Final report for GNC18-269
Project Information
The incorporation of cover crops (CC) in agricultural cropping systems has increased by 422% over the past eight years. This rapid increase in adoption is primarily driven by producers' knowledge of the ability cover crops to reduce sediment, increase soil heath, and to reduce nitrogen (N) loss from the landscape. The ability of cover crops to scavenge N has been well documented within the scientific community, and the decomposition and release of this N has garnered more interest amongst researchers in recent years. While the rate at which cover crop shoot biomass decomposes has been investigated, what remains unknown information is the partitioning of cover crop residue N in to soil N pools after release and the bioavailability of decomposed cover crop N across the growing period of subsequent crops. Therefore the proposed objectives of this study are to use 15N labeled isotope techniques to I.) investigate the decomposition rates of cereal rye shoot and root biomass, II.) investigate the synchrony of cover crop N release with peak N demand growth stages for maize, III.) determine the partitioning of decomposed cover crop N amongst the soil inorganic, organic, and microbial biomass N pools; among three of the most common soil orders within the North Central region. 15N labeled cereal rye root and shoot biomass will be used to track cover crop biomass N into the various soil N pools. Results from this study will enhance the knowledge surrounding cover crop root and shoot biomass decomposition and N availability, potentially allowing for producers across the North Central SARE region to advance their understanding of how cover crops affect N availability to cash crops after termination, which could lead to more effective adaptive fertilizer management.
The incorporation of cover crops into agricultural cropping systems across the North Central region has been steadily increasing over the past ten years. However, the portion of acres under cover crop production remains relatively low compared to the total number of cultivatable acres across the region. While there is ample knowledge surrounding cover crop environmental benefits such as nitrogen (N) scavenging, reduction of surface water contamination, and erosion control, hesitation in cover crop adoption could be linked to the lack of knowledge surrounding agronomic benefits provided by cover crops, specifically the release and bioavailability of N scavenged during cover crop growth. Therefore, the learning outcomes of this study are to 1.) elucidate the transition of decomposed cover crop N into the soil inorganic, organic, and microbial biomass N pools, 2.) to determine the bioavailability of that N over the duration of maize and soybean growing seasons, and 3.) investigate the synchronicity of bioavailable cover crop N with periods of peak growth and N demand in maize and soybean cropping systems. Accomplishing our learning outcomes could lead to the education of producers, trainers (i.e. certified crop advisors, government agency training officers), and the scientific community on the bioavailability of cover crop N to subsequent cash crops. Finds from this project will increase the knowledge of crop production in agricultural systems including cover crops and potentially allow for more effective adjustment of fertilizer management plans to account for bioavailable N released during cover crop decomposition and more positive economic cover crop experiences.
Cooperators
- (Researcher)
- (Researcher)
Research
Specific Research Objectives
- Investigate the dynamics of nitrogen release from cereal rye shoot and root biomass
- Investigate synchronicity of cover crop reside nitrogen bioavailability during periods of maize peak nitrogen demand within soil orders common to the North Central region.
- Determine the partitioning of decomposed cover crop nitrogen amongst soil inorganic, organic, and microbial biomass nitrogen pools across soil orders common to the North Central region
Approach and Methods
The primary strategy of this project was to use an aerobic incubation coupled with stable isotope techniques to understand the partitioning of cereal rye (CR) Residue N into the different soil N pools. The study took place within the Purdue Soil Ecosystem and Nutrient Dynamics Laboratory housed in Lilly Hall of Life Sciences at Purdue University in West Lafayette, Indiana. The experimental design consisted of a complete randomized block design with fifteen treatments (3 soil orders x 5 cover crop (CC) treatments) replicated four times. The five CC treatments will include a control (no CR biomass), 15N Enriched CR Shoot Biomass, 15N Enriched CR Root Biomass, Non-Enriched CR Shoot Biomass, and Non-Enriched CR Root Biomass. Cereal Rye was selected because of its high affinity for scavenging nitrogen, and its popularity within the North Central region (1).
