Final report for GNE16-118
Project Information
Summary:
Bee declines pose a serious risk to the human food supply, crop production, wild plant diversity, and the commercial bee industry and have, therefore, caused both local and global concerns about bee health. Pathogens and parasites that are harmful to bees, including RNA viruses, microsporidian fungi, mites, nematodes, and parasitic flies, pose a serious threat to bee population dynamics as they manipulate distributions, behaviors, survival, and overall abundance. Management of these pathogens and parasites requires a detailed understanding of the cyclic trends in pathogen and parasite prevalence and a better understanding of the factors impacting these patterns and tendencies, such as seasonal forcing, behavioral shifts, life cycles, pathogen and parasite strategies, as well as their interactions.
The purpose of this project was to improve understanding of pathogen and parasite epidemiology and transmission in bee communities to improve pathogen prevalence predictability and, in turn, management strategies. Under this mission, we more specifically addressed (1) how the influence of pathogens and parasites differ between honey bees and bumble bees, (2) the seasonal prevalence of pathogens and parasites in foraging honey bees and bumble bees, (3) the impact of differing overwintering strategies on pathogen and parasite prevalence in the spring in honey bees and bumble bees, (4) the relationship between foraging experience or range and pathogen prevalence, and (5) the relationship between geographic distance and the structure of pathogen and parasite prevalence among honey bee and bumble bee populations. Through integrating information on spatial properties of pathogen communities, likelihood of horizontal transmission and the seasonal prevalence of pathogens across bee taxa with different social and behavioral structures (honey bees and bumble bees), we can better understand the patterns of pathogen and parasite transmission as well as the periods for which these pathogens and parasites pose the greatest threat in combination with other seasonal trends (i.e. Varroa intensity, population size, etc) to the bee community. With this improved understanding, we can define applicable control strategies for bee pathogens and parasites to ensure sustained and high productivity of crops and wild plants as well as reduce the price of pollination services.
Towards this goal, honey bees (Apis mellifera) and bumble bees (Bombus impatiens) were collected across two overwintering periods (fall 2015 - spring 2016 and fall 2016 - spring 2017), one full active season (early spring to fall 2016), and an additional early season collection (2018) across six sites within Centre County, Pennsylvania. Honey bee and bumble bee samples were screened for Deformed Wing Virus (DWV), Black Queen Cell Virus (BQCV), and fly parasitism. Additionally, bumble bees were screened for Nosema sp. and Sphaerularia bombi. Results suggest that unique seasonal trends exist for pathogens and parasites that are consistent between honey bees and bumble bees. DWV peaks in prevalence in the fall and BQCV peaks in prevalence during mid-summer. In bumble bees, fly parasitism also peaks in the fall. Overall, honey bees exhibited higher prevalence of both viruses compared to bumble bees. While both honey bees and bumble bees reduced viral prevalence through the overwintering process, bumble bees did so to a greater extent suggesting a potential purge of pathogens. Thus, honey bees may have a larger impact on pathogen transmission, compared to bumble bees, as they could be harboring these viruses during the winter period and spreading them back to the native bee community each year in the spring.
The overall goal of this project was to improve understanding of pathogen and parasite epidemiology and transmission in bee communities to better predict pathogen prevalence and spread. Utilizing this information, more informed management strategies and decisions can be defined and adopted. As many factors can impact host-pathogen/parasite relationships as well as pathogen and parasite patterns and tendencies, it is vital that we improve our understanding of when pathogens and parasites pose the greatest threat and how these pathogens and parasites are being transmitted within the bee community. To fully understand the epidemiological impact of these pathogens/parasites and manage their spread, we need to understand how temporally based host strategies and behaviors impact pathogen and parasite susceptibility. To accomplish this goal, we addressed the following questions:
- What is the pathogen prevalence in bumble bees and honey bees and how do they differ?
- Is there a seasonal pattern to pathogen and parasite prevalence in bees and does this differ between bee taxa (honey bees and bumble bees)?
- How do different overwintering strategies impact the survival of pathogens and parasites through the winter?
- Is there a relationship between foraging experience or range and pathogen and parasite prevalence?
- How does geographic distance impact pathogen and parasite prevalence among honey bee and bumble bee populations at a local scale?
Agricultural sustainability hinges on pollination services. Worldwide, 75% of the crops that humans consume (Klein et al. 2007) and 80% of wild plants (Potts et al. 2010) rely on pollinators for successful reproduction. The majority of pollination services are provided by bees (Free et al. 1993). Pollination services in agriculture have relied heavily on commercial honey bees, nonetheless native bees, including bumble bees and the thousands of species of solitary bees, perform vital pollination services. Native bees can be more efficient pollinators than honey bees, enhance crop production alongside honey bees, and provide sufficient pollination in lieu of honey bees for many agricultural crops (Garibaldi et al. 2013, Winfree et al. 2008). The pollination services of native bees alone are valued at $3.07 billion in the United States (Losey and Vaughan 2006). Thus, bee conservation is an important local and global issue as precipitous losses of honey bees and population declines of native bees threaten the sustainability of crop production, native plant diversity, the commercial bee industry, and the human food supply (McMahon et al. 2018, VanEngelsdorp and Meixner 2010).
