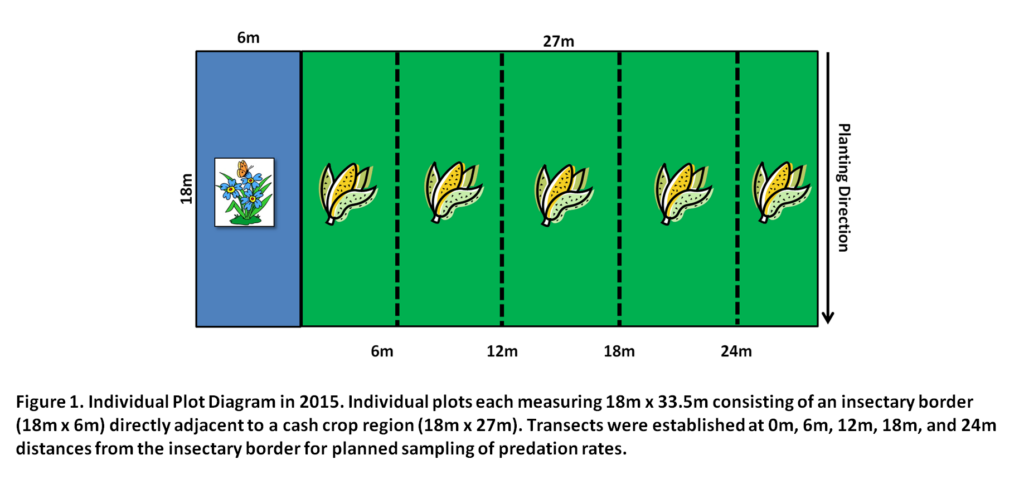
Final Report for GNE14-081
Project Information
Agronomic crop farms are often highly disturbed, lacking alternative resources to support natural enemies which are vital in suppressing pests below economically damaging levels. Under these circumstances, natural enemy survival may be reduced. However, deliberately introducing floral resources into the farmscape represents a conservation biological control approach that can support natural enemies through the provision of nectar and pollen. While natural enemies can be supported by alternative plant resources, they often exhibit preferences toward specific plant species. Therefore, insectary plant mixtures can be designed to target specific important natural enemies. Buckwheat (Fagopyrum esculentum) and cowpea (Vigna unguiculata), which have been reported as resource-provisioning for important natural enemies such as the spotted lady Beetle (Coleomegilla maculata) and the minute pirate bug, (Orius insidiosus), were established in monocultures and a biculture to assess their impacts on the insect community. To measure the impacts of resource provisioning and diversification on the insect community, insects were surveyed weekly via sweep net sampling and biweekly using sentinel traps to monitor predation. Results indicate that predators increase in abundance with increasing number of inflorescences. Buckwheat appears to be especially attractive to natural enemies as indicated by their higher abundances. Results from this study can inform decisions when selecting potential insectary plant species to support natural enemies.
Introduction:
Insect natural enemies play a critical role in suppressing agricultural pests, but they also rely on plants for alternative food and shelter when their prey is scarce. Many agricultural landscapes have become more and more simplified and are now lacking in alternative plant resources that can be used by natural enemies. Although primarily predatory or parasitic, many important natural enemy species depend on resources provided by non-crop plants, such as nectar, pollen, alternative prey for food and shelter survive, especially during periods of limited prey availability. Natural enemy survival in these landscapes may be reduced because the resources on which they depend are declining or have become absent. One approach to support natural enemies that are key to suppressing agricultural pests may be to deliberately introduce resource-producing "insectary plants" into the landscape to promote natural enemies and pest suppression. These insectary plants provide nutrient rich nectar and pollen to predators and parasitoids and can potentially therefore be used to enhance the abundance of natural enemies and their ability to suppress insect pests. Using insectary plants to promote the presence of beneficial insects in the field represents a sustainable approach to pest management that can result in reduced reliance on pesticides which can safeguard grower health and the environment.
