Final report for GNC21-322
Project Information
Organic vegetable growers are interested in utilizing the ‘soil block’ method for transplant production as an alternative to plastic flats. The soil block method compresses growing media into a freestanding block in contrast to the cells of a plastic flat. Anecdotal evidence of soil block grown transplants with increased vigor and root development exists, but limited research has been conducted to evaluate these claims. Furthermore, identifying commercial growing medium for certified organic transplant production is needed. The objective of this study was to compare growth parameters and root development of cucumber (Cucumis sativus) and pepper (Capsicum annum) transplants grown in soil blocks and plastic flats, in combination with four commercially available certified organic media (Beautiful Land Products ‘Soil Blocking Mix’, Purple Cow Organics ‘Seed Starter Mix’, Cowsmo ‘Green Potting Soil’, and Vermont Compost Company ‘Fort Vee’). A volume-based 50% peat, 25% compost, 12.5% perlite, and 12.5% vermiculite growing medium was also evaluated. A split-plot randomized complete block design with four replications was used with growing method as the whole plot factor and medium as the subplot factor. ‘Marketmore 76’ cucumbers and ‘Yankee Bell’ peppers were seeded in 50-cell flats and soil blocks made with Johnny’s Selected Seeds Stand-up 12 Soil Blocker. Data were collected on growth parameters by destructively sampling cucumbers three weeks after seeding, and peppers five, six, and seven weeks after seeding. Root development was evaluated using WinRHIZO at the last sampling. Cucumber and pepper transplants performed differently in soil blocks and flats. Cucumbers grown in flats had a significantly greater dry weight than those grown in soil blocks, by 20% in 2022 and by 38% in 2023. In contrast, pepper transplants grown with the soil block method had between 50% and 130% greater dry weight in the final sampling in 2022. Cucumber and pepper transplants grown with Cowsmo ‘Green Potting Soil’ performed poorly, with an up to 144% lower dry weight and up to 167% lower root surface area than transplants grown with the other media. Root development correlated with shoot development, without a specific advantage in soil blocks, although differences in root system architecture should be investigated. The evaluated Beautiful Land Products, Purple Cow Organics, and Vermont Compost Company media can all be considered suitable for growing certified organic vegetable transplants in both soil blocks and flats.
Our findings are relevant for certified organic vegetable transplant producers in the Midwestern U.S. looking to use regionally produced growing media, potentially limiting shipping costs and carbon footprint. Very limited research is available regarding the soil block method, even as it maintains popularity amongst small and medium-sized growers. More information and continued research on soil blocks is of great value, establishing optimal bulk density parameters and improving understanding of the impact this method has on vegetable transplant performance.
Demonstration workshops will be held at the Iowa State Horticulture Greenhouses and during on-farm extension visits. The developed curriculum will increase farmer’s knowledge of successful organic vegetable transplant production methods, the availability of good quality organic media, and increase awareness of the soil block technique as an alternative technique. In the workshop farmers will improve their organic transplant production skills, including appropriate fertilization, watering, and compaction of cells. This will lead to the action outcome of farmers adopting more successful production practices for transplants. These learning and action outcomes will be evaluated through follow up interviews with workshop attendees.
Outreach specifically targeted at beginning farmers will improve extension education for this historically under-served group, allowing for future on-farm problem solving and collaboration. This project will also spur further research on organic transplant production as more questions arise from collaboration with farmers and the results of the experiment. The project results will be printed in extension publications, published as an article in a scientific journal, and be a chapter in the graduate student’s thesis. These publications will lead to citations from other researchers and a continued increase in knowledge, awareness, and skill development of good organic transplant production practices. A recorded video of the soil block technique will also be made available online to increase learning opportunities for those not able to attend the in-person demonstration workshop, again increasing skill building and knowledge of organic growers.
Cooperators
Research
Environmental conditions
The experiment was conducted in the Iowa State University Department of Horticulture greenhouse in Ames, IA, USA (lat. 42.020370°N, long. -93.633790°W) in 2022 and 2023. Day and night temperatures were maintained between 21˚C and 23˚C. Supplemental light was provided by 1000 W high-pressure sodium lamps (AgroMax; Summerdale, AL, USA) to maintain a 16H daylight period with a light intensity of at least 350 µmol·m-2·s-1 at bench height.
Plant material
Bell pepper (Capsicum annum, 'Yankee Bell') and cucumber (Cucumus sativus L., 'Marketmore 76') seeds were sown in either plastic propagation trays (referred to as flats) or soil blocks on 17 Jan 2022 and 16 Jan 2023. Flats with 50 cells (Product Code: 720463; T.O. Plastics, Otsego, MN,USA) were cut in half to create 25-cell flats and filled by hand with desired growing medium. Soil blocks were made as described by Coleman (1995). Each growing medium was hand mixed in a plastic tub with water at a ratio of three to one (by volume). Additional water was added as needed to achieve the necessary consistency for block release and growing medium adherence. The moistened growing medium was mounded and the Stand-up 12 Soil Blocker (Product ID: 7861.0; Johnny’s Selected Seeds, Fairfield, ME,USA) was repeatedly pressed into the growing medium until each block well was filled. Blocks were released into a web-bottom carrying tray (Item#CF 2; Nolt’s Greenhouse Supplies, Charles City, IA, USA) and arranged so each tray held 25 soil blocks. Cell and block size were chosen from available products to ensure the most equal size possible, taking into consideration the compression of the growing medium with the blocking tool. Flat cells measured 4.83 cm x 4.83 cm x 6.03 cm (140.7 cm3) and soil blocks measured 4.76 cm x 4.44 cm x 4.13 cm (87.3 cm3).
Pepper transplants were fertilized with 150 mg·L-1 Aqua Power™ 5-1-1 (JH Biotech, Inc., Ventura, CA, USA) 38 and 45 days after seeding (DAS) in 2022 and 2023. Cucumber transplants did not receive supplemental fertilization.