Pathogens and parasites have been identified as drivers of biodiversity loss among numerous wildlife populations (Altizer et al. 2003), including bees (Cameron et al. 2011; Evison et al. 2012). As is the case with many wildlife diseases, the epidemiological dynamics surrounding pathogen and parasite prevalence within the bee community is quite complex. Not only are a diverse assortment of pathogens, ranging from RNA viruses to microsporidian fungi (Evans and Schwarz 2011; Macfarlane et al. 2015; Evison et al. 2012), and parasites, including mites (Sammataro et al. 2000), flies (Schmid-Hempel et al. 1990; Core et al. 2012), and nematodes (Macfarlane et al. 2015; Tripodi et al. 2018) harmful to bees, but these pathogens and parasites each utilize unique strategies ranging in host breadth, transmission routes, and overall virulence (Schmid-Hempel 2011). Furthermore, current research shows that many of these pathogens and parasites can readily be shared through pathogen spillover/spillback among numerous bee taxa (Singh et al. 2010; Furst et al. 2014; McMahon et al. 2015; Ravoet et al. 2014). Horizontal transmission of pathogens and parasites occurs during foraging bouts with flowers operating as hotspots for transmission and pollen acting as the vehicle by which pathogens and parasites are transported back to natal colonies, thus creating an ideal scenario for cross-species transmission or pathogen spillover/spillback (Singh et al. 2010; Graystock et al. 2015). To date, the directionality of transmission, or the part that managed bees play in transmission to native bee communities and in turn native bees to managed bees, is poorly understood. While numerous bee taxa are inflicted with these pathogens and parasites, they appear to vary in terms of susceptibility and pathogenicity. For instance, viruses can be more virulent to honey bees than solitary bees, Megachile rotundata and Colletes inaequalis (Dolezal et al. 2016).
Many factors apart from the host-parasite relationship can modify pathogen and parasite patterns and thus lead to to cyclic pathogen outbreaks, such as population density, seasonal behavioral trends, the presence of other parasites, and climate (Altizer et al. 2006). These various factors can lead to cyclic trends through variably altering the probability of infection, host susceptibility, and pathogen viability and transmission potential. As bees have cyclic variation in their behaviors, such as differing periods of diapause, reproduction, and foraging, it is likely that there are cyclic patterns to the pathogens and parasites they carry. Trait differences in bees such as colony growth rate and life-cycle phases will likely impact species-specific pathogen prevalence and the composition of the host community will influence whether pathogens/parasites persist. For example, if a species has a relatively short active period, this may reduce their exposure and their pathogen loads.
Pathogen/parasite survival during the overwintering period may differ depending on a taxa’s overwintering behavior. Honey bee colonies are perennial, meaning they are active throughout the entire year, although workers are genetically distinct and reduced in number during the winter. Bumble bees, alternatively, have an annual cycle where the colony dies each year leaving the next year’s queens (gynes) to survive the winter alone. Thus, it could be hypothesized that honey bees, whereby many individuals survive the winter together, are more likely to maintain pathogens and parasites as there are more susceptible individuals compared to bumble bees. During the active season, honey bee colonies also maintain a greater number of individuals per colony compared to bumble bees and many solitary bees.
Conservation of bee populations as well as sustenance of the commercial bee industry will require an improved understanding of the epidemiological dynamics, including population and community level patterns and transmission properties of pathogens and parasites across a range of bee taxa, in order to develop and apply effective management strategies. However, management is hindered by our lack of knowledge about the complex epidemiological dynamics of pathogen and prevalence and transmission in bee communities. In particular, we lack a thorough understanding of how pathogens and parasites are transferred between species across time and space as well as how various factors impact species-specific patterns of pathogen prevalence. In this study, we sought to improve epidemiological understanding of prevalence and transmission patterns of pathogens and parasites among bee taxa by examining trends in honey bees and bumble bees. More specifically, we documented (1) the seasonal prevalence of pathogens and parasites in foraging honey bees and bumble bees, (2) the impact of differing overwintering strategies on pathogen and parasite prevalence in the spring, (3) the relationship between foraging experience and range and pathogen prevalence, and (4) the relationship between geographic distance and the structure of pathogen prevalence among local bee populations. To achieve this goal, we performed a large-scale pathogen survey, identifying the change in prevalence of viruses (Deformed Wing Virus (DWV) and Black Queen Cell Virus (BQCV)), microsporidians, and nematodes across two overwintering periods, an active season (March-October) and multiple years (2015-2018) in Centre County, Pennsylvania.
