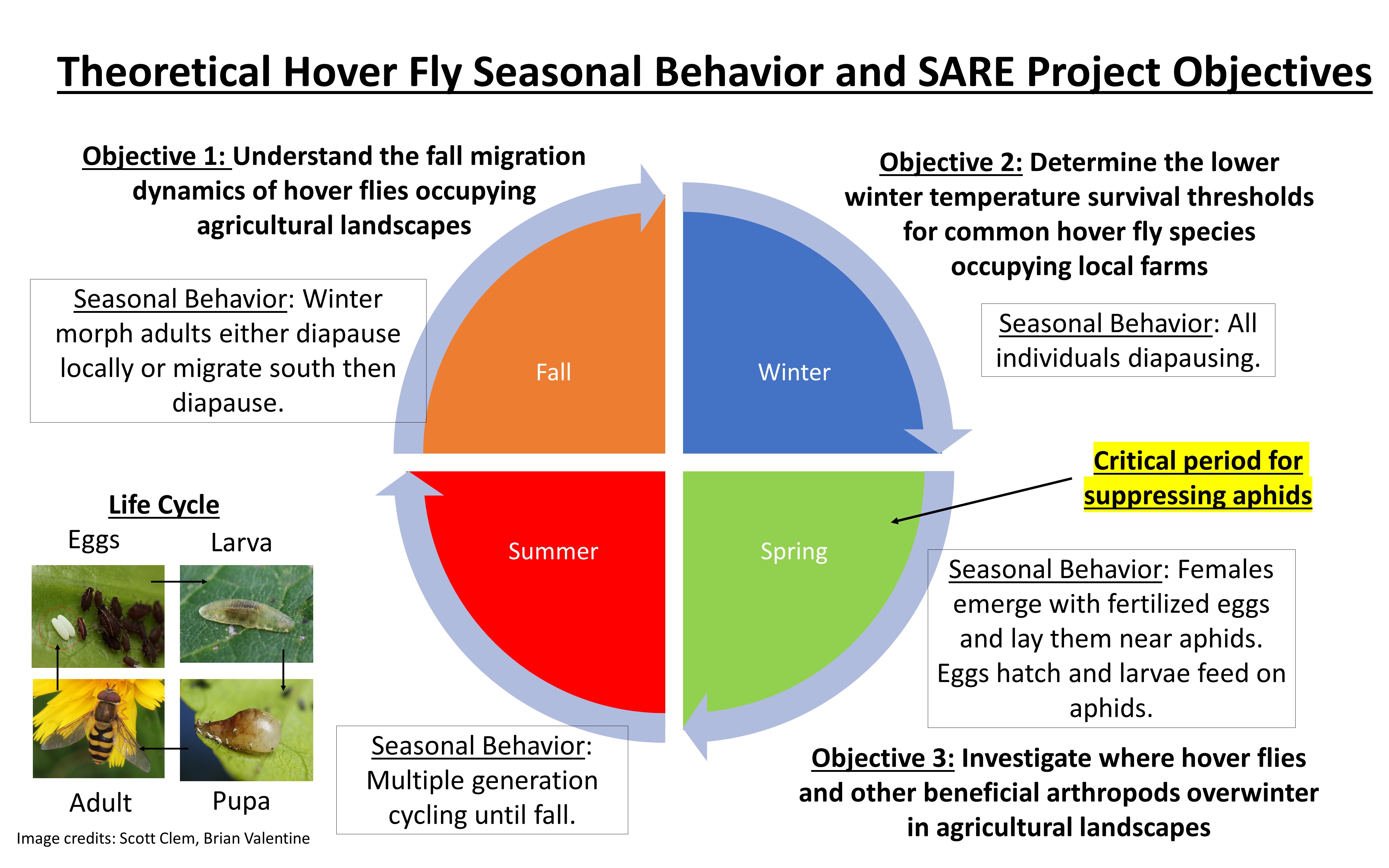
Figure 1: Outline of SARE project objectives as they relate to hover fly seasonal behaviors and hover fly overwintering strategies
Research
Objective 1: understand the fall migration dynamics of hover flies occupying agricultural landscapes
This objective was addressed using stable isotope analysis. Stable isotopes are natural variations of chemical elements like hydrogen (deuterium; δ2H) that fluctuate predictably on a north-south gradient throughout North America. Isotope ratios are expressed as parts per thousand using delta notation (δ), which is a positive or negative number that is a relative difference to an international standard. Insects accumulate isotopes into their tissues as larvae, which are permanently fixed in adults, thus giving a signature of where they originated. These isotope ratios can therefore be used as markers indicating where individual flies originated (Hobson and Wassenaar 2008). For example, if a fly larva developed near Winnipeg, Canada then flew south as an adult, it would possess the isotopic signature of that region of Canada.