Soil and Cover Crop Biomass Bulk Collection
Soil Collection
Bulk soil for use in the incubations was collected from fields at three different Purdue Agronomic Centers (PACs). All fields from which the soils were collected had the same tillage management history (no-till), the same crop rotation history (corn-soybean rotation), were all entering the same phase of the crop rotation (coming out of soybeans going into corn), and none of the fields had ever had cover crops in the past. The soils used in this experiment are representative of three of the most common soil orders within the North Central SARE region (Alfisol, Entisol, and Mollisol). The from each location were collected in bulk to a depth of 20 cm (8 in), which is the recommended depth for soil fertility analysis in the state of Indiana. Following collection, samples were transported to the laboratory in a cooler, sieved to pass through a 2mm mesh to provide a uniform soil for incubation, and then stored in a refrigerator at 4°C prior to use.
Cover Crop Biomass Collection
This study was performed simultaneously with another 15N project being conducted at the Purdue University Agronomy Center for Research and Education and utilized 15N enriched CR biomass grown as part of that study. CR biomass was collected following chemical termination in the spring, a common practice amongst producers in the North Central region, to capture any effect of chemical termination on cereal rye decomposition. 15N enriched and non-enriched CR shoot biomass were collected from their respective plots by clipping the entire aboveground portion of the plant at the soil surface. Once collected, shoot biomass was immediately placed in refrigeration until use. Cereal rye root biomass was collected in bulk by digging and collecting soil to a depth of 20 cm. Once collected, the soil containing the root biomass was transferred to a wash table where roots were rinsed free of soil and then placed into refrigeration until use. Immediately prior to use, root and shoot biomass was sized to 8mm to ensure even distribution of residue within the incubation cups.
Incubation Preparation and Procedure
Prior to incubation a sub sample of each soil was used to determine 1/3 bar water retention (field capacity moisture content) for the three soils. During incubation, soil moistures were maintained at 40% of their respective field capacity and ambient air temperature was maintained at 23.9°C ± 0.4°C, which is recommended for work considering microbial biomass nitrogen (3). The containers used for the incubation were 250ml specimen cups, with a parafilm cover containing 8 holes in a circular pattern to allow for airflow. Incubation samples were prepared by adding each soil on a 100g-dry soil basis, as calculated by the current moisture status of the soil. Cereal rye biomass was added to its respective treatments with shoots and roots being incubated separately to observe the release of nitrogen from the above and belowground portions of the plant independently. Pre-sized (8mm) CR shoot biomass was added at 0.1% by weight relative to the dry soil mass, which is representative of CR biomass equivalent to 2,000 kg ha-1, a value that is common within the North Central region and has previously been used in the scientific community (2). Within the scientific literature it is common to find shoot-to-root biomass ratios for cereal rye of 2:1; thus, root biomass was added at 0.05% by weight relative to the dry soil mass representing 50% of the shoot biomass used.
Sampling Timeline, Processing, and Analysis
Sampling and Processing
Incubations were sampled at six different times according to growing degree day (GDDC) accumulation (calculated with temperature in Celsius) which would correspond to critical growth stages of maize in a field setting. Sampling occurred at the following GDD: 0, 167 (average GDD accumulation between CC termination and maize planting), 278 (maize at the 6-leaf stage), 431 (maize 12-leaf stage), 945 (maize tasseling stage), and 1667 (maize physiological maturity). At each sampling, incubation cups were removed from the incubator and destructively sampled. The soils were analyzed for total nitrogen, inorganic nitrogen, and microbial biomass nitrogen. All values were extrapolated to a kilogram per hectare basis for relevance to the production agriculture community.