Cooperators
- (Researcher)
- (Researcher)
- (Researcher)
- (Researcher)
- (Researcher)
- (Researcher)
- (Researcher)
Research
Sample collection: Bumble bees (Bombus impatiens) and honey bees (Apis mellifera) were net collected while nest searching or foraging during the active season (March to October) at multiple time periods across four years (2015, 2016, 2017, and 2018) at several sites in Centre County, Pennsylvania. In 2015, bumble bees were collected at 5 sites (Bellefonte, Colyer Lake Area, Penn State University Park Campus, Russell E Larson Agricultural Research Farm, and Tussey Mountain) at two time points: spring (late April) and fall (late September). Honey bees were collected at all of these sites, but only in the fall in 2015. In 2016, bumble bees and honey bees were collected at six sites (University Park Airport Area, Bellefonte, Colyer Lake Area, Penn State University Park Campus, Russell E Larson Agricultural Research Farm, and Tussey Mountain) at four time points (spring (late March/early April), summer 1 (early June), summer 2 (mid-late July), and fall (mid-September/late October)). In 2017, bumble bees and honey bees were collected at four sites (Colyer Lake Area, Penn State Campus, Russell E Larson Agricultural Research Farm, and Tussey Mountain) at one time period, spring (April), and in 2018, bumble bees were collected at three sites (Colyer Lake Area, Russel E Larson Agricultural Research Farm, and Tussey Mountain) at one time period, spring (April) (Figure 1). The spring bumble bees were newly emerged queens, whereas those collected at other time periods were primarily workers. The summer 1 time period was selected based on Bombus impatiens worker emergence. Samples were kept alive on ice until they could be stored at -80oC for molecular and microscopic analyses. Our sampling scheme allowed us to examine the prevalence of pathogens and parasites throughout a whole active season as well as across two overwintering periods. In addition, we assessed the pathogen and parasite prevalence of spring bumble bee queens across all four years. Collecting samples from flowers rather than colonies, further enabled us to examine the amount and trends with which pathogen/parasite prevalence are shared, at the community level, in the field.
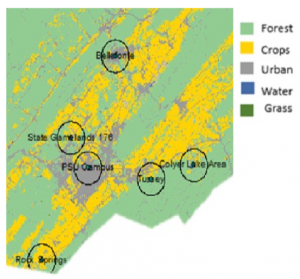
Figure 1: Location and landscape cover of sampling sites used across all four years (2015, 2016, 2017, and 2018) in Centre County, Pennsylvania.
Assessing the overall and seasonal prevalence of pathogens and parasites: Bumble bee and honey bee samples were individually screened for the presence of Deformed Wing Virus (DWV), Black Queen Cell Virus (BQCV), and fly parasitism. Additionally, bumble bees were screened for Chronic Bee Paralysis Virus (CBPV), Sac Brood Virus (SBV), Kashmir Bee Virus (KBV), and Israeli Acute Bee Paralysis Virus (IAPV) in 2015 as well as Nosema spp., and Sphaerularia bombi across all years. Only DWV, BQCV, fly parasitism, Nosema spp., and Sphaerularia bombi were screened after 2015. This was because DWV and BQCV were found to be the most common viruses in Centre County during the 2015 sampling, fly parasitism could be assessed during dissections, Nosema spp. has been suggested as a major pathogen causing declines in bumble bees (Cameron et al. 2011; Cameron et al. 2016), and Sphaerularia bombi could be easily assessed during Nosema spp. screens. Overall, a total of 1,055 bees were screened (432 Apis melliferaand 623 Bombus impatiens) for DWV, BQCV, and parasitic flies, 168 Bombus impatiens were screened additionally for IAPV, KBV, SBV, and CBPV.
RNA was extracted from the abdominal cavities of ~15 bees per time period per site per year using a phenol (Qiazol)-chloroform extraction followed by DNase remove with the Invitrogen TURBO DNA-free kit. The RNA extraction was performed on the abdominal cavity with the gut removed to assess tissue where viral replication occurs and to avoid attaining viral signal from food remaining in the gut. RNA was converted to cDNA using the High-Capacity cDNA Reverse Transcription Kit from Applied Biosystems. To assess for RNA viruses, RT-PCR was performed on converted bee cDNA using established primers for Deformed Wing Virus (DWV), Black Queen Cell Virus (BQCV), Chronic Bee Paralysis Virus (CBPV), Sac Brood Virus (SBV), Kashmir Bee Virus (KBV), and Israeli Acute Paralysis Virus (IAPV) (Singh et al. 2010; Muli et al. 2014). Furthermore, elongation factor-1α (EF1α) (Hines et al. 2006), a housekeeping gene, was utilized to confirm that a negative result was not an artifact of a failed extraction. All RT-PCR trials included a positive and a negative control. Gel electrophoresis was run to detect the presence or absence of each virus. Samples collected post 2015 were only screened for DWV and BQCV as these were found to be the most common viruses in bumble bees in Centre County in 2015. The presence of parasitic flies in bumble bees were assessed using dissections of the abdomen. Parasitic flies will lay their eggs into and the larvae will develop in the abdomens of bees, therefore, during dissections, larvae could be observed. Further, during dissections, guts were removed to detect Nosema spp. spores and Sphaerularia bombi. Guts were homogenized in 100 ul of phosphate-buffered saline (PBS) using a pestle. Twelve ul of homogenate was placed on a slide and visualized using phase-contrast microscopy at 400x.