Three species of common aphidophagous hover flies (Allograpta obliqua, Eupeodes americanus, and Syrphus ribesii; Figures 2A-C) were sampled twice a month from restored prairies in Urbana, IL beginning in July and ending in October when they were no longer active. These species were chosen because they are all common and either they or congeneric relatives have exhibited migratory behaviors (Aubert et al. 1976; Skevington 2016). Ten specimens per species were collected each sampling period either through hand-netting or the use of interception malaise traps. In addition, approximately twenty individual hover flies of A. obliqua and E. americanus were reared from egg to adult in the lab on locally captured aphids. Upon emergence, adults were immediately frozen. Because the water within the local aphids was their only deuterium source, these individuals provide a reliable local isotopic signature that can be used to compare with wild-caught individuals. Thus, we could determine whether wild individuals were locals or immigrants. Wings and legs were removed from each insect and sent to the Hobson lab at Western University in London, Ontario for isotopic analysis. The comparative equilibrium approach (Wassenaar and Hobson 2003) was implemented to distinguish exchangeable from unexchangeable hydrogen found within the insect chitin. Isotope data were examined using a one-way ANOVA to compare average isotopic signatures within each sampling period and the known locals.
Objective 2: determine the lower winter temperature survival thresholds for common hover fly species occupying local farms
Methods
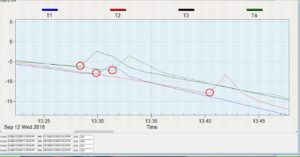
For this objective, we tested and compared the cold tolerance of three common hover fly species, Allograpta obliqua, A. exotica, and Eupeodes americanus. These two genera were selected because, based on isotope results, they represent migratory and non-migratory species. We sought to explore whether non-migratory Allograpta species exhibited greater cold-tolerance than the migratory Eupeodes americanus. Cold-tolerance was investigated by examining individual insect supercooling points. A supercooling point (SCP) is a sub-zero temperature measurement that denotes the point at which an insect’s body freezes, representing its lower lethal temperature. It is signified by a brief spike in temperature caused by the heat of ice crystallization and can be detected with a thermocouple, as seen in Figure 2.
The protocol for these tests is partially based on that of Carillo et al. 2004, who developed a simple, efficient way for investigating insect cold tolerance. Four 29x29x29 cm cubes were constructed with 5.08 cm (2 in) polystyrene insulation board glued together with PL-300 foam board adhesive, and a 2.54 cm (1 in) hole was drilled into the center. These cubes are calibrated so that objects placed into the center hole are subject to a cooling rate of -0.5oC per minute in a -80oC freezer. Living specimens were captured from local prairies in Urbana, IL when outdoor temperatures were between 18-27oC which was usually between 7:30-10AM, placed in 2-dram glass vials, brought into the lab, and allowed to acclimate to lab temperatures for one hour. During this time, flies were examined for wing wear as a proxy for age; if any portion of their wings were missing, they were not tested. Flies were then removed from vials, attached to a nickel-chromium thermocouple using high-vacuum grease, and placed into a plastic 1.7 mL microcentrifuge vial with a small hole drilled into the side as a lead for the thermocouple wire (Figure 3). A small piece of cotton ball was then placed into the top of the vial to secure the fly and the thermocouple. The vial was placed into one of the polystyrene cubes which had been in a growth chamber set at 0oC for 24hrs. A No 6.5 rubber stopper was secured into the top of the hole, and the thermocouple was connected to a HH374 Type K 4-channel data-logger. Finally, four of these assemblages were placed into a -80oC freezer and the fly temperature was recorded for 1.5 hours. Data was then extracted from the data logger and each flies’ supercooling point was noted (Figure 2; red circles). Four flies were tested per day, and the entire procedure was repeated 20 times during September and October 2018. A total of 78 flies were tested: 24 A. obliqua, 11 A. exotica, and 43 E. americanus. A generalized linear mixed model with daily high temperature designated as a random variable was implemented to compare average supercooling values between the species. Additionally, we compared the SCP between specimens collected in late August/early September vs. early October using a generalized linear model to see if there was a seasonal factor.