The specific objectives the study are:
- Evaluate the effects of plant-based cover crop resources on the survival and longevity of two key generalist predators (Ongoing).
- Determine cover crop growth, development, and plant resource quality established in the field (Completed).
- Assess the foliar arthropod community associated with buckwheat, cowpea and a buckwheat-cowpea biculture compared to a fallow control (Completed).
- Monitor predation on pest insects within the cover crop strips and adjacent corn crop (Completed).
- Investigate dispersal of arthropods between the cover crop borders and cash crop plots (Ongoing).
Objective 1: Natural Enemy Longevity
We originally intended to investigate the effects of resource plants on the survival on the two key generalist predators the pink spotted ladybeetle and the insidious flower bug. While we did manage to start colonies of pink spotted ladybeetle, we were not able to successfully harvest enough eggs for continued experiments. The pink spotted ladybeetle colony lasted several months, however they did not lay sufficient amounts of eggs to sustain the colony. We tried different cage materials such as paper towel, kimwipes and other paper textures to simulate their natural egg laying locations, to encourage egg laying behavior, but we were not successful. We then decided to switch our focus to the insidious flower bug, which was reportedly easier to rear. To date, we have been able to successfully start and maintain a breeding colony, however more time is needed to complete greenhouse experiments necessary to evaluate insect performance. Progress toward this objective will continue and will be updated.
Objective 2: Insectary Establishment
Each year, the weedy fallow, buckwheat monoculture, cowpea monoculture and buckwheat-cowpea biculture treatments were established in the field in early June. Three weeks after planting , we began sampling to assess the insectary cover crop establishment. Sampling included weekly measurements of cover crop plant density, height, leaf number, total number of buckwheat floral inflorescences and cowpea extrafloral nectaries and the presence of weed species. We also recorded accumulated growing degree days. Data has been analyzed for this objective
Objective 3: Insect Community Effects
Assessment of the insect community was conducted in both years. Insects were collected from the insectary border weekly using sweep net sampling. Timed counts were done in standing corn plots to assess the abundance of natural enemies. All samples have been identified, counted and data analyzed
Objective 4: Predation Effects
Predation was assessed using European corn borer sentinel insect eggs placed in the field every two weeks. Currently, all collected eggs have been examined, recorded, entered and has been analyzed
Objective 5: Predator Dispersal
To observe dispersal between insectary borders and the corn main crop, insectary borders were marked using egg white protein and an additional color coded fluorescent dye to determine the particular insectary treatment that a marked insect has visited. The first year's batch of recaptured specimens have been processed, however, the second year data will be processed after visiting my collaborator's laboratory in January. This objective is ongoing and will be updated
Cooperators
Research
Field Establishment
To assess the potential of cover crop species mixtures to support natural enemies and enhance pest suppression in adjacent corn, Zea mays, we established field experiments at the Russell E. Larson Agricultural Research Center, Rock Springs, PA in 2014 and 2015. The experimental site consisted of 16 experimental treatment plots, each measuring 18.2 m x 13.7m, arranged in a randomized complete block design. Each block was replicated 4 times. In 2014, treatment plots consisted of two areas, an insectary border region (13.7m x4.5m) and an adjacent corn crop (13.7m x 13.7m). In 2015, the experimental layout was similar, however treatment plot sizes were larger, where the insectary border and cash crop portion of each individual plot was 18.2m x 4.5m and 18.2m x 27.4m, respectively.