Growing media
The product information of all growing media evaluated specifically stated their appropriateness for use with the soil block method and flats and approval for use in certified organic production by the Organic Materials Review Institute (OMRI). The growing media evaluated were: Purple Cow Organics® ‘Seed Starter Mix’ (Middleton, WI, USA), Cowsmo, Inc. ‘Green Potting Soil’ (Cochrane, WI, USA), Beautiful Land Products® ‘Soil Blocking Mix’ (Tipton, IA, USA), Vermont Compost Company® ‘Fort Vee’ (Montpelier, VT, USA), and an in-house formulated custom lab mixture. In the text, the growing media will be referred to as Purple, Cowsmo, BLP, Vermont, and Lab, respectively. Lab was comprised of 50% peat (Sphagnum Peat Moss 0128; Premier Tech, QC, Canada), 25% compost, 12.5% perlite (Therm-O-Rock East, Inc., New Eagle, PA, USA), and 12.5% vermiculite (Premium Grade; Sungro Horticulture, Agawam, MA, USA) by volume. Compost was supplied by the Iowa State University Compost Facility (Ames, IA, USA), and made using certified organic methods from plant material and livestock manure. New growing media were purchased directly from the supplier or from an approved retailer in 2022 and 2023 (Gardener’s Supply Company, Burlington, VT, USA).
Before seeding, in 2022 and 2023, samples of each growing medium were sent to AgSource Laboratories, LLC (Lincoln, NE) for nutrient analysis by saturated media extraction method. In 2023, we performed additional physicochemical tests (bulk density and water holding capacity) to better understand differences found amongst treatments in the previous year. The bulk density of each growing medium in flats was measured by hand-filling 50-cell flats of known volume with the growing medium, emptying the contents into a beaker, and oven-drying the sample. For the soil block method, blocks were made, weighed, and oven dried. Bulk density was calculated by dividing the oven-dry weight by the volume of the sample (Ali 2010). To determine the water holding capacity of each growing medium ten grams of air-dried medium were placed in a funnel with Whatman #1 filter paper, wetted with deionized water until fully saturated, covered with plastic wrap, left to drain for six hours, and weighed (Roberston et al. 1999). The water holding capacity was calculated by first finding the water mass, by subtracting the dry medium weight from the wet medium weight. The water mass value for each medium was then divided by the dry medium weight, and multiplied by 100, resulting in the percent water holding capacity. Bulk density and water holding capacity measurements were averaged over three replicates to account for random variation in growing medium samples. Chemical and physicochemical properties for each growing medium are reported in Table 1 and Table 2, respectively. Additionally, temperature probes (HOBO U12-008; Onset Computer Corporation, Bourne, MA, USA) were deployed one DAS to 38 DAS in 2023 to record the growing media temperature on an hourly basis. Probes were placed 2 cm below the medium surface in the center of the block or cell in the interior of the tray. Due to supply constraints, probes were placed in one flat and one soil block in one replication of BLP, Vermont, and Lab, for a total of six probes.
On a weekly basis for the duration of pepper transplant growth, data was collected on the pH and electrical conductivity (EC) of the leachate of each growing medium by performing the pour-through extraction method (Torres et al. 2010). Pour-through leachate data was only collected from flats. The lack of division between soil blocks prohibited proper leachate collection, so pour-through extraction was not performed on soil blocks. Additionally, pour-through was not performed on flats containing cucumbers due to the short growing time. Deionized water quantities were adjusted to ensure 50 mL of leachate was extracted from each flat after the removal of pepper transplants from destructive sampling.
Experimental design
The experimental design was a split-plot randomized complete block design with four replications. The transplant method was the whole plot factor and growing medium was the subplot factor. Pepper and cucumber transplants were grown with each growing medium in four 25-cell plastic trays and in four 25-block carrying trays (peppers, n=1000 and cucumbers, n=1000).
Growth parameters
Seedling emergence data was collected for cucumbers 10 DAS and for peppers 15 DAS by counting the number of emerged seedlings in each flat and tray of soil blocks. Plant count data was collected at the time of the first destructive sampling of peppers, 36 DAS, and cucumbers, 22 DAS, by counting the number of plants in each flat and tray of soil blocks.
Five cucumber plants were selected for destructive sampling 22 DAS from the center of the flat or soil block carrying tray, leaving the exterior plants as guard plants. Pepper transplants were destructively sampled three times: 36 DAS, 43 DAS, and 50 DAS. At each sampling, three peppers plants were selected from the center of the flat or soil block tray, leaving the exterior plants as guard plants. Pepper destructive sampling data is only presented from the final sampling, 50 DAS.
Growth measurements collected include plant height and stem diameter. Plant height was measured from the surface of the growing medium to the apical meristem with a ruler. Stem diameter was measured 1 cm below the cotyledon leaves with digital Vernier calipers (VWR International, LLC, Radnor, PA, USA).
After growth parameter measurements were taken, the roots of each cucumber and pepper transplant were thoroughly and carefully washed to remove remaining growing medium. After washing, plants were placed in a forced-air oven at 67˚ C for 7 d until a constant weight was reached. The whole plant dry weight was recorded. Dry cucumber whole plant tissue was ground to a uniform size and sent to Ward Laboratories, Inc. (Kearney, NE, USA) for plant tissue analysis (Table 4). Pepper plant tissue was not analyzed because supplemental fertilization remediated growing medium induced nutrient deficiencies in the plant tissue.
Root system parameters
One transplant was reserved from the final pepper sampling and from the cucumber sampling from each treatment for further root system analysis. The day following destructive sampling, roots were removed at the crown from each plant and placed in water to rehydrate. Roots were prepared, scanned (Epson Perfection V800; Epson America, Inc., Los Alamitos, CA, USA), and analyzed with the WinRHIZO® Reg software (Regent Instruments Inc., QC, Canada) according to methods described by Suchoff et al. (2017) for root surface area. In 2023, data of pepper roots from soil blocks were not analyzed due to damage during sampling. Therefore, pepper root analysis was only performed in 2022.