Microbial Biomass Nitrogen
Microbial biomass nitrogen was determined using a modified version of the method from Voroney, Brookes, and Beyaert (3). Immediately upon sampling the incubation cups, a 25±0.05g sample was weighed into a 125ml Erlenmeyer flask, then 75ml of 0.5M K2SO4 extracting solution is added. The flasks were covered with parafilm and then placed on an oscillating shaker for 1 hour. Following the shake the solution was filter through Whatman 42 filter paper, all extractant was collected and stored in the freezer in 20ml scintillation vials until analysis. Simultaneously to these operations, a second 25±0.05g soil sample was weighed into 50ml centrifuge tubes and placed in desiccators with a beaker containing 50ml of chloroform (CHCl3) and a few boiling stones. A vacuum pump was then used to place the desiccator under vacuum and bring the chloroform to a boil. After two minutes of boiling, the vacuum was turned off and the desiccators with the chloroform fumes were allowed to sit in a dark fume hood for 24 hours. After the 24 hour period, the centrifuge tubes containing the soils were removed from the desiccators and extracted as described above.
Just prior to colorimetric analysis the samples were oxidized in an autoclave with persulfate at 121°C for 30 minutes in order to convert all nitrogen in the extractant to NO3-N according to the procedure outlined by Chantigny et al. (4). Samples were then analyzed colorimetrically for NO3-N concentrations using a SEAL AQ2 Discrete Analyzer (SEAL Analytical US, Mequon, Wisconsin, US).
Total Inorganic Nitrogen
After the soil for microbial biomass nitrogen (MBN) was collected, the remainder of the soil was allowed to air-dry for a minimum of 7 days, and then stored in air-tight bags until needed for analysis. In order to determine the total inorganic nitrogen in the soil, a 5±0.05g sample of air-dried soil was weighed into a 50ml centrifuge tube and 50ml of 2M KCl extracting solution was added. The tubes were then capped and placed on an end-to-end shaker on high-speed for 30 minutes. After shaking, the tubes were allowed to settle for 5 minutes before filtering the supernatant through Whatman 42 filter paper. All extractant was collected and stored in the freezer in 20ml scintillation vials until analysis. Colorimetric analysis of soil NO3-N and NH4-N was conducted using a SEAL AQ2 Discrete Analyzer.
Soil Total Nitrogen
Percent soil total nitrogen was analyzed via dry combustion using a Flash 2000 Series Elemental Analyzer (ThermoFisher Scientific, Waltham, Massachusetts, U.S.). A subtraction method was used to determine the nitrogen contained in the soil organic nitrogen pool according to equation 1.
Soil Organic N kg ha-1 = Soil Total N kg ha-1 - Soil Inorganic N kg ha-1 - Soil Microbial Biomass N kg ha-1
[Eq. 1]
15N Stable Isotope Analysis and Calculations
All soil fractions from each sampling date were submitted to the Cornell Stable Isotope Laboratory for isotopic analysis of 15N concentrations. All liquid extractants were freeze dried to obtain a weighable solid material. All samples were then submitted to isotopic ratio mass spectrometry facilitated by a dry combustion instrument. The recovery of 15N in each soil N fraction will be determined using a mass balance approach (equation 2):
%N from enriched CR biomass = (atom %15N ETRT – atom %15N NETRT) / (atom %15N ECR Biomass – atom %15N NECR Biomass)
[Eq.2]
where ETRT equals the analyte from the enriched treatment, NETRT equals the analyte from the non-enriched treatment, ECR equals enriched cereal rye, and NECR equals non-enriched cereal rye.
Works Cited
- Cover Crop Survey. 2017. Conservation Technology Information Center/ Sustainable Agriculture Research and Education. http://www.ctic.purdue.edu/Cover%20Crops/. Accessed 17 Mar. 2018.
- Sullivan, D.M., Datta, R., Andrews, N. and Pool, K.E., 2011, March. Predicting plant-available nitrogen release from cover crop residues. In Proceedings of the Western Nutrient Management Conference(Vol. 9, pp. 55-60)
- Voroney, R. P., Brookes, P. C., & Beyaert, R. P., 2007. Soil Microbial Biomass C, N, P, and S. In Soil Sampling and Methods of Analysis (2nd ed.). Boca Raton, FL: CRC Press.
- Chantigny, M. H., Angers, D. A., Keiser, K., & Kalbitz, K. (2007). Extraction and Characterization of Dissolved Organic Matter. In Soil Sampling and Methods of Analysis (2nd ed.). Boca Raton, FL: CRC Press.