Figure 2: Hover fly supercooling points (red circles).
Figure 3: Protocol for Objective 2 freeze-tolerance experiments.
Objective 3: investigate where hover flies and other beneficial arthropods overwinter in agricultural landscapes
We sought to determine if hover flies and other beneficial arthropods preferentially overwinter within cultivated soybean fields or restored prairie field margins. Five privately-owned organic soybean farms in central Illinois with adjacent semi-natural prairie buffer-zones were chosen for this project. Soybean was selected as a crop because it is extensively grown throughout Illinois and subject to aphid problems. Furthermore, we selected among organic properties because of the lack of insecticides and because they are required to maintain buffer zones between their fields and conventional fields.
At each site, ten 60x60 cm emergence tent traps were placed 20 m into the field from the outside edge, and ten were placed 5 m into the prairie strip field boundary (Figure 4). These traps cover an area of soil and capture insects occupying that area. Traps were placed in a line, each 1 m from one another and parallel to the traps in the opposing habitat (Figure 4). Trap bottles were supplied with 350mL of 20% propylene glycol to euthanize and temporarily preserve specimens; a few drops of surfactant (dish soap) were also added to eliminate surface tension. Tents were placed at sites in early March 2018. Arthropods were extracted every two weeks from March 28-April 26, 2018 and placed in 70% ethanol. All predators and parasitoids were then identified to species or morphospecies. Paired t-tests were used to compare average species richness and abundance per site between the two types of habitat (n = 5).
Figure 4: Protocol for Objective 3 field experiment.
*Results presented here have not yet been published and therefore may be subject to change; we do not expect these changes to be extensive.*
Objective 1: understand the fall migration dynamics of hover flies occupying agricultural landscapes
Our isotope results suggest that two common aphidophagous Midwestern hover fly species migrate south during the fall. Based on isotope maps in the literature (Hobson et al. 2012; Wassenaar and Hobson 1998), we can infer that the more negative the δ2H values, the further north an insect originated. It was determined that local δ2H values within hover fly wing and leg chitin reliably range between -90 and -130 δ2H. We found that certain individuals of both Eupeodes americanus and Syrphus ribesii collected during autumn exhibit markedly negative δ2H values outside the range of known locals and/or those collected during expected non-migratory July weeks. Eupeodes americanus displayed the most dramatic pattern, with most individuals during late August and early/middle September exhibiting δ2H values between -140 and -181 δ2H, while those collected during July and late September/early October exhibited local signatures (Figure 5A). Most Syrphus ribesii exhibited local δ2H signatures, but there was a general pattern that δ2H became more negative as the season progressed (Figure 5B). Two individuals, specifically, exhibited the highly negative values of -138 δ2H and -147 δ2H during September suggesting that they traveled from a northern latitude. These results imply that E. americanus and S. ribesii are both partial migrants, with some individuals overwintering locally and others traveling south, E. americanus being by far the most dramatic. The third species we examined, Allograpta obliqua exhibited mostly local signatures throughout the season with little pattern suggestive of a migration, the exception being the second week of July (Figure 5C). Unexpectedly, three individuals exhibited the values of -129 δ2H, -130 δ2H, and -140 δ2H at this time point which were the most negative values from that species observed the entire season. It is unknown why these individuals had such low values during an expected non-migratory time period, but we suspect this was a dispersal event due to an environmental phenomenon like weather.