Statistical analysis
Analysis of variance was performed with the PROC GLIMMIX procedure in SAS (version 9.4; SAS Institute, Cary, NC, USA). Data from each year and week of pepper transplant sampling were analyzed separately due to significant interactions between years and between weeks. Pour-through extraction data from each year was analyzed separately by week, due to a significant week by media interaction. All response variables were tested with method and growing medium as the fixed terms and replication as the random term. When no significant interaction was found for a response variable, the effect of the fixed terms was analyzed individually by partitioning method and media variables with the ‘slice’ statement. The least significant differences were found for response variables at P ≤ 0.05.


|
Table 1. Saturated media nutrient concentrations (in g·kg⁻¹) of five organic growing media in 2022 and 2023. |
||||||
|
Media |
Nitrate-N |
Ammonium-N |
P |
K |
Ca |
Mg |
|
|
2022 |
|||||
|
BLPi |
4.2 |
139.0 |
53 |
509 |
87 |
50 |
|
Cowsmo |
5.1 |
3.6 |
61 |
471 |
25 |
14 |
|
Lab |
151.0 |
24.7 |
27 |
1432 |
66 |
39 |
|
Purple |
251.0 |
26.3 |
31 |
256 |
153 |
141 |
|
Vermont |
454.0 |
5.1 |
35 |
528 |
533 |
142 |
|
|
2023 |
|||||
|
BLP |
20.0 |
95.5 |
50 |
609 |
82 |
48 |
|
Cowsmo |
9.0 |
13.8 |
36 |
640 |
48 |
25 |
|
Lab |
26.3 |
17.6 |
41 |
1005 |
64 |
35 |
|
Purple |
194.0 |
6.0 |
13 |
1089 |
249 |
116 |
|
Vermont |
364.0 |
6.9 |
49 |
697 |
424 |
124 |
|
iBLP represents Beautiful Land Products ‘Soil Blocking Mix’ (Tipton, IA), Cowsmo represents Cowsmo ‘Green Potting Soil’ (Cochrane, WI), Lab represents a custom lab mixture of 50% peat, 25% compost, 12.5% perlite, and 12.5% vermiculite by volume, Purple represents Purple Cow Organics ‘Seed Starter Mix’ (Middleton, WI), and Vermont represents Vermont Compost Company ‘Fort Vee’ (Montpelier, VT). |
||||||
|
Table 2. Bulk density and water holding capacity (WHC) of five organic growing media in 2023. |
|||
|
|
Bulk Density (g·cm-3) |
|
|
|
Media |
Flat |
Soil Block |
WHC (% of volume) |
|
BLPi |
0.06 fii |
0.21 d |
680 a |
|
Cowsmo |
0.14 e |
0.31 c |
390 bc |
|
Lab |
0.14 e |
0.37 b |
287 c |
|
Purple |
0.16 e |
0.46 a |
296 bc |
|
Vermont |
0.13 e |
0.40 b |
402 b |
|
iBLP represents Beautiful Land Products ‘Soil Blocking Mix’ (Tipton, IA), Cowsmo represents Cowsmo ‘Green Potting Soil’ (Cochrane, WI), Lab represents a custom lab mixture of 50% peat, 25% compost, 12.5% perlite, and 12.5% vermiculite by volume, Purple represents Purple Cow Organics ‘Seed Starter Mix’ (Middleton, WI), and Vermont represents Vermont Compost Company ‘Fort Vee’ (Montpelier, VT). iiMeans followed by the same letter within the same column and year are not significantly different (P≤0.05). |
|||
|
Table 3. pH and electrical conductivity (EC) of five growing media in 2022 and 2023.i |
|||||||||||||
|
|
pH |
|
EC (mS·cm-1) |
||||||||||
|
Media |
Week 1 |
Week 2 |
Week 3ii |
Week 4 |
Week 5 |
Week 6 |
|
Week 1 |
Week 2 |
Week 3 |
Week 4 |
Week 5 |
Week 6 |
|
|
|
|
|
|
|
|
2022 |
|
|
|
|
|
|
|
BLPiii |
5.7 biv |
5.9 b |
- |
5.9 c |
6.0 b |
6.1 c |
|
6.29 b |
4.29 b |
2.84 b |
2.21 b |
1.41 bc |
1.04 |
|
Cowsmo |
6.8 a |
7.0 a |
- |
7.3 a |
7.3 a |
7.4 a |
|
4.77 c |
3.44 bc |
2.77 b |
2.18 b |
1.93 b |
1.40 |
|
Lab |
6.8 a |
6.9 a |
- |
7.2 a |
7.3 a |
7.3 a |
|
6.48 b |
4.62 b |
2.94 b |
2.19 b |
1.88 b |
1.24 |
|
Purple |
5.2 c |
5.4 c |
- |
6.1 b |
6.1 b |
6.5 b |
|
5.65 b |
2.94 c |
1.64 c |
1.47 c |
1.16 c |
0.98 |
|
Vermont |
5.1 c |
5.3 c |
- |
5.6 c |
6.0 b |
6.1 c |
|
7.82 a |
6.66 a |
5.16 a |
3.87 a |
2.69 a |
1.18 |
|
|
|
|
|
|
|
|
2023 |
|
|
|
|
|
|
|
BLP |
5.6 c |
5.7 d |
5.4 d |
5.6 d |
5.7 d |
6.2 e |
|
6.36 b |
5.23 b |
4.75 bc |
2.98 |
2.20 c |
1.78 d |
|
Cowsmo |
6.4 b |
6.7 b |
6.8 b |
6.9 b |
7.0 b |
7.2 c |
|
6.31 b |
5.18 b |
4.79 bc |
3.74 |
3.91 a |
3.19 a |
|
Lab |
6.8 a |
7.0 a |
7.1 a |
7.3 a |
7.5 a |
7.7 a |
|
6.15 b |
5.05 b |
4.23 c |
2.89 |
2.83 b |
1.96 b |
|
Purple |
6.4 b |
6.5 c |
6.7 b |
6.9 b |
7.1 b |
7.4 b |
|
7.55 a |
6.50 a |
5.82 a |
3.73 |
3.21 ab |
1.64 bc |
|
Vermont |
5.5 c |
5.6 d |
5.7 c |
6.2 c |
6.4 c |
6.8 d |
|
7.39 a |
6.38 a |
5.55 ab |
3.13 |
2.75 bc |
1.40 cd |
|