Results and Discussion
This NCR-SARE grant funded the isotopic analysis of the samples collected throughout the duration of this experiment, which allowed for the determination of the fate of nitrogen from cereal rye above and belowground biomass.
Objective 1: Investigate the dynamics of nitrogen release from cereal rye shoot and root biomass
Results indicate that both root and shoot N decomposition reach a plateau during the growing season. For roots, N decomposition peaks between 167 and 278 GDDC (corn planting and corn V3), before plateauing for the remainder of the season. On average, approximately 8% of CR root N is decomposed and enters the soil in any form by 1667 GDDC (physiological maturity). For shoots, N decomposition continues much later into the season until about 945 accumulated GDDC following chemical termination before plateauing through physiological maturity. On average, about 85% of CR shoot nitrogen decomposes and enters the soil by 1667 accumulated GDDC. Average CR whole plant N decomposition is highly driven by CR shoot decomposition and thus follows the same trends as the shoot N decomposition. Approximately 67% of CR whole plant N is decomposed into the soil by the time of physiological maturity. Figure 1 demonstrates the patterns of N decomposition for roots and shoots across the two soil types, and whole plant N decomposition averaged across soils.
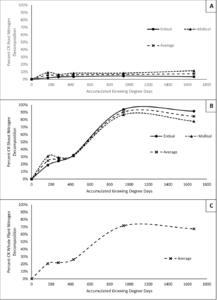
Results for the rate of CR N decomposition for both shoots and roots are highly dynamic and show how the influx of N to the soil from CR changes over the courses of the growing season. Both roots and shoots had an early peak in N decomposition rate at the 167 accumulated GDDC sampling time before steadily decreasing through the 278 to 431 GDDC samplings, followed by another peak in CR N decomposition in roots just after the 431 GDDC (Corn V6) samplings and in shoots at the 945 accumulated GDDC (Maize VT) sampling. Again, average CR whole plant N decomposition rate in highly influenced by the shoots and thus follows the same trends as the shoots (figure 2). As a note, negative rates of CR N decomposition could be partially explained due to a dilution effect occuring in the analysis. Meaning that at the time of the negative rate, mineralization of soil organic matter outweighed mineralization of the CR resulting in a depletion of nitrogen from CR within the given soil N pool.
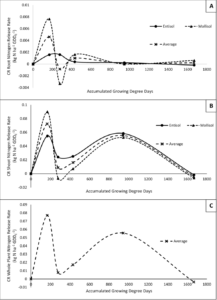
The combination of results for percent N decomposed and N decomposition rate indicate that the majority of N entering the soil from CR does not occur until later in the growing season as the plant is transitioning from vegetative to reproductive growth.
Objective 2: Investigate synchronicity of cover crop nitrogen release and bioavailability with periods of peak nitrogen demand for maize
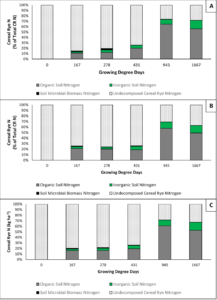
For objectives 2 and 3 of the results, root and shoot data were combined to create a total N decomposition for each soil order, as well as an average across soil types. Bioavailable CR N is the portion of CR N that decomposes and is then mineralized through microbial processes resulting in plant available nitrate and ammonium. The results from our investigation indicate that 6% or less of CR whole plant N becomes bioavailable by 431 accumulated GDDC which corresponds to an in-field maize growth stage of V6 which is when maize N demand begins to increase linearly. At the 945 accumulated GDDC (Maize VT) sampling, which corresponds to the time in-field when maize transitions from vegetative to reproductive growth, only an average of 10% of CR N is bioavailable. Further, on average, by the sampling corresponding to maize physiological maturity (1667 accumulate GDDC) just 14% of CR whole plant N had become bioavailable (Figure 3).