In early June, cover crops were established as insectary borders containing buckwheat (Fagopyrum esculentum)(BW), cowpea (Vigna unguiculata)(CP), a buckwheat-cowpea biculture (MX), or a weedy fallow treatment (WF) along the edges of corn cash crop plantings. In monoculture plots, buckwheat was planted at the recommended seeding rate of 61.6kg/ha. Cowpea was planted at rate of 78kg/ha, a rate higher than recommended to compensate for poor germination observed during a preliminary study. The seeding rate of the biculture consisted of an adjusted 16.8kg/ha for buckwheat and 48kg/ha for cowpea to reduce the likelihood of buckwheat dominating the mixture as observed in a preliminary study. The weedy fallow treatment consisted of naturally occurring weed species as a control. Untreated, non-transgenic corn was planted immediately after the insectary border at the recommended rate of 81,500 plants per hectare. We began data collection 3 weeks after planting, when insectary borders and corn had germinated, and continued through mid-September, when we ended of the study
Insectary Resource Abundance
To monitor resource abundance of the insectary border areas, we measured plant features such as plant density, height, number of true leaves, number of inflorescences, and extrafloral nectaries per plant. Data collection started three weeks after planting and just prior to bloom. We measured density by randomly tossing a 1/4 meter quadtrat into the insectary border and counted all of the plants. At each sample location we chose one plant to measure the growth characteristics mentioned above.
Sweep Net Sampling
To survey the insects attracted to the insectary border, we began sweep net sampling three weeks after the borders were planted. We sampled weekly and close to midday when insects were most active. We placed the captured insects into bags and froze them until ready for identification and counting. During identification, all insects were identified to their lowest taxonomic level.
Timed Counts
To survey predator abundance within the standing corn, we conducted timed counts. Three-minute timed counts were conducted weekly. During a three minute timed counts we examined a row of corn and recorded natural enemies such as the pink spotted lady beetle and the insidious flower bugs.
Sentinel Predation
We examined predation rates using sentinel prey eggs of the European corn borer. Predation assays were conducted biweekly throughout the duration of the study within the insectary border and in the adjacent corn area of the plot. European corn borer eggs were purchased and prepared by recording the number of eggs per mass and gluing individual egg masses to index card backing. Sentinel predation assays each year approximately 5 weeks after planting when corn and insectary border treatments were established. We collected the sentinel egg masses after 48 hours of field exposure and examined them under microscope for signs of predation.
Insect Dispersal and ESLIA Protocol
While it is important to survey the abundance of natural enemies and pest suppression offered by natural enemies within insectary borders and main crop, it is also important to determine whether natural enemies supported by resource producing plants actually move between the insectary border and the main crop. To do this we decided to measure dispersal from the insectary border to the main crop. This as accomplished by using a technique called mark-capture. During the bloom window of the insectary borders, just before they were terminated, we applied a solution of egg whites to the insectary border. Any arthropod within the insectary border would be covered in the egg white protein. This protein can be later collected and tested for the presence of the egg white protein. This would confirm that the insects came from the insectary border. After the insectary borders were removed, we conducted recapture trials for weeks after initially marking with the egg white protein where we recaptured all ladybeetles and insidious flower bugs within the main crop over timed intervals. The mark-recapture method and ELISA testing was done with the help of my collaborator Dr. James Hagler at the USDA-Arid Land Research Center in Arizona. The ELISA, or enzyme linked immunosorbent assay is a procedure where, the egg white proteins covering potentially marked insects captured from our insectary borders are rinsed of the protein. We then use a series of reagents of enzymes that would bind to the egg white protein and a pigmented protein that turns blue when bound. This test allows rapid large scale detection of these natural enemies that are potentially marked with the chicken egg protein. This further tells us which insects travelled between insectary borders for nectar and pollen and back to the main crop to attack pest insects. To further confirm this we did a second level of protein tagging where we tagged the aforementioned sentinel insect eggs with a solution of milk and tested for the presence of the milk protein using the similar ELISA technique. This second layer would confirm that natural enemies were in fact feeding on the bait eggs we set out into the field. Taken together these tests show the degree to which insects move between insectary borders and the main crop as well as how many of those marked fed on our bait insect eggs.
1. Effects on Longevity
No data is currently available from this study on the direct effects of floral provisioning on natural enemy longevity as we are currently rearing Orius insidiosus for use in the small scale green house study.