ipH and EC measurements collected by performing the pour through method (Torres et al. 2010) in central data cells of plastic flats containing pepper plants. iiWeek three pH data was not collected. iiiBLP represents Beautiful Land Products ‘Soil Blocking Mix’ (Tipton, IA, USA), Cowsmo represents Cowsmo ‘Green Potting Soil’ (Cochrane, WI, USA), Lab represents a custom lab mixture of 50% peat, 25% compost, 12.5% perlite, and 12.5% vermiculite by volume, Purple represents Purple Cow Organics ‘Seed Starter Mix’ (Middleton, WI, USA), and Vermont represents Vermont Compost Company ‘Fort Vee’ (Montpelier, VT, USA). ivMeans followed by the same letter within the same column and year are not significantly different (P≤0.05). |
|||||||||||||
|
Table 4. Cucumber whole plant nutrient concentrations from five organic growing media in 2022 and 2023. |
|||||
|
Media |
N (%) |
P (%) |
K (%) |
Ca (%) |
Mg (%) |
|
|
2022 |
||||
|
BLPi |
5.1 aii |
1.34 a |
7.3 b |
1.1 c |
0.55 c |
|
Cowsmo |
1.7 c |
0.75 c |
5.1 d |
0.7 c |
0.39 d |
|
Lab |
4.2 b |
0.83 c |
8.4 a |
1.0 c |
0.49 cd |
|
Purple |
5.1 a |
0.96 b |
5.9 c |
2.1 b |
1.30 a |
|
Vermont |
5.8 a |
0.95 b |
6.3 c |
3.1 a |
0.73 d |
|
|
2023 |
||||
|
BLP |
4.4 b |
1.12 a |
6.5 ab |
1.2 c |
0.46 c |
|
Cowsmo |
1.6 e |
0.62 c |
5.3 c |
0.7 d |
0.41 d |
|
Lab |
2.1 d |
0.67 c |
6.6 a |
0.7 d |
0.39 d |
|
Purple |
3.6 c |
0.60 c |
6.5 ab |
1.4 b |
0.62 b |
|
Vermont |
4.9 a |
0.86 b |
6.2 b |
2.5 a |
0.68 a |
|
iBLP represents Beautiful Land Products ‘Soil Blocking Mix’ (Tipton, IA, USA), Cowsmo represents Cowsmo ‘Green Potting Soil’ (Cochrane, WI, USA), Lab represents a custom lab mixture of 50% peat, 25% compost, 12.5% perlite, and 12.5% vermiculite by volume, Purple represents Purple Cow Organics ‘Seed Starter Mix’ (Middleton, WI, USA), and Vermont represents Vermont Compost Company ‘Fort Vee’ (Montpelier, VT, USA). iiMeans followed by the same letter within the same column and year are not different (P≤0.05). |
|||||
|
Table 5. Emergence and plant count of cucumber and pepper transplants grown in different growing media in soil blocks (SB) and flats (F) in 2022 and 2023.i |
||||||
|
|
|
Cucumber |
|
Pepper |
||
|
Method |
Media |
Emergence |
Plant Count |
|
Emergence |
Plant Count |
|
|
|
|
|
2022 |
|
|
|
F |
BLPii |
24 aiii |
24 ab |
|
23 a |
23 ab |
|
SB |
BLP |
20 a |
20 abc |
|
21 ab |
21 b |
|
F |
Cowsmo |
25 a |
25 a |
|
23 a |
24 a |
|
SB |
Cowsmo |
16 b |
17 d |
|
23 a |
24 a |
|
F |
Lab |
25 a |
25 a |
|
23 a |
24 a |
|
SB |
Lab |
16 b |
18 cd |
|
15 c |
17 c |
|
F |
Purple |
24 a |
24 a |
|
23 a |
24 a |
|
SB |
Purple |
22 a |
23 ab |
|
18 bc |
23 ab |
|
F |
Vermont |
25 a |
24 a |
|
24 a |
25 a |
|
SB |
Vermont |
16 b |
19 bcd |
|
22 a |
23 a |
|
|
|
|
|
2023 |
|
|
|
F |
BLP |
24 |
24 |
|
23 ab |
24 |
|
SB |
BLP |
24 |
25 |
|
15 d |
23 |
|
F |
Cowsmo |
24 |
24 |
|
22 ab |
23 |
|
SB |
Cowsmo |
23 |
24 |
|
17 cd |
24 |
|
F |
Lab |
25 |
25 |
|
24 a |
24 |
|
SB |
Lab |
23 |
24 |
|
17 cd |
24 |
|
F |
Purple |
24 |
25 |
|
23 ab |
23 |
|
SB |
Purple |
24 |
24 |
|
19 bc |
23 |
|
F |
Vermont |
24 |
24 |
|
22 ab |
23 |
|
SB |
Vermont |
21 |
21 |
|
14 d |
23 |
|
iEmergence data collected 10 days after seeding (DAS) cucumbers and 15 DAS peppers. Plant count data collected at time of first sampling, 22 DAS cucumbers and 36 DAS peppers. All numbers out of 25 total cells or blocks. iiBLP represents Beautiful Land Products ‘Soil Blocking Mix’ (Tipton, IA, USA), Cowsmo represents Cowsmo ‘Green Potting Soil’ (Cochrane, WI, USA), Lab represents a custom lab mixture of 50% peat, 25% compost, 12.5% perlite, and 12.5% vermiculite by volume, Purple represents Purple Cow Organics ‘Seed Starter Mix’ (Middleton, WI, USA), and Vermont represents Vermont Compost Company ‘Fort Vee’ (Montpelier, VT, USA). iiiMeans followed by the same letter within the same column and year are not different (P≤0.05). |
||||||
Results
Growing media properties
pH and EC. The pH increased on average by 0.8 in 2022 and 0.9 in 2023 throughout the weeks of sampling (WOS) (Table 3). Lab displayed a higher pH than the other growing media in all six WOS in both years, except Cowsmo had a similar pH to Lab in all WOS in 2022. In 2022, Purple and Vermont had the lowest pH in 1 and 2 WOS, and in 6 WOS Vermont (6.1) and BLP (6.1) had the lowest pH. In 2023, BLP and Vermont consistently had the lowest pH.