Objective 3: Determine the partitioning of decomposed cover crop nitrogen amongst soil inorganic, organic, and microbial biomass nitrogen pools across soil orders common to the North Central region
As seen in the results for objective 2, only 14% of whole plant CR N becomes bioavailable after 1667 accumulated GDDC. Thus, it is important to understand the fate of the remaining 86% of CR whole plant N by maize physiological maturity. Our results illustrate that the largest portion (average 53%) of CR N that enters the soil remains in the organic form, meaning that it has been decomposed and sized in such a manner that it is no longer visible to the naked eye, but has yet to go through the microbially driven mineralization process and thus remains unavailable to the plant. There is a small portion of CR N that is incorporated into the soil microbial biomass that fluctuates throughout the season and while in the microbial biomass remains unavailable to the subsequent crop. Lastly, an average of 33% of CR N remains in the undecomposed CR biomass. See figure 3 for seasonal trends in CR N within the different soil N pools.
Educational & Outreach Activities
Participation Summary:
Richard Roth has disseminated the results of this research project to producers, agriculture professionals and fellow scientists at approximately 20 extension and scientific meetings. Scientific events include the 2018, 2019, and 2020 International Tri-Society meetings (where he was awarded 2nd, 1st, and 3rd place awards for his presentations, respectively), the 2018, 2019, and 2020 Midwest Cover Crops Council meetings (where both producers and scientists were present), the 2019 Illinois Nutrient Research and Education Council meeting (awarded people's choice for his poster presentation), and the 2019 Universities Council on Water Resources meeting (awarded 1st place for graduate student research competition). Other educational and extension engagements include a two seminars with Purdue’s Department of Agronomy, the 2020 Hamilton County Indiana Soil and Water Conservation District annual meeting, the 2021 Purdue Extension Soil Health Webinar Series, along with several other extension engagements across Indiana and Illinois. Additionally, Dr. Shalamar Armstrong has presented the findings from this research at approximately 50 outreach and extension engagements across the North Central Region reaching producers, certified crop advisers, agriculture industry professionals, and fellow scientists throughout the funding period. Richard Roth, Dr. Shalamar Armstrong, and the rest of the members of the Armstrong Soil Ecosystem and Nutrient Dynamics Lab at Purdue University will continue to disseminate the results of this project through and beyond the end of the project funding period. In addition, at the time of publishing this report, the authors have submitted one peer-reviewed journal article for publication and are awaiting the review process.
List of Oral and Poster Presentations by Richard Roth:
- Roth R.T. and S.D. Armstrong. 2021. Nutrient Returns from Soil Health Practices. In Purdue Extension, Conservation Cropping Systems, Natural Resources Conservation Services, Howard and Tipton County Soil and Water Conservation Districts Soil Health Webinar Series. Virtual.
- Roth R.T. and S.D. Armstrong. 2020. Fate of Nitrogen from Cereal Rye Root and Shoot Biomass. In Annual Meetings ASA. Virtual.
- Roth, R.T. and S.D. Armstrong. 2020. Cover Cropping in the Midwest: A SEND Lab Research Overview. In Purdue Agronomy Graduate Student Organization Graduate Student Seminar Series. Virtual.
- Roth, R.T. and S.D. Armstrong. 2020. Impact of Cereal Rye on Nitrogen Fate and Cycling, Water Quality, and Cash Crop Yields. In Hamilton County Soil and Water Conservation District Pesticide Applicator and CCA Training. Noblesville.
- Roth, R.T. and S.D. Armstrong. 2020. Is Cereal Rye and Effective Conduit for Sustainable Intensification of Agriculture in the Midwest?. In Purdue Agronomy Graduate Student Organization Graduate Student Seminar Series. West Lafayette.
- Roth, R.T., Reeling, C.J., Armstrong, S.D.. 2019. Stochastic Analysis of Economic Risk and Uncertainty Following Cover Crop Adoption . In Annual Meetings ASA. San Antonio.
- Roth R.T. and S.D. Armstrong. 2019. Understanding Cereal Rye Nitrogen Decomposition and Its Transition into Inorganic and Organic Soil Nitrogen Pools . In Annual Meetings ASA. San Antonio.
- Roth R.T. and S.D. Armstrong. 2019. Digital Cover Crop Phenotyping. In Purdue University College of Agriculture Digital Agriculture Roundtable. West Lafayette.