Assessment of foraging experience and range: For a subset of bumble bee samples, we measured wing wear and the marginal cell length. We used wing wear as a measure of amount of bumble bee foraging experience, thus as an approximation for age and degree of field exposure. The marginal cell length is a measure of body size (Medler 1962; Plowright and Jay 1968). Wings were imaged using an Epson Perfection V600 Photo computer scanner and aligned to a template of a wing with no wing wear (full wing) which was used to measure the area of the wing missing using photoshop along with marginal cell length.
What is the pathogen prevalence in bumble bees and honey bees and how do they differ?
Overall, we found that viral prevalence of DWV and BQCV was consistently higher in honey bees compared to bumble bees (Figure 3). Of all pathogens, BQCV was the most prevalent pathogen for both honey bees and bumble bees (honey bees: 51.74%; bumble bees: 14.77%), followed by DWV (honey bees: 45.6%; bumble bees: 11.24%), and parasitic fly occurrence (honey bees: 0%; bumble bees:7.38%). Unlike bumble bees, honey bees were more likely to be infected in general than not, and more often were infected with both DWV and BQCV than either pathogen alone (Figure 2). These data suggest that honey bees are likely to act as a reservoir for pathogens shared among bee communities. Previous research suggests that even at the low prevalence observed in bumble bees, pathogens and parasites can negatively impact population health, especially in concert with other stressors, such as pesticides and poor nutrition (McMahon et al. 2015). Honey bees and bumble bees experienced co-infections more than expected (honey bees: p=2.2e-16, probability of co-infection=0.2853828; bumble bees: p=0.03093, probability of co-infection=0.05778491). This may be driven by the potential for stressed bees to harbor heavier and more diverse pathogen loads (Chen et al. 2004; Carrillo-Tripp et al. 2016).
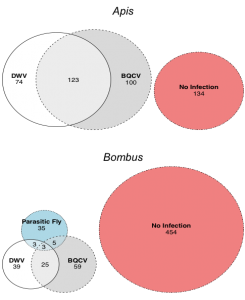
Figure 2: Individual occurrence of pathogens and parasites and the incidence of co-infections in honey bees and bumble bees.
Is there a seasonal pattern to pathogen and parasite prevalence in bees and does this differ between bee taxa (honey bees and bumble bees)?
Honey bees and bumble bees significantly differed in DWV prevalence (z=11.703, p<2e-16), p=0.00028). Bumble bees and honey bees appear to follow similar trajectories in the seasonality of these pathogens (Figure 3). However, DWV prevalence was not significantly different in honey bees throughout the active season (X2=2.951, p=0.15160), while in bumble bees, there was a significant difference in DWV presence over time (Julian dates) (X2=24.252, p=0.00028) with a peak occurring in the fall (Figure 3). In addition, the presence of DWV differed between years (X2=18.010, p=3.61e-05), with the highest prevalence occurring in 2015 and the least in 2018. The seasonal peak for BQCV was different from DWV, occuring during the middle of summer. This temporal pattern is apparent in both honey bees and bumble bees with there being a significant effect of time for both respectively (honey bees: X2=31.702, p=4.84e-06; bumble bees: X2=87.693, p=1.19e-14) (Figure 3). Overall, honey bees and bumble bees differ in BQCV prevalence (z=11.257, p<2e-16). Furthermore, both site (X2=34.813, p=2.63e-07) and year (X2=7.831, p=0.0125) had significant effects on BQCV presence. The average prevalence of BQCV was greatest in 2016 and least in 2018.
These results suggest that there are predictable seasonal trends in pathogen prevalence for both pathogens. The differences observed in the seasonal trends between DWV and BQCV could be the result of viral strategy (chronic vs. acute). With a chronic viral strategy, the expected seasonal trend would be to slowly increase throughout the season as more individuals become infected. DWV is thought not to be highly lethal (Chen and Siede 2007) which may help support its persistence as a chronic virus, thus leading to the observed season increase. With an acute viral strategy, the expected seasonal trend would be a peak during the time period when the virus has opportune circumstances (ex. pulse in susceptible individuals) and then to taper off as infected individuals die without infection of further susceptible individuals. BQCV could be operating under such a strategy. Host phenology could explain the timing of the peak of BQCV, as it peaks mid-summer when both bumble bee and honey bee colonies reach peak worker abundance and brood rearing. This correlation with peak brood production makes sense given that BQCV is primarily considered to impact the brood (Chen and Siede 2007). Varroa abundance could explain the trend of DWV peaking at the end of the season, as Varroa populations increase and likely more frequently transmit DWV in the fall (Degradi-Hoffman and Curry 2004; Martin 2001).