2. Cover Crop Establishment.
In 2014, the insectary borders planted in early June in each of the treatments emerged about 5 days after planting and data collection began two weeks after planting. Figure 2.1 depicts cover crop resource production, measured as the number of inflorescences (left) or extrafloral nectaries (right) per 0.25m2, each week after planting. Buckwheat in both the monoculture and mixture treatments began to flower 5 weeks after planting, reaching peak blooms of ~700 and ~400 inflorescences per 0.25m2 at 7 weeks after planting, respectively. Cowpea nectary structures began developing shortly after planting in the stipels of each trifoliate leaf. Cowpea nectary production reached ~250 and ~50 nectaries per 0.25m2 before termination 7 weeks after planting in the monoculture and mixture treatments, respectively. Buckwheat inflorescences and cowpea extrafloral nectaries were lower in the mixture treatments due to lowered seeding rate of each. Cowpea development was slowed in the mixture treatment likely due to vigorous growth by buckwheat leading to increased competition with cowpea. Moving forward, this effect can be minimized by further lowering the seeding rate of buckwheat, while increasing the rate of cowpea in the mixture. The weedy fallout treatment contained no observable floral or extra floral nectary resources.
In 2015, seeding rates were adjusted in the mixture treatments to allow cowpea to establish better than in 2014. Results from 2015 were similar to 2014 in that mean density of buckwheat inflorescences were higher in buckwheat monoculture compared to cowpea, mixture and weedy fallow plots . Cowpea extrafloral nectaries were monoculture plots compared to the biculture treatment on all dates sampled, despite increasing the seeding rate of cowpea in mixtures to reduce the likelihood of buckwheat dominating the mixture treatments in 2015.
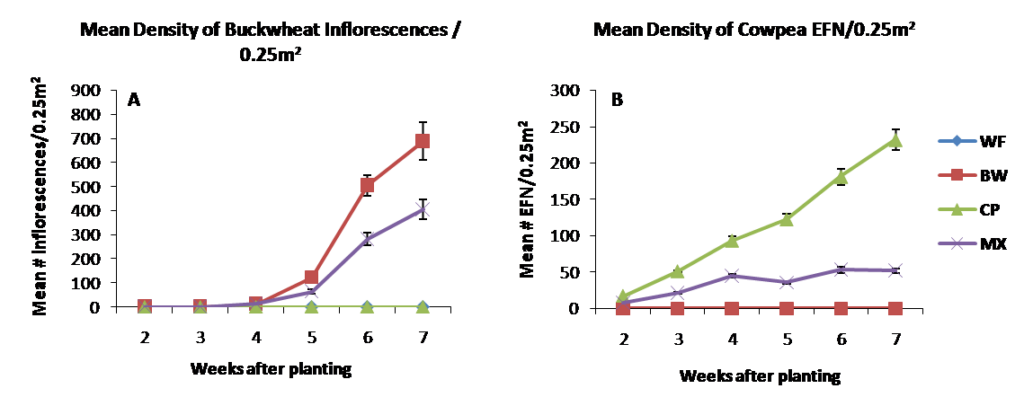 Figure 2.1 Resource density measured as mean number of buckwheat inflorescences (A) and cowpea extrafloral nectaries (B) per 0.25m2 in 2014.
Figure 2.1 Resource density measured as mean number of buckwheat inflorescences (A) and cowpea extrafloral nectaries (B) per 0.25m2 in 2014.
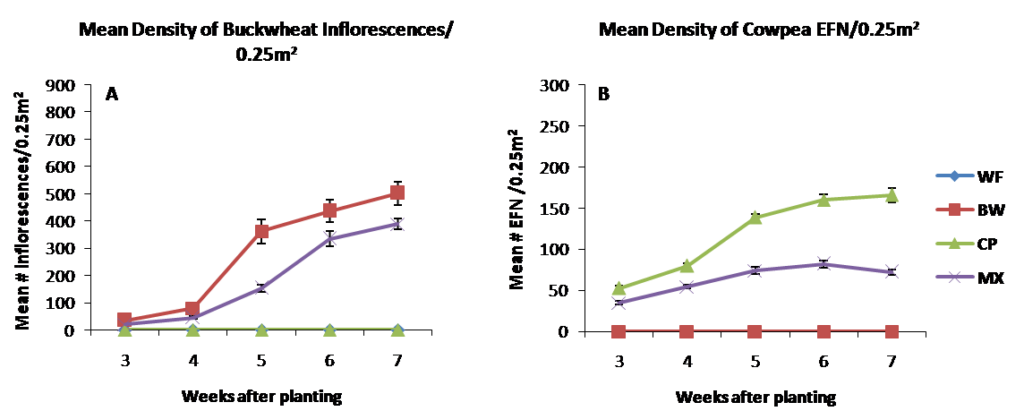 Figure 2.2 Resource density measured as mean number of buckwheat inflorescences (A) and cowpea extrafloral nectaries (B) per 0.25m2 in 2015.