The EC of all media decreased on average by 5.03 mS·cm-1 in 2022 and 4.76 mS·cm-1 in 2023 throughout the six WOS (Table 3). In 2022, Vermont exhibited a higher EC than the other media from one WOS (7.82 mS·cm-1) until five WOS (2.69 mS·cm-1). In 2022, the EC of Purple reached 1.64 mS·cm-1 in three WOS, while the other growing media did not reach an EC below 2.00 mS·cm-1 until five WOS (BLP, Cowsmo, and Lab) or six WOS (Vermont). In 2023, all growing media reached an EC below 2.00 mS·cm-1 in six WOS, except Cowsmo, which had an EC of 3.19 mS·cm-1 in the final WOS.
Bulk density. Growing media bulk density was between 76% and 111% greater in soil blocks than in flats (Table 2). In soil blocks, Purple displayed the greatest bulk density (0.46 g·cm-3), while BLP had the lowest (0.21 g·cm-3). In flats, all media displayed a similar bulk density.
Water holding capacity. Water holding capacity differed between growing media. BLP (680%) exhibited the greatest water holding capacity, while Lab (287%) exhibited a lower water holding capacity compared to BLP and Vermont (Table 2).
Cucumber and pepper emergence and plant count
Cucumbers. In 2022, cucumbers seeded in Cowsmo, Lab, and Vermont showed between 42% and 46% higher emergence in flats than in soil blocks (Table 5). In soil blocks, cucumber emergence was 23% greater in BLP and 32% greater in Purple than in Cowsmo, Lab, and Vermont. In flats, cucumber emergence was similar between media. Cucumber plant count was higher in flats compared with soil blocks with Vermont, Lab, and Cowsmo. No differences were found in cucumber emergence and plant count between treatments in 2023.
Peppers. In 2022, peppers seeded in Lab and Purple showed a 45% and 26% higher emergence in flats than soil blocks, respectively (Table 5). Pepper plant count was similar between flats and soil blocks for all media in 2022, except Lab produced a 38% lower plant count in soil blocks than flats. Across all media in 2023, pepper emergence was 33% higher in flats than in soil blocks, while no differences between the media were found. Pepper plant count was similar between all treatments in 2023.
Transplant growth parameters
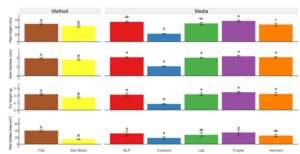
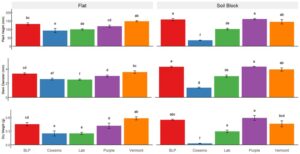
Dry weight. Cucumber dry weight was greater in flats than soil blocks by 20% in 2022 (Fig. 1) and 38% in 2023 (Fig. 2). All media produced cucumbers with similar dry weight in 2022, except Cowsmo grown cucumbers exhibited between 90% and 103% lower dry weight than all other media. In 2023, cucumbers grown with Lab and Cowsmo exhibited a lower dry weight than cucumbers grown with BLP, Purple, and Vermont.
In 2022, pepper dry weight was between 50% and 130% greater in soil blocks than flats for all media, except Cowsmo. Cowsmo grown peppers had a similar dry weight in both methods in 2022 (Fig. 3), but dry weight was consistently lower than all other treatments in both years. In 2022, Purple in soil blocks (1.22 g) and Vermont in soil blocks (1.18 g) produced the largest pepper dry weights. In 2023, the largest pepper dry weights were found in BLP in soil blocks (0.55 g), Purple in soil blocks (0.59 g), and Vermont in flats (0.58 g) (Fig. 4). In 2023, no differences in dry weight were found between peppers grown in flats and soil blocks.
Plant height. In 2022, cucumber plants were 13% taller in flats than in soil blocks (Fig. 1). In 2023, Cowsmo and Vermont grown cucumber plants were 39% and 30% taller in flats than soil blocks, respectively, while all other media produced plants with a similar height in both methods (Fig. 2). Cucumber plants grown in BLP (68 mm) and Purple (71 mm) were the tallest in 2022. In 2023, the tallest cucumber plants were found in BLP in flats (78 mm) and soil blocks (85 mm) and Vermont in flats (90 mm). Cucumber plants in Cowsmo were 48% to 96% shorter than all other media in both years.
In 2022, pepper plants were between 47% and 74% taller in soil blocks than flats in all media, except Cowsmo, which produced peppers with a similar height in both methods (Fig. 3). In 2023, BLP and Purple produced taller peppers in soil blocks than in flats, by 18%, and 30%, respectively (Fig. 4). Pepper plants were tallest with Vermont, Purple, and BLP in soil blocks, and, in 2023, Vermont in flats as well. Cowsmo in soil blocks consistently produced peppers that were between 97% and 166% shorter than all other media in either method.