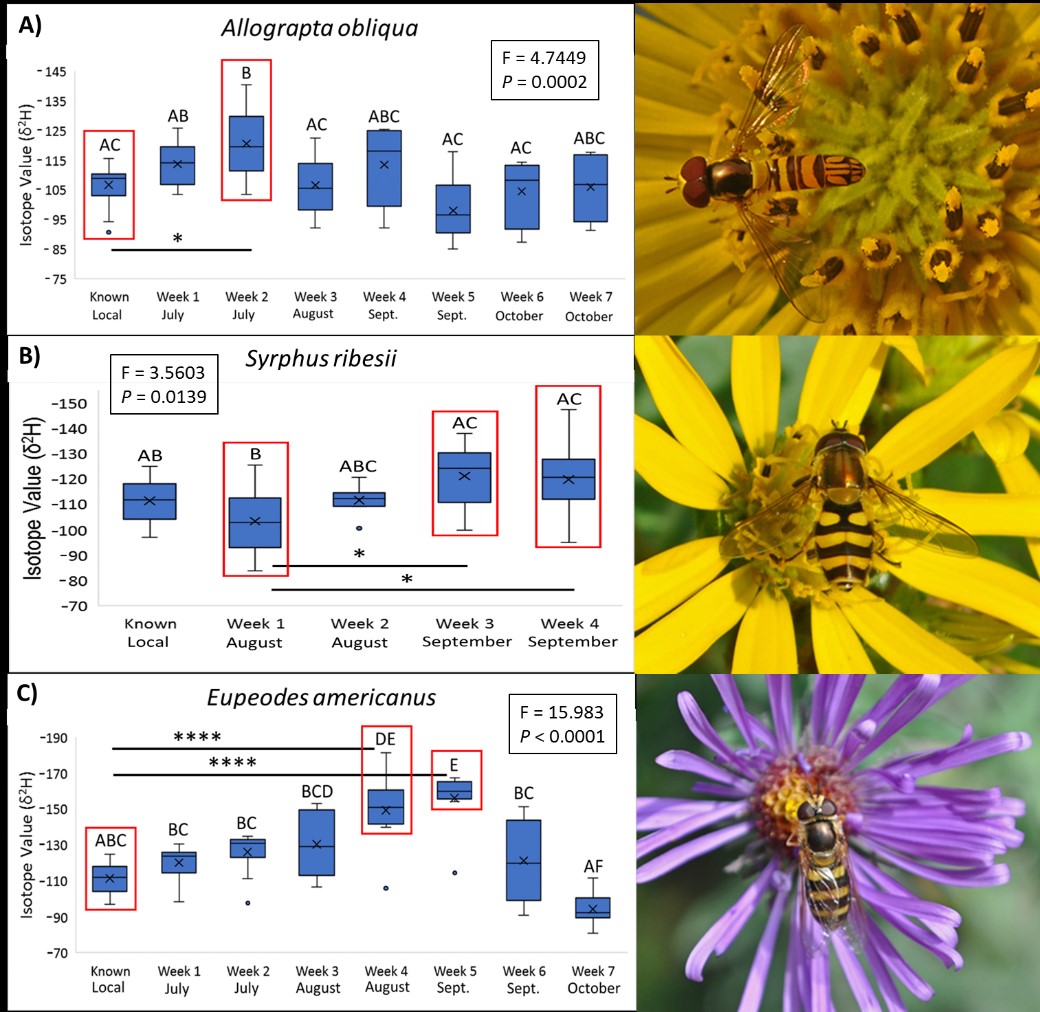
Figure 5: Results from 2017 stable isotope analyses and one-way ANOVA tests for A) Allograpta obliqua, B) Syrphus ribesii, and C) Eupeodes americanus over the season. Y-axes represent isotope ratio values; the more negative the value, the further north an individual likely developed.