Figure 2.2 Resource density measured as mean number of buckwheat inflorescences (A) and cowpea extrafloral nectaries (B) per 0.25m2 in 2015.
Insect Community Effects
Sweep Net Survey
In 2014, we surveyed arthropods weekly using sweep nets. The most commonly collected natural enemies included crab spiders, the pink spotted ladybeetle and the insidious flower bug. There was a significant effect of treatment on spider abundance. In general, we found crab spider abundance to be greater in buckwheat and biculture treatments compared to cowpea and weedy fallow. Lady beetles appeared to be more abundant in the buckwheat and biculture treatments, by 7 weeks after planting, but these differences were not statistically significant for any week sampled in this year. Insidious flower bugs were more abundant in buckwheat and biculture borders compared to cowpea and weedy fallow beginning 5 weeks after planting. There were also some notable pest species captured in sweep net samples. These included pests such as aphids , the tarnished plant bug and the potato leafhopper. There were no significant treatment effects on aphids. Tarnished plant bugs were more abundant in buckweat and biculture treatments compared to weedy fallow and cowpea when sampled 5, 6 and 7 weeks after planting. Potato leafhoppers were significantly impacted by insectary border treatments and were more abundant in cowpea insectary borders compared to the other treatments (figure 3.1).
In the 2015 study year, results were similar to 2014, the most commonly collected predatory arthropods included crab spiders, pink spotted ladybeetles and minute pirate bugs. Crab spiders were again, more abundant in the buckwheat and biculture borders compared to weedy fallow and cowpea 7 weeks after planting. In 2015, there were no significant effects of insectary treatment on pink spotted ladybeetle abundance although they appeared to be more abundant in buckwheat and biculture by 7 weeks after planting. There was a significant effect of insectary treatment on insidious flower bug abundance. Insidious flower bugs were more abundant in buckwheat monocultures and biculture compared to weedy fallow and cowpea monoculture on all dates sampled. Patterns with pest species were similar to those observed in 2014.The most commonly captured herbivorous insects included aphids, tarnished plant bugs and potato leafhoppers. In 2015, aphids were more abundant in the weedy fallow compared to buckwheat , cowpea and the biculture treatment throughout the duration of the study. Tarnished plant bugs were more abundant in buckwheat and the biculture treatments, which both contained buckwheat inflorescences when sampled 7 weeks after planting. Potato leafhoppers were captured only in the cowpea monoculture treatment and, as such, was significantly more abundant in the cowpea insectary treatment compared to other treatments on all days sampled, with abundance peaking at week 7 (figure 3.2).
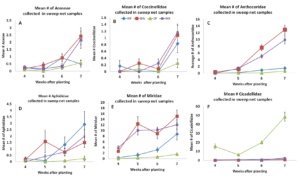 Figure 3.1 Mean number of (A.) Araneae, (B.) Coccinellidae, (C.) Anthocoridae, (D.) Aphididae, (E.) Miridae, (F.) Cicadellidae collected from sweep net samples collected from flowering insectary strips in 2014. Buckwheat, cowpea, and biculture treatments are denoted using squares, triangles, and Xs, respectively.