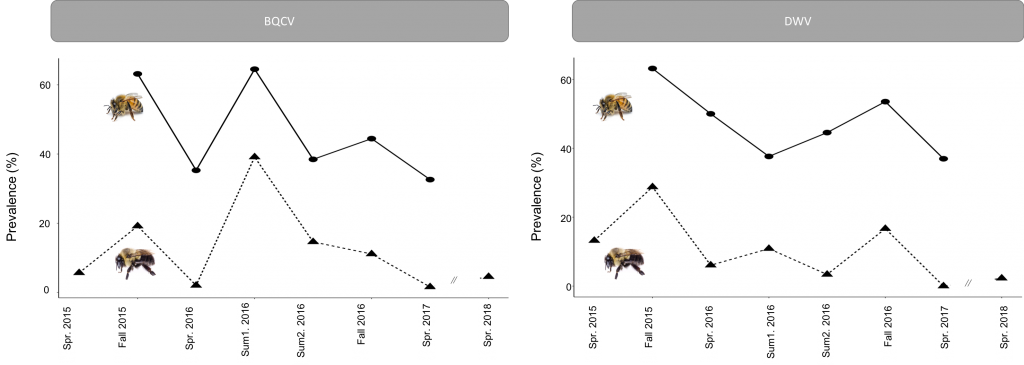
Figure 3: Seasonal prevalence of BQCV and DWV for honey bees (solid lines) and bumble bees (dashed lines). Fall was September/October. Spring was March/April. Summer 1 was early June. Summer 2 was mid-July.
How do different overwintering strategies impact the survival of pathogens and parasites through the winter?
Using a chi-squared test, pathogen prevalence was compared across the overwintering period between fall and spring time periods individually within each taxon. Our results suggest that pathogen prevalence in honey bees does not significantly change through the overwintering period (from fall to spring) from 2015 to 2016 nor from 2016 to 2017 for DWV (2015-2016: X2=0.57134, p=0.4497; 2016-2017: X2=2.825, p=0.09281). However, the pathogen prevalence of BQCV in honey bees did differ from 2015 to 2016 (X2=3.6739, p=0.05527), but not from 2016 to 2017 (X2=1.3656, p=0.2426). Alternatively, in bumble bees, the pathogen prevalence of both viruses (DWV and BQCV) significantly changes across all overwintering periods (DWV: 2015-2016: X2=14.699, p=0.0001261; 2016-2017: X2=9.8328, p=0.001714; BQCV: 2015-2016: X2=12.698, p=0.000366; 2016-2017: X2=3.7113, p=0.05405) (Figure 4). Overall, the data suggest that bumble bees have a seasonal pathogen reduction or purge of pathogens while honey bee populations retain pathogens. The purge of pathogens observed in bumble bees may be due to a bottleneck effect whereby bumble bees that are infected may be less likely to survive overwintering and, as they are in a solitary state, further transmission cannot occur. Alternatively, bumble bee queens may be better at fighting off and purging acquired pathogens than workers. Given the much higher spring prevalence in honey bees and overall pathogen prevalence in general, this means honey bees potentially act as a reservoir maintaining pathogens in the bee community. The retention of these pathogens in honey bees may therefore negatively impact levels in the native bee community repetitively year after year.
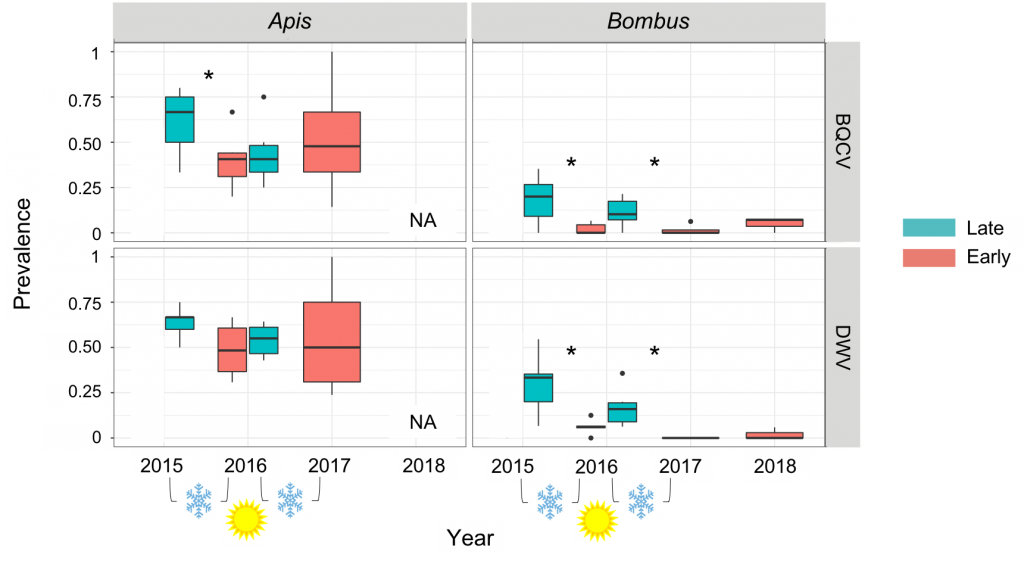
Figure 4: Viral prevalence of DWV and BQCV in honey bees and bumble bees over two overwintering periods (Fall 2015 to Spring 2016 and Fall 2016 to Spring 2017). Fall (Late) is represented with the color teal and Spring (Early) is represented with the color salmon.