Stem diameter. Cucumber stem diameter was 7% larger in flats than in soil blocks in 2022 (Fig. 1). In 2023, Cowsmo and Vermont produced cucumbers with 20% and 14% larger stem diameters in flats than in soil blocks, respectively, while all other media produced a similar stem diameter in both methods (Fig. 2). In 2023, Vermont in flats displayed a larger cucumber stem diameter than all other treatments. Cowsmo in soil blocks consistently produced cucumbers with a smaller stem diameter than all other media in either method.
In 2022, peppers in soil blocks exhibited a 26% larger stem diameter than in flats (Fig. 3). In 2023, peppers grown with BLP, Lab, and Purple had larger stem diameters in soil blocks than in flats (Fig. 4). The largest pepper stem diameters were found in BLP, Vermont, and Purple in 2022, and with those same media in soil blocks in 2023. Cowsmo in soil blocks consistently produced smaller pepper stem diameter than all other media in either method.
Root surface area. Cucumber root surface area was 92% and 118% larger in flats than in soil blocks in 2022 (Fig. 1) and 2023 (Fig. 2), respectively. Cowsmo in flats and soil blocks produced between 171% and 193% smaller pepper root surface area than all other treatments. Pepper root surface area with Purple produced 52% to 193% larger root surface area than all other treatments (Fig. 3). In 2023, no differences between media were found in cucumber root surface area.
Discussion
Peppers grown in soil blocks tended to have lower emergence at 15 DAS than those in flats, seen across all media in 2023 and with Lab and Purple in 2022 (Table 5). Although, pepper plant count between methods was not different at the time of plant count data collection (36 DAS), apart from Lab in 2022. The lack of continued disparity from emergence (15 DAS) to plant count (36 DAS) between methods indicates a delay in pepper emergence in soil blocks, as opposed to a reduction in germination. The seedling environment as determined by the transplant method, specifically the growing medium temperature and bulk density, may help explain the delay in emergence found in peppers in soil blocks.
Flats used were made of black plastic, which conducts heat from artificial lighting and solar radiation into the growing medium. The growing media within flats was on average 3˚C higher as compared with soil blocks, 21.3˚C in flats and 18.5˚C in soil blocks. This increase in medium temperature is similar to what is found in containerized production in black pots (Ingram et al. 2003; Markham et al. 2011). An increase in growing medium temperature, without exceeding an ideal range, can lead to more rapid germination and seedling emergence (Cantliffe 1998). Research found ‘Dasher II’ cucumbers to reach 80% germination two DAS at 15˚C, 1.5 DAS at 20˚C, and 0.75 DAS at 25˚C (Jennings and Saltveit 1994). This reduction in hours to germination in temperatures above 20˚C has also been found in hot peppers (Capsicum baccatum var. pendulum), reaching 50% germination after 10 DAS at 15˚C, eight DAS at 20˚C and 7.5 DAS at 25˚C (Silva et al. 2012). Therefore, the faster emergence of peppers in flats as compared to soil blocks may be partly explained by the higher medium temperature with that method.
An additional factor which may have impacted pepper emergence rates was the difference in growing medium bulk density between methods. The bulk density of the growing media were between 137% to 237% higher in soil blocks as compared to the same medium in flats. The compression of moistened growing media into the blocker tool, required to make soil blocks, helps explain the higher bulk density. A higher bulk density imparts an increase in overall compaction and a reduction in available oxygen, as pore space is reduced, and medium solids make up a higher proportion of the block after compression (Hanks and Thorp 1956; Sabahy et al. 2015; Gruda et al. 2013). Without adequate pore space for air and water, root respiration and root metabolic activity are reduced, and overall root growth is inhibited (Green 1976). Furthermore, research has shown pepper seedling emergence to be slowed by three to five days in field plots with higher compaction (Fawusi 1978). Research has also shown an oxygen reduced environment, as found with a higher bulk density, increased the number of days required to reach 50% germination in Brassicaceae, Apiaceae, Asteraceae, and Amaranthaceae seeds (Yasin and Andreasen 2016), as most seeds require an aerobic environment to germinate (Cantliffe 1998). The greater bulk density in soil blocks, with a proportionate decrease in the available air and water pore space, may have contributed to a slower emergence of peppers in soil blocks as compared to flats.
As research on soil blocks is limited, bulk density comparison between this study and others is not possible. Further, soil block making is highly variable, using a hand-tool and varying levels of compression. Considering the importance of bulk density for root growth and seed germination, an evaluation of appropriate block making technique and ideal block bulk density has the potential to optimize soil blocks for transplant production. Future research should compare transplants grown in flats and soil blocks with the same bulk density to better elucidate differences found in transplant performance and seedling emergence.
Although peppers grown in soil blocks experienced a delay in emergence, at 50 DAS peppers grown in soil blocks often outperformed peppers grown with the same media in flats. In 2022 soil block grown peppers with BLP, Lab, Purple, and Vermont displayed a greater plant height, stem diameter, and dry weight than in flats with the same media (Fig. 3). And, in 2023, peppers in soil blocks displayed a greater plant height (BLP and Purple), dry weight (Purple), and stem diameter (BLP, Lab, and Purple) than those grown in flats (Fig. 4).
In contrast to what was found in peppers, cucumbers showed no difference in emergence between methods in 2023 (Table 5). Additionally, cucumbers grown in flats had a larger dry weight than those in soil blocks (Fig. 2). An emergence delay, resulting in a slower growth rate and final dry weight, may have occurred in cucumbers in soil blocks, but not be represented in the data. Cucumber emergence data was collected at 10 DAS, while cucumbers are known to germinate and emerge in two DAS (Jennings and Saltveit 1994). Collecting emergence data at 10 DAS may not have accurately captured a delay. Therefore, it is possible that a reduction in growing medium temperature and increased bulk density in soil blocks may have resulted in a slower cucumber growth rate which could not be overcome before destructive sampling at 22 DAS.
The overall study findings showed peppers performing better in soil blocks and cucumbers performing better in flats. This may partly be explained by a delay in emergence in soil blocks, which peppers were able to overcome with a longer transplant growth period, while cucumber were not. The research available on soil blocks in vegetable production is mostly limited to evaluations of the in-field performance and yield from soil block and flat grown transplants. Therefore, we are unable to compare our results to other work.