Figure 3.1 Mean number of (A.) Araneae, (B.) Coccinellidae, (C.) Anthocoridae, (D.) Aphididae, (E.) Miridae, (F.) Cicadellidae collected from sweep net samples collected from flowering insectary strips in 2014. Buckwheat, cowpea, and biculture treatments are denoted using squares, triangles, and Xs, respectively.
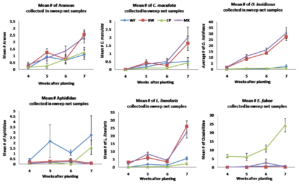 Figure 3.2 Mean number of (A.) Araneae, (B.) Coccinellidae, (C.) Anthocoridae, (D.) Aphididae, (E.) Miridae, (F.) Cicadellidae collected from sweep net samples collected from flowering insectary strips in 2015. Buckwheat, cowpea, and biculture treatments are denoted using squares, triangles, and Xs, respectively.
Figure 3.2 Mean number of (A.) Araneae, (B.) Coccinellidae, (C.) Anthocoridae, (D.) Aphididae, (E.) Miridae, (F.) Cicadellidae collected from sweep net samples collected from flowering insectary strips in 2015. Buckwheat, cowpea, and biculture treatments are denoted using squares, triangles, and Xs, respectively.
Timed Counts
In 2014, the pink spotted ladybeetle was initially found on July 10th and peaked by July 30th before declining toward the end of the study. Pink spotted ladybeetle numbers were significantly more abundant in corn plots adjacent to buckwheat insectary borders compared to those bordering cowpea throughout the study. However were similar when compared to weedy fallow and biculture treatments. Insidious flower bug was initially found on July 18th and began to decline by July 30th. Insectary border treatment did not appear to affect insidious flower bug numbers.
In 2015, neither the pink spotted lady beetle nor the insidious flower bug were impacted by insectary border treatment.
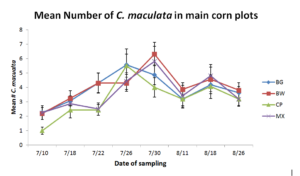 Figure 4. Mean number of Pink Spotted Ladybeetles found during three-minute timed counts
Figure 4. Mean number of Pink Spotted Ladybeetles found during three-minute timed counts
Rates of Predation
To test the effects of insectary borders on rates of predation in the field, we used sentinel prey at increasing distances away from the insectary border. However, these distances had no significant effect on proportion of prey eggs attacked therefore was removed. Figure 5.1 shows cumulative predation by all predators in the field over the course of the study. In general predation rates within the corn crop adjacent to the various insectary borders were similar with about 55-60% of eggs being attacked by chewing and sucking predators which were most notably the pink spotted ladybeetle and the insidious flower bug. Predation within the insectary border was likely due to nectar and pollen resources provided by each of the insectary border plants. Weedy fallow did contain approximately 50% predation which was likely due to less nectar and pollen available in weedy fallow treatment.
Figure 5.2 summarized the cumulative predation by all predators in the field in 2015. Similar to 2014, there were similar levels of predation within the corn crop adjacent to insectary borders. Predation within the insectary borders were however unlike 2014. We instead saw higher levels of predation in the buckwheat and biculture treatments rather than just the weedy fallow seen in 2014. It is possible that the larger area of the insectary borders could have served as a stronger signal to attract more natural enemies. This is consistent with the fact that we found overall higher numbers of natural enemies including spider, pink spotted ladybeetles and insidious flower bugs in 2015 compared to 2014
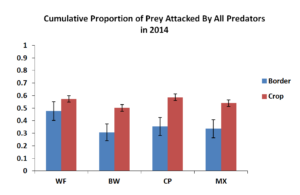 Figure 5.1 Mean Proportion of Prey Attacked by All Predators in 2014. Blue bars represent sentinel eggs placed within the insectary borders while red bars represent eggs place in the main corn crop.