Is there a relationship between foraging experience or range and pathogen and parasite prevalence?
Neither foraging experience nor body size had a significant impact on viral prevalence in bumble bees (foraging experience: t=-1.1602, df=132.59, p=0.2481; body size: t=-0.36655, df=110.04, p=0.7147). Further, when viruses were tested separately, there was still no relationship between either DWV prevalence or BQCV prevalence and foraging experience (DWV: t=0.58771, df=52.763, p=0.5592; BQCV: t=-1.2457, df=55.99, p=0.2181) or body size (DWV: t=-0.67115, df=48.857, p=0.5053; BQCV: t=0.069996, df=52.576, p=0.9445) (Figure 5). However, while the result is not significant, the average body size is consistently slightly greater for bees where virus, be it DWV or BQCV, is present (Figure 5). In addition, the presence of parasitic fly larvae was marginally significantly related to wing wear (t=-2.0548, df=18.764, p=0.05409), as may be expected given their higher prevalence later in the season, but not significantly related to body size (t=0.81487, df=17.885, p=0.4259) (Figure 5). These results suggest that direct viral transmission may be playing a more prominent role than horizontal viral transmission during foraging activities. Perhaps this is not unexpected, as both DWV and BQCV can be acquired within the nest either from mother to offspring or from nestmates prior to infection in the field.
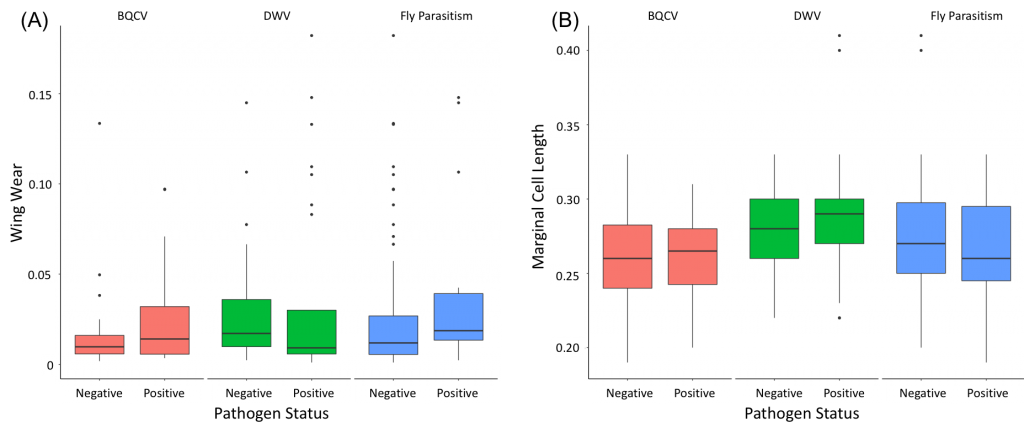
Figure 5: Examination of the effect that horizontal transmission via foraging impacts overall pathogen and parasite prevalence. (A) Effect of foraging experience (wing wear) in B. impatiens on pathogen and parasite (BQCV, DWV, and fly parasitism) status (negative or positive). (B) Effect of foraging range (marginal cell length) in B. impatiens on pathogen and parasite (BQCV, DWV, and fly parasitism) status (negative or positive).
How does geographic distance impact pathogen and parasite prevalence among honey bee and bumble bee populations at a local scale?
We deduced whether particular pathogens or parasites are spatially structured at a local scale by examining the relationship between the geographic distance between each of our sites and the difference in pathogen or parasite prevalence. We determined that DWV prevalence is similar across sites for honey bees (R = 0.055, p=0.55) and only marginally different for bumble bees (R=0.16, p=0.073), suggesting spatial homogeneity of DWV at this scale. However, we found signal of localized spatial structuring of BQCV prevalence in honey bees (R=0.25, p=0.0067) and bumble bees (R=0.24, p=0.0076). While the spatial structuring was apparent in honey bees only during the summer 2 (July) time period, in bumble bees it was evident only during the summer 1 (June) time period. This indicates that at these time periods, sites that are closer together experience more similar BQCV prevalence. Additionally, we observed marginal spatial structure in parasitic fly prevalence in bumble bees (R=0.17, p=0.061) (Figure 6). Overall, these results suggest that at the scale of our study, sampling across a 30 km region in central Pennsylvania, there is localization at certain time periods for BQCV, but not DWV nor parasitic fly prevalence in honey bees and bumble bees. Interestingly, localization of pathogen and parasite prevalence is more evident in bumble bees than honey bees. It is possible that further spatial differences in pathogen prevalence occur at a more local scale or perhaps across broader geographic regions. Addressing the role of the landscape in pathogen levels is a focus of a grant following from this research.