The second aim of this study was to evaluate the suitability of four commercially available certified organic growing media. Many differences were found between growing medium nutrient concentrations and physical properties. The results of this investigation show that BLP and Purple performed similarly to Vermont, while Cowsmo stunted cucumber and pepper plant growth, and Lab performed better only than Cowsmo.
BLP displayed the lowest bulk density when used in both flats and soil blocks (Table 2), but still increased by 250% in soil blocks compared with in flats. Additionally, BLP exhibited the highest water holding capacity out of the media evaluated (680%). The companies which developed the products evaluated in this study list medium ingredients without proportions. BLP was the only medium that did not contain compost and appeared to have a higher proportion of peat than the other mixes. Peat is highly porous, with a low bulk density and a high water holding capacity (Kubota et al. 2013). When comparing soilless medium mixes, studies have found that an increase in the proportion of compost in the mix correlates with an increase in bulk density (Chrysargyris et al. 2019; Wilson et al. 2002). Additionally, higher proportions of compost in relation to peat in a mix can lower the water holding capacity of the medium (Papafotiou et al. 2005). Our study supports the previous findings. Yet, properties are variable by type of compost used and, more specifically, particle size (Carlile et al. 2019). Growing medium for the soil block method is most often recommended to possess a high water holding capacity and fibrous materials, such as peat, to help hold the block form together (Coleman 1995; Kasten 2019). BLP meets this criterion. The other media evaluated also held together well in block form, although longevity of block cohesion was not within the parameters of this study.
The EC of all the growing media was far above the general guideline of 2 mS·cm-1 for optimum plant growth (Ozores-Hampton et al. 1998), and often above 4 mS·cm-1, which can limit plant growth (Ozores-Hampton et al. 1998; Willumsen 1997). One of the challenges with soilless medium mixes containing compost is the risk of high EC, but great variability exists depending on the components and process used to make the compost (Balliu et al. 2017). The EC of all the growing media reduced each week of sampling, as irrigation leached excess salts and the growing plants took up nutrients. Even though most of the growing media had an EC outside the optimum range (<4.0 mS·cm-1) in the first two to three weeks of pepper growth, peppers exhibited healthy growth and no signs of salinity stress. This finding may help inform growers and researchers that guidelines should be revisited for EC limits to plant health in soilless mixes.
Cowsmo performed poorly, with transplants displaying lower plant height, stem diameter, and dry weight in both cucumbers and peppers compared with almost all other media with either method. Visible stunting occurred in cucumbers and peppers grown in Cowsmo. Despite similar bulk density and water holding capacity with the other growing media, Cowsmo displayed 177% to 193% lower total plant available nitrogen (nitrate-N and ammonium-N) concentration than the other media in 2022 and 63% to 177% lower in 2023 (Table 1). Nitrogen is one of the most essential elements for plant growth, contributing to photosynthesis, protein formation, and overall growth. Symptoms of nitrogen deficiency in seedlings include stunting and chlorosis (Uchida 2000). Tissue analysis confirmed a lower amount of plant available nitrogen in Cowsmo, with cucumber plant tissue containing up to 105% lower percentage of nitrogen when grown in Cowsmo than in all other media (Table 4).
The media did not perform consistently in flats and soil blocks, resulting in a significant method by media interaction for most pepper growth parameters. While peppers grown with BLP, Purple, and Lab in soil blocks performed better than in flats across most metrics in both years, Vermont performed better in flats than in soil blocks across some metrics. Additionally, Purple performed well in soil blocks, with the greatest plant height, stem diameter, and dry weight in 2023 (Fig. 4). But, in flats, peppers grown with Purple had the smallest plant height, stem diameter, and dry weight in both years and smallest root surface area in 2022, excluding Lab and Cowsmo. These inconsistencies in pepper performance, depending on both medium and method, led to an inability to parse out a clear method or media affect. As such, the data is presented for each method by media variable when an interaction was found, in contrast to the whole plot and subplot factor presented independently in other parameters.
BLP, Purple, and Vermont can all be considered suitable for growing certified organic vegetable transplants in both soil blocks and flats, while Cowsmo should undergo further investigation before continued usage. Our results are contrary to what was found in an on-farm trial, which found Cowsmo grown broccoli and tomato transplants compared similarly or better to Vermont and a different growing media from Beautiful Land Products (Liddle 2021). Unfortunately, no information regarding growing media analysis from the trial is available to compare with the products used in this study. Although new media was purchased each year during this experiment, variability has been reported within retail potting mix brands and many commercially available products (Wiberg et al. 2006).
The claims surrounding soil block grown transplants assert that there is an increase in the vegetable transplant root system, due to increased lateral root growth and a lack of root circling (Coleman 1995; Pill and Stubbolo 1986; Tresemer 1983). Our findings do not support these claims, as we did not see a general increase in root surface area in transplants grown with the soil block method. Instead, root growth of peppers and cucumber correlated with shoot growth, irrespective of method. Cucumbers had a greater root surface area in flats in both years, corresponding with a greater plant height, stem diameter, and dry weight in flats. Additionally, in 2022, pepper root surface area did not show differences between methods, except for Purple, which showed a greater root surface area in soil blocks as compared to flats. Further research should look specifically at the architecture of the root system to further understand the impact the soil block method has on vegetable transplant root development.
The lack of physical barriers between soil blocks is claimed to reduce root circling by allowing for air-pruning (Coleman 1995; Pill and Stubbolo 1986; Tresemer 1983). We did not witness air pruning, as the air separating blocks became filled with surrounding media during watering. If underwatering was used, instead of the traditional overhead watering which we performed, the air gaps between blocks may be preserved and allow for air-pruning. The presence of air gaps may also limit the tendency of transplants to grow into neighboring soil blocks. If this occurs, separating the blocks for transplanting would result in a large amount of root damage, leading to reduction in yields. Others have indicated this tendency as a challenge with soil blocks (Greer and Adam 2005). To prevent this from occurring, establishing the correct size of block and time to transplant is essential. Many favor transplanting relatively young transplants (Balliu et al. 2017), which may be encouraged by the need to stop transplant growth before roots outgrow their individual block.