Figure 5.1 Mean Proportion of Prey Attacked by All Predators in 2014. Blue bars represent sentinel eggs placed within the insectary borders while red bars represent eggs place in the main corn crop. 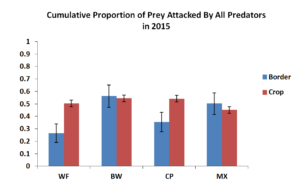 Figure 5.1 Mean Proportion of Prey Attacked by All Predators in 2015. Blue bars represent sentinel eggs placed within the insectary borders while red bars represent eggs place in the main corn crop.
Figure 5.1 Mean Proportion of Prey Attacked by All Predators in 2015. Blue bars represent sentinel eggs placed within the insectary borders while red bars represent eggs place in the main corn crop.
5. Dispersal Effects
In 2014. We captured 1052 pink spotted ladybeetles and 1291 insidious flower bugs to test for the presence of the protein mark. However, few individuals were positively marked with either protein. Due to low recovery of positively marked predators, it is difficult to determine how insectary treatment and/or transect influence predatory abundance in corn cash crop after termination of the resource rich border. The positive chicken marks, 22 (2.09%) pink spotted ladybeetle and 15 (1.16%) insidious flower bug, confirm that there is some degree of movement by predators that visited insectary borders into corn after termination of the insectary border. Positive milk marks indicate 15 (0.99%) C. maculata and 37 (2.86%) O. insidiosus preyed on marked sentinel eggs. No predator tested positive for both marks which would have confirmed that an individual both dispersed and preyed on marked sentinels.
Data for the 2015 is currently being process and will be completed when we are able to visit our collaborator in January.
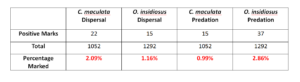 Table 1. Total number and proportion of C. maculata and O. insidiosus that were collected and tested positive for the presence of a dispersal and predation protein.
Table 1. Total number and proportion of C. maculata and O. insidiosus that were collected and tested positive for the presence of a dispersal and predation protein.
Over the course of the research project,concepts and results of this study were presented at a variety of academic and nonacademic events. Research presentations were done at the annual National Entomological Society of America meeting in 2014 and 2015. I also presented this research at the Entomological Society of America's Eastern Branch meeting in 2015 and will be presenting again as an invited speaker in March 2017. In addition to our ESA meetings, I regularly present this research at Penn State's Annual Sustainable Agriculture Cropping Systems Symposium yearly. I have also presented this research at numerous farmer advisory board meetings, field days and extension events often garnering much interested and inquiry into insectary border plants or mixtures that might help on different farmer's farms. These events each focused on grower adoption of cover crops mixtures aimed at improving ecosystem functions such as weed suppression, pest management and soil fertility. These events attracted both local and international farmers interested in learning of the costs and benefits cover crop use. Feedback from my presentations suggest that there is quite a bit of interest in both the academic and farming community in using these insectary plants to suppress on farm pests.
The potential impacts of this type of research could help reduce the amount of synthetic inputs that are currently being used on farm. Natural enemies if supported can be responsible for a high degree of pest suppression. Currently pesticides are expensive to develop, costly to apply due to equipment requirements and are toxic to many organisms in the environment. Many pesticides can cause non target effects in predatory insects and pollinators. This may lead to further decline of our beneficial insect populations. Giving this type of research serious consideration could begin to offset some of the declines we have been seeing.
Education & outreach activities and participation summary
Participation summary:
Along with ESA meetings and Penn State Symposiums (see above) I have presented this research at numerous farmer advisory board meetings, field days and extension events often garnering much interested and inquiry into insectary border plants or mixtures that might help on different farmer's farms. These events each focused on grower adoption of cover crops mixtures aimed at improving ecosystem functions such as weed suppression, pest management and soil fertility. These events attracted both local and international farmers interested in learning of the costs and benefits cover crop use. Concepts from this research were also incorporated into and Entomology course I instructed in the Summer of 2016 as a part of the Upward Bound Migrant Program as a part of the Federal TRIO Programs.
We are also currently creating natural enemy fact sheets that will contain information on how to identify, attract and sustain common natural enemies that farmers and gardeners may see in their gardens and farms. This will be updated as soon as they are finished.