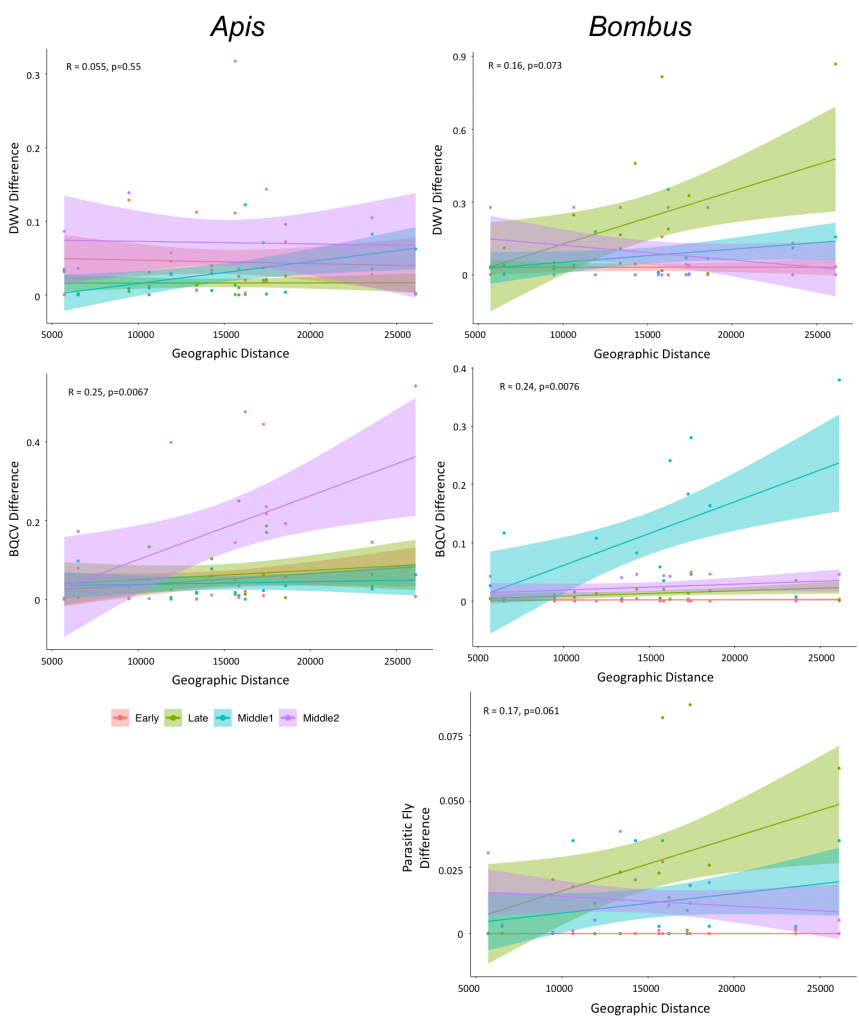
Figure 6: Examination of the spatial structure of pathogen prevalence at a local scale in Centre County, Pennsylvania. Early represents Spring, Late represents Fall, Middle 1 represents Summer 1, and Middle 2 represents Summer 2.
The results of this project add to the current literature and shed further light on the implications of pathogen spillover/spillback between honey bees and bumble bees. Similar to previous studies, the prevalence of DWV and BQCV were higher throughout all time periods in honey bees with bumble bees tracking the same seasonal trends, but at a lower prevalence. The higher prevalence in honey bees may be indicative of a closer association between these viruses and honey bees compared to bumble bees. Depending solely on prevalence, honey bees are more likely to be the primary reservoir for these two viruses, but they may also be more negatively impacted by these viruses. Furthermore, while honey bees retain fairly high viral prevalence from winter to spring, bumble bees were more likely to purge pathogens, reducing pathogen loads to levels near zero each spring. This means honey bees are likely to facilitate the spread of viruses to native bee communities each year in the spring and act as a highly infected reservoir capable of infecting native bees year-round. These data thus help to build expectations for community-level effects on shared pathogen loads.