A natural progression of this work is to analyze the performance of soil block grown vegetable transplants in the field. Our research was limited to the greenhouse phase and data on in-field performance would be revelatory, allowing for an investigation of the claims of a reduction in transplant shock and increased yields when using this method.
Making soil blocks is a time-consuming and labor-intensive process, especially when compared with the relative ease of filling a flat. The benefit of on-farm plastic reduction can be a significant driver for many growers looking for alternatives to flats (FAO 2021), but the labor cost of using soil blocks on a commercial scale should be investigated further. Additionally, by compressing growing media when soil blocks are made, more growing media is used per block than per cell. This additional cost and resource use should be considered, especially as peat continues to be a major component of most growing media. Peat is a non-renewable resource under threat of over-exploitation (Nesse et al. 2019), therefore, any reduction in the amount of media used can result in resource conservation and environment benefit.
Conclusion
Our work demonstrated that pepper transplants performed better in soil blocks, while cucumber transplants performed better in flats. A significant advantage to the root system of transplants grown in soil blocks was not found, although future research should examine the differences in root system morphology produced between flat and soil block grown transplants. More work needs to be done to examine the in-field performance of soil block grown vegetable transplants and assess transplant shock. Cowsmo stunted pepper and cucumber growth, while BLP, Purple, and Vermont produced healthy and vigorous transplants. Our findings are relevant for certified organic vegetable transplant producers in the Midwestern U.S. looking to use regionally produced growing media, potentially limiting shipping costs and carbon footprint. Very limited research is available regarding the soil block method, even as it maintains popularity amongst small and medium-sized growers. More information and continued research on soil blocks would be of great value, establishing optimal bulk density parameters and improving understanding of the impact this method has on vegetable transplant performance.
Educational & Outreach Activities
Participation summary:
I gave an oral presentation of the research project and first year results at the American Society of Horticultural Sciences Annual Conference on August 2, 2022.
The research was also presented at the Graduate Program in Sustainable Agriculture Research Symposium on May 4, 2022. This also included a graduate student competition, where I place 2nd.
I presented in a session at the Great Plains Growers Conference on January 13, 2023 to an audience of mostly farmers and growers. This was a full, 45 minute session where I gave background on the topic and presented first year findings.
Additionally, I have given three tours of the project to interested researchers and student recruiters.
Three videos have been made about different aspects of the project, available to the public through YouTube. The first discusses and shows how to make soil blocks, the second discusses the pour thru method for understanding growing media qualities, and the third shows the plants during data collection and reviews our findings.
In April 2023, I participated in a full day workshop for Master Gardener's of Iowa. I gave an introduction to transplant production methods and guide a hands-on portion on the making of soil blocks to an audience of gardeners and growers. As part of the workshop, I developed a factsheet and informational handout for participants.
I also presented the research in the form of a poster at the International Society of Horticultural Sciences Annual Conference in June 2023 in Montreal, Canada to an international audience of researchers. In February 2024, I also presented the poster at the Marbleseed Annual Conference in Wisconsin and won the People's Choice Award.
Finally, a journal article covering the results of this project was published in HortScience in 2024.
Project Outcomes
Using the soil block method for transplant production decreases plastic use, providing economic and environmental benefits for farmers. Using a long-lasting, metal tool instead of plastic flats, growers are able to reduce their costs and do not need to rely on purchasing and transporting new plastic flats every few years.
With the skills and knowledge to produce healthy, vigorous vegetable transplants, growers are also able to increase their economic returns. High quality transplants leads to high quality crops, which sell for higher prices. Growers who participated in my talks, workshops, and poster presentations learned about what makes a quality growing media as well as other, general considerations for growing good transplants. This knowledge can be used to improve overall transplant production, regardless of plastic flat or soil block use.
Additionally, having the confidence that a regionally produced growing media is of similar (if not greater) quality as a product from the Northeast, North Central growers can increase their economic, environmental, and social sustainability by purchasing these products. When goods are purchases from a local company, the money is often cycled within the local economy. These local dollars build social sustainability, growing communities and building resiliency. Shipping costs and emissions are also reduced and the products become less expensive, providing economic and environmental advantage.
After this project my awareness, skills, and attitude about sustainable agriculture have increased. I was aware of the soil block method for transplant production previously, but did not consider the all of the advantages such a technique could afford small-scale growers looking to increase their sustainability. Making soil blocks with a reusable tool instead of the flimsy plastic trays most common on farms opened my eyes to other ways farmers can reduce plastic use. I am now more aware of all the ways plastic is commonplace and depended on by small-scale (oftentimes organic) growers. Some may think it is impossible to grow efficiently without this plastic (such as in plastic mulch), but by showing that transplants can be successfully grown with significantly less plastic, I am now more open to the other changes in behavior that would be needed to reduce plastic on the farm. Additionally, I have develop my skill of making soil blocks and experienced the nuances that come with growing transplants in this way, such as their irrigation needs.
My advisor has experienced similar changes in awareness and skill. Having the opportunity to discuss transplant production with many growers and others throughout this project has given him (and myself) a greater understanding of the challenges regional growers have in producing quality transplants. We have become aware of networks which grown transplants for each other and buying clubs for purchasing growing media in bulk quantities, amongst other things. Coming from an academic environment, one may have a limited view of what is necessary for 'proper' transplant production, relying on expensive greenhouses, inline fertigation, and expensive lighting. Yet, most growers in our region do not have access to these amenities. In our increasing awareness of the practices used by growers in our area, we are better able to tailor extension materials and develop research projects which benefit them and increase their sustainability.