These migration data are important for improving crop pollination efficiency and conservation biological control. In knowing that these flies migrate south during the fall, we recommend that farmers maintain healthy populations of common, fall-flowering plants like goldenrod (Solidego sp.) and frost aster (Symphyotrichum pilosum) in their field margins. This will help provide sufficient resources for hover flies foraging for winter refugia and traveling south during fall, boosting their winter survival and the ecological services they provide in the spring. Any flies emerging from refugia or traveling north during the spring will be seeking to lay eggs among early-forming aphid colonies. Eggs will then hatch, and larvae will extinguish aphid colonies before they become outbreaks.
Objective 2: determine the lower winter temperature survival thresholds for common hover fly species occupying local farms
With an average supercooling point of -6.17oC, E. americanus is less cold-tolerant than A. obliqua (-7.64oC; t = -2.182, P = 0.0828) and A. exotica (-9.15oC; t = -3.214, P = 0.006) (Figure 6). Additionally, there were no significant differences between specimens collected in late August/early September compared to early October (Eupeodes: t = 0.486, P = 0.630, Allograpta: t = -0.564, P = 0.57646). These results provide a potential mechanism for why Allograpta species are much less likely to migrate compared to E. americanus. Eupeodes americanus cannot tolerate the cold as well as Allograpta, so they must migrate south.
All three of these species exhibited supercooling points that were not extremely low, suggesting that they are freeze intolerant. It is therefore likely that populations of these insects will be knocked back by harsh winters like 2019, when temperatures reached -26oC. During these years, farmers may expect northward-moving spring migrants to provide more biological control.
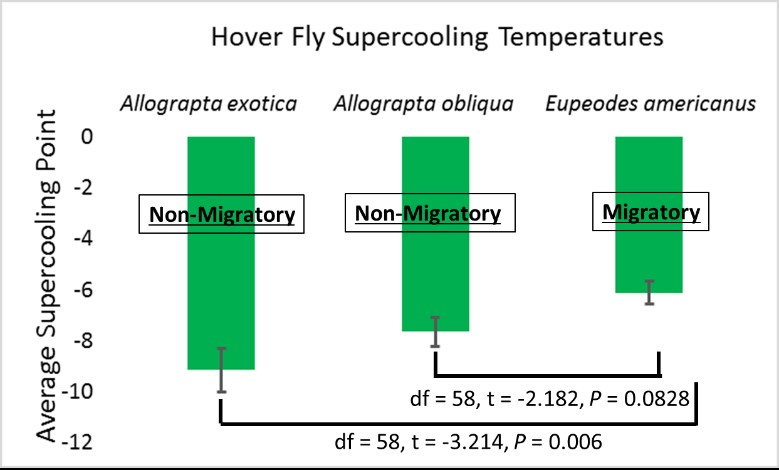
Figure 6: Results from Objective 2 freeze-tolerance experiments.
Objective 3: investigate where hover flies and other beneficial arthropods overwinter in agricultural landscapes
Results
We captured a plethora of predacious and parasitoidic arthropods (Table 1), but only one was a hover fly. Analyses reveal that semi-natural prairie-strip field borders generally supported greater abundance and species richness of overwintering predaceous and parasitoidic arthropods compared to arable organic soybean fields (Table 1).