All articles, publications and presentations are credited the the NE SARE for making this research possible.
Hinds, J and Barbercheck, M. 2016. The role of insectary plants in promoting pest suppression by key generalist natural enemies. 6th Annual Sustainable Agricultural Cropping Systems Symposium. Poster Presentation.
Hinds, J. 2015. Impacts of cover crop diversification on attraction, dispersal, and pest suppression by generalist predators. Entomological Society of America - Annual National Meeting. Presentation.
Hinds, J and Barbercheck, M. 2015. The role of insectary plants in promoting pest suppression by key generalist natural enemies. 5th Annual Cropping Systems Symposium. Poster Presentation.
Hinds, J. 2015. The role of insectary plants in promoting pest suppression by key generalist natural enemies. Entomological Society of America - Eastern Branch Meeting. General Poster Submission. Poster Presentation.
Hinds, J. 2014. Investigating the role of plant diversification on attraction and pest suppression in an insectary border. Entomological Society of America National Meeting. 10 Minute Student Paper Competition. First place winner. Presentation.
Hinds, J. 2014. Floral resources support higher abundances of beneficial insects. Bar Charts & Barbeque. Penn State University, University Park. April 25, 2014. Poster Presentation.
Hinds, J. 2014. Floral resources support higher abundances of beneficial insects. 4th Annual Triad Sustainable Cropping Systems Symposium. Penn State University, University Park. March 21, 2014. Poster Presentation.
Barbercheck, M.E., Hinds, J. 2014. Preserving beneficial predators in the home garden. Penn State Ag Progress Days. Russell E. Larson Agricultural Research Center. Rock Springs, PA.
2015 Pennsylvania State University. Office of Educational Equity. Workshop for Upward Bound College Preparation Seminar. July 16, 2015.
2015 Pennsylvania State University. Office of Educational Equity. Information Session for TRIO Students. March 18, 2015.
Project Outcomes
Not Applicable
Farmer Adoption
This research is regularly presented at farmer focused advisory board meetings, field days and extension events focused on grower adoption of cover crop mixtures aimed at improving ecosystem functions such as weed suppression, pest management and soil fertility. These events have attracted both local and international farmers interested in learning of the costs and benefits cover crop use. Farmers frequently show interest in using insectary plant species to suppress pests on their farms. They often ask about management strategies of insectary crops, what species to use and even the timing. Another farmer suggested not only using insectary plants to suppress pests, but to preserve soil, help with erosion and even harvest them for sale as some can be sold as ornamental plants. Another farmer who was in the process of writing a SARE FArmer-Rancher Grant proposal invited me to their farm to present on the potential impacts of the using insectary plants to attract beneficial insects. These experiences suggests to me that there is a lot of interest in this work.
Areas needing additional study
There are many areas that could use additional study. Basic research into discovering plant species that can serve as insectary species. Many of these are already currently being used as cover crops. However many times we do not know of the insects, both natural enemy and pest, associated with potentially insectary plant species. Work should also be done to evaluate how economically important natural enemy species perform in the presence of these insectary plant species. Similar to the concept of the longevity trials described above. It is important to demonstrate the the natural enemy species is not only attracted to the insectary plant, but also receive a nutritional benefit seen through increased survival, fecundity, or potential to attack more prey. Further work could also be done on the types of plant species that work well as a mixture. In this study we saw that buckwheat often outcompetes cowpea. Is there a plant that could pair better with buckwheat or cowpea so that both are represented? In addition to researching plant species and the insects they attract, more research could go into timing and management strategies. Insectary plants should be suited to the farmer's desired growing window, including peak bloom, termination and even the potential to become a weed.
Information Products
- Impacts of cover crop diversification on attraction, dispersal and pest suppression by generalist predators
- The role of insectary plants in promoting pest suppression by key generalist natural enemies.
- Preserving Beneficial Predators in the Home Garden
- Floral resources support higher abundances of beneficial insects
- Investigating the role of plant diversification on attraction and pest suppression in an insectary border