- Roth R.T. and S.D. Armstrong. 2019. Cover Crop Impact on Nitrogen Fate and Cash Crop Yield. In Universities Council on Water Resources Annual Conference. Snowbird.
- Roth R.T.,G. Lacey, S.D. Armstrong. 2019. Determining the Fate of Cereal Rye Nitrogen within the Soil Profile. In Annual Meetings SSSA. San Diego.
- Roth R.T.,G. Lacey, S.D. Armstrong. 2018. Understanding Cereal Rye Nitrogen Decomposition and Its Transition into Inorganic and Organic Soil Nitrogen Pools. In Annual Meetings ASA, CSSA, SSSA. Baltimore.
Project Outcomes
With a dearth of knowledge surrounding the fate of nitrogen within the soil following decomposition, there was much to learn from the experiment. From this study, we now know that the majority (53%) of cereal rye nitrogen that enters the soil over the first growing season following chemical termination enters the organic nitrogen pool, and that the second largest portion (33%) remains undecomposed by the time of maturity for the subsequent crop. This research also gave us insights on the rate at which cereal rye nitrogen enters each of the soil nitrogen pools. Key takeaways for this study include: 1) an average of only 8% of cereal rye root nitrogen decomposes within 1667 accumulated GDDC, while 85% of cereal rye shoot nitrogen decomposes during the same timeframe, 2) though 67% of cereal rye whole plant nitrogen decomposed over 1667 accumulated GDDc, only 15% of the cereal rye whole plant nitrogen became bioavailable, and 3) the majority of cereal rye whole plant nitrogen that became bioavailable was observed to happen between the 945 (Maize VT) and 1667 (Maize R6) accumulated GDDC, which corresponds to the in-field timing of maize transitioning from vegetative to reproductive growth through physiological maturity.
Gaining this knowledge and understanding of the fate of nitrogen from cereal rye above and belowground biomass has driven the Armstrong Soil Ecosystem and Nutrient Dynamics (SEND) Lab at Purdue University to become more ambitious in pursuing research regarding adaptive nitrogen fertilizer management strategies to overcome in-field issues that have been previously observed for corn following cereal rye.
Furthermore, in presentation of these results, it has been enlightening to hear producer and agriculture professional perspectives on the issues concerning nitrogen availability to corn following cereal rye. The Armstrong SEND Lab believes that cereal rye is one conduit towards sustainable intensification of agricultures within the North Central Region, and the insights gained through presenting these findings have brought to the forefront the primary issues producers are facing with these production systems, and driven our research program towards addressing these issues. Research projects and ideas derived from this study include: starter nitrogen and late season nitrogen applications in corn following cereal rye, precision planting of cereal rye, overwintering legumes, monitoring of nitrogen mineralization utilizing in-situ soil sensors.
As we increase our knowledge surrounding the timing and fate of cereal rye nitrogen decomposition, we increase our understanding of the feasibility of assessing a nitrogen credit to a cereal rye cover crop. Enhancing our knowledge of cereal rye nitrogen decomposition and availability could aid producers in adapting the timing of nitrogen fertilizer applications to achieve competitive maize grain yield relative to a non-cereal rye treatment, without increasing the overall amount of nitrogen applied. Moving towards more nitrogen efficient management in cereal rye field while maintaining or increasing levels of productivity is crucial as the agricultural community continues striving towards feeding the ever-growing global population and reducing their environmental footprint.
Based on results of this study, coupled with previous research, it is our recommendation that producers do not rely on nitrogen scavenged by a cereal rye cover crop to be available for crop uptake during the growing season subsequent to chemical termination. Further, producers should focus on the use of adaptive nitrogen fertilizer management within cereal rye containing production systems to overcome any potential issues of nitrogen immobilization that can occur during cereal rye biomass decomposition. Investments should be made in research similar to that conducted in this experiment except instead of cereal rye using different species of cover crops, as well as research regarding in-field adaptive cereal rye management (nitrogen fertilization, planting/termination strategy, etc.) to help maximize production within cereal rye containing production systems.