Table 1: Average predaceous and parasitoidic arthropod species richness and abundance per site in field borders and cultivated soybean fields (paired t-test, df = 4)
|
Average Arthropod Species Richness |
|||
|
Arthropod Group |
Semi-natural field border |
Cultivated Field |
Test Statistic |
|
Predaceous beetles |
17.4 ± 0.78 |
11.4 ± 0.78 |
t = 4.602 P = 0.01* |
|
Total predators |
22.0 ± 1.19 |
14.8 ± 1.19 |
t = 4.272 P = 0.0129* |
|
Parasitoids |
15.2 ± 2.97 |
9.8 ± 2.97 |
t = 2.401 P = 0.0743 |
|
Total predators and parasitoids |
37.2 ± 3.55 |
24.6 ± 3.55 |
t = 3.658 P = 0.0216* |
|
Average Arthropod Abundance |
|||
|
Arthropod Group |
Semi-natural field border |
Cultivated Field |
Test Statistic |
|
Predaceous beetles |
112 ± 21.38 |
54.4 ± 21.38 |
t = 5.043 P = 0.007** |
|
Aranea |
29.2 ± 6.82 |
10.2 ± 6.82 |
t = 2.077 P = 0.106 |
|
Total predators |
160.4 ± 25.82 |
71.2 ± 25.82 |
t = 4.678 P = 0.009** |
|
Parasitoids |
62.2 ± 12.24 |
42.6 ± 12.24 |
t = 1.18 P = 0.302 |
|
Total predators and parasitoids |
223.6 ± 33.4 |
113.8 ± 33.4 |
t = 3.69 P = 0.021* |
Discussion
It is intriguing that only one hover fly and no bees were captured in our experiment, especially given that four out of the five properties we examined were CRP CP-42 properties designed specifically for supporting pollinators. This is contrary to previous findings in Europe that hover flies readily overwinter in agricultural fields (Raymond et al. 2014). Organic soybean may not have provided sufficient prey for hover flies in the growing season which may explain why we captured so few. Alternatively, grassland field borders and restored prairies may not be ideal overwintering habitat for these insects. Hover flies are known to overwinter in decomposing leaf litter in woodland habitat, so it is possible these habitats are more integral for hover fly winter survival.
Our results back the claim that semi-natural properties support overwintering beneficial predaceous and parasitoidic arthropods (Table 1). This is likely due to the capacity of field margins to provide more stable and complex habitat compared to arable fields. Temporary, highly disturbed agricultural fields only support a subset of the arthropods in semi-natural field margins. While the link between habitat/predator diversity and pest suppression can vary on case-by-case basis, we recommend that farmers incorporate at least some of these habitats into their management schemes. In the midst of rampant habitat disturbance and loss in the Midwest, semi-natural prairie habitats are essential for maintaining biodiversity in landscapes.
Literature Cited
Aubert J., J-J. Aubert, P. Goeldlin 1976. Douze ans de captures systematiques de Syrphides (Diptères) au col de Bretolet (Alpes valaisannes). Bull Soc Ent Suisse 49:115–142.
Bugg, R.L., Colfer, R.G., Chaney, W.E., Smith, H. a., & Cannon, J. 2008. Flower Flies (Syrphidae) and Other Biological Control Agents for Aphids in Vegetable Crops. Division of Agriculture and Natural Resources, 8285(May), 1–25.
Carrillo, M. A., Kaliyan, N., Cannon, C. A., Morey, R. V., & Wilcke, W. F. 2004. A simple method to adjust cooling rates for supercooling point determination. Cryo-Letters, 25(3), 155–160.
Colley, M.R., and J.M. Luna. 2000. Relative attractiveness of potential beneficial insectary plants to Aphidophagous Hoverflies (Diptera: Syrphidae). Environ. Entomol. 29(5):1054-1059.
Frank, T. 1999. Density of adult hoverflies (Dipt., Syrphidae) in sown weed strips and adjacent fields. J. Appl. Ent. 123:240-244.
Hobson, K.A., and L.I. Wassenaar. 2008. Tracking Animal Migration with Stable Isotopes. Terrestrial Ecology Series 2. Academic Press, London, England.
Hobson, K. A., Soto, D. X., Paulson, D. R., Wassenaar, L. I., & Matthews, J. H. 2012. A dragonfly (δ2H) isoscape for North America: A new tool for determining natal origins of migratory aquatic emergent insects. Methods in Ecology and Evolution, 3(4), 766–772. https://doi.org/10.1111/j.2041-210X.2012.00202.x
Hondelmann, P., and H-M Poehling. 2007. Diapause and overwintering of the hoverfly Episyrphus balteatus. Entomologia Experimentalis et Applicata 124:189–200.
Nelson, E.H., B.N. Hogg, N.J. Mills, and K.M. Daane. 2012. Syrphid flies suppress lettuce aphids. BioControl 57:819–826.
Orford, K.A., I.P. Vaughan, and Jane Memmott. 2015. The forgotten flies: the importance of non-syrphid Diptera as pollinators. Proc. R. Soc. B 282:20142934.
Ouin, A., P. Menozzi, M. Coulon, A.J. Hamilton, J.P. Sarthou, N. Tsafack, A. Vialatte, and S. Ponsard. 2011. Can deuterium stable isotope values be used to assign the geographic origin of an auxiliary hoverfly in south-western France? Rapid Commun. Mass Spectrom. 25:2793–2798.
Raymond, L., Sarthou, J. P., Plantegenest, M., Gauffre, B., Ladet, S., & Vialatte, A. (2014). Immature hoverflies overwinter in cultivated fields and may significantly control aphid populations in autumn. Agriculture, Ecosystems and Environment, 185, 99–105. https://doi.org/10.1016/j.agee.2013.12.019
Rotheray, G. E. 1983. Feeding behavior of Syrphus ribesii and Melanostoma scalare on Aphis fabae., 34, 148–154.
Safarzoda, S. C.A. Bahlai, A.F. Fox, and D.A. Landis. 2014. The role of natural enemy foraging guilds in controlling cereal aphids in Michigan wheat. PLoS ONE 9(12):e114230.
Skevington, J. 2016. Personal communication, October 13, 2016.
Verheggen, F.J., L. Arnaud, S. Bartram, M. Gohy, and E. Haubruge. 2008. Aphid and plant volatiles induce oviposition in an aphidophagous hoverfly. J Chem Ecol 34:301–307.
Wassenaar, L. I., Hobson, K. A., & Obson, K. E. A. H. 1998. Natal origins of migratory monarch butterflies at wintering colonies in Mexico: New isotopic evidence. Proceedings of the National Academy of Sciences, 95(26), 15436–15439. https://doi.org/10.1073/pnas.95.26.15436
Wassenaar, L. I., & Hobson, K. A. 2003. Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isotopes in Environmental and Health Studies, 39(3), 211–217. https://doi.org/10.1080/1025601031000096781
Wratten, S.D., M.H. Bowie, J.M. Hickman, A.M. Evans, J.R. Sedcole, and J.M. Tylianakis. 2003. Field boundaries as barriers to movement of hover flies (Diptera: Syrphidae) in cultivated land. Oecologia 134:605–611
Educational & Outreach Activities
Participation Summary:
I presented on topics related to this project at various informal and formal meetings. I gave a poster on conservation biological control and how to identify hover flies and other predaceous insects at the Midwest Organic and Sustainable Education Service (MOSES) Organic Growers Field Day in 2017, the University of Illinois Farm-to-Fork event in February of 2018, and at the Farming in Balance: Bringing back Wildlife to the Farm farming symposium in June 2018 and 2019. At these meetings, I also distributed handouts and used a display of pinned insects to help farmers with identification. Results from this project were presented at the 9th annual International IPM meeting in Baltimore, MD, the SARE Our Farms Our Future meeting in St. Louis, MO, the 2018 and 2019 Entomological Society of America meetings in Vancouver, CAN and St. Louis, MO, the 2019 Ecological Society of America meeting in Louisville, KY, the North Central Entomological Society webinar "Pollinators and Biological Control: Overlap and Reciprocal Insights," and the 2020 Graduate Students in Ecology and Evolutionary Biology (GEEB) symposium on the University of Illinois campus. I presented to a variety of audiences including academics, extension experts, IPM professionals, farmers, and land managers.
Project Outcomes
By understanding the winter survival strategies of understudied hover flies (Syrphidae), this project aimed to provide growers specific recommendations on methods to increase and maintain these beneficial insects on their property post-harvest to maximize the pest suppression and pollination benefits. We expect our findings to greatly contribute to agricultural sustainability, and we fully expect future researchers to expand upon our work. Hover fly migration, particularly, is a phenomenon which has been seldom described in North America and there is much left to uncover.
This project taught me to think about my research in the context of sustainable agriculture. In meeting several central Illinois farmers, I have learned a lot about how they think and what concerns them the most. This certainly gives me a perspective that I suspect many of my fellow graduate students do not have.
Information Products
- A quick guide to beneficial predacious insects (Fact Sheet)