In addition, this research provides important information regarding pivotal time periods that are highly stressful to bee communities where management strategies could be introduced to decrease the negative impacts of pathogens and parasites. We have revealed that seasonal trends exist for pathogens and parasites that are consistent between honey bees and bumble bees, with DWV and fly parasitism peaking in prevalence in the fall, and BQCV during mid-summer. These patterns can be used for building management strategies and epidemiological models for these bees. Future studies should investigate the potential drivers of these seasonal trends, such as honey bee and bumble bee seasonal demographics and population growth, Varroa mite seasonal growth, and seasonal immune response levels.
Education & Outreach Activities and Participation Summary
The Pennsylvania State University Great Insect Fair
Participation Summary:
Presentations:
The results of this research project have been presented at various scientific meetings and to beekeepers. The scientific meetings attended included the 2017 Annual Entomological Society of America Meeting and the 2016 XXV International Congress of Entomology Meeting. In addition, we were able to share the results of this project with beekeepers at the Pennsylvania State Beekeepers Association Annual Meeting as well as at the 2016, 2017, and 2018 Penn State Center for Pollinator Research Symposia. These presentations have sparked interest and conversation about the implication that honey bees could be spreading pathogens to native bees as well as about the potential best management strategies.
Oral Presentations:
- Ezray, B.D., Hines H.M. (2018) Spatial and temporal dynamics in bumble bees: from mimetic coloration to epidemiological patterns. Willamette University Behavioral Ecology Classroom Seminar. Salem, OR.
- Hines, H.M., Ezray, B.D. (2018) Modelling pathogen dynamics in bumble bees: Overview of projects in the Hines lab.The Pennsylvania State University Center for Pollinator Research Spring 2018 Symposium. State College, PA.
- Ezray, B.D., Hines H.M. (2017) Pathogen dynamics in bee communities.The Pennsylvania State University Center for Pollinator Research Spring 2017 Symposium. State College, PA.
- Ezray, B.D., Hines H.M. (2017) Pathogen dynamics in bee communities. Annual Meeting of the Entomological Society of America Student Oral Ten Minute Paper Competition. Denver, CO.
- Ezray, B.D., Hines H.M. (2016) Understanding pathogen dynamics in bee communities. XXV International Congress of Entomology Student Oral Ten Minute Paper Competition. Orlando, FL.Hines H.M.
- Ezray, B.D., Hines H.M. (2016) Seasonality of pathogens in bumble bees and honey bees.The Pennsylvania State University Center for Pollinator Research Spring 2016 Symposium. State College, PA.
- Ezray, B.D., Treanore, E.D. (2016) The tricks and treats of pollinator health: from poor nutrition to pathogens.The Pennsylvania State University Science Café. State College, PA.
Posters:
- Ezray, B.D,McCormick, E.C. *, Hines, H.M. (2018) Pathogen dynamics in honey bee and bumble bee communities.Poster Presented at: The Pennsylvania State Beekeepers Association Annual Meeting. State College, PA. * presented by McCormick E.C. Poster
Outreach Products:
As a product of this project, a course curriculum for the Summer Academy for Creative Studies Upward Bound Math and Science program (Upward Bound) and an outreach activity and informational handout for the Annual Pennsylvania State University Great Insect Fair were created.
Upward Bound: Together with my research advisor, Dr. Heather Hines, and the Pennsylvania State University Center for Pollinator Research, I led the effort to create and instruct a pollinator biology course for Upward Bound. Upward Bound is a college preparatory program for underrepresented, first-generation college bound high school students (syllabus displayed in Figure 7). The course curriculum covered the breadth of areas in the life sciences (i.e. ecology, evolution, biodiversity, conservation biology, disease dynamics, molecular biology, behavior, and physiology) with a focus on pollinators and pollinator health. To cover the multiple facets of the life sciences and expose students to the types of work done in each of these subfields, each week, we addressed a different field of biology with interactive and engaging activities involving a combination of field, lab, and classroom instructional experiences. The combination of experiences provided real world examples of application of learned concepts and inspired students, promoted scientific curiosity, gave examples of the vast opportunities for scientific research, and solidified the importance of pollinators including bees.
Upward-Bound-Syllabus
Figure 7: Syllabus of pollinator biology course used in 2018 Summer Academy for Creative Studies Upward Bound Math and Science program.
Great Insect Fair: An outreach activity to teach children about the many factors that are causing bee declines was created and exhibited at the Pennsylvania State University 2017 Great Insect Fair. The outreach activity used a piece of yellow tissue paper with a bee drawn on it to represent a bee colony and a variety of knick-knacks to represent different stressors. For example, diseases were signified by bouncy balls, varroa mites were symbolized as basketball shaped erasers, pesticides were represented by a water spray bottle, and poor nutrition was exemplified by removing a pillar of fake flowers from underneath the bee colony. Visitors could place the different stressors on the tissue paper until it eventually ripped. Then, by talking through what the visitor had just done, we were able to explain both verbally and visually how it is the interaction of stressors and not one stressor alone that is likely causing bee declines. While this concept can be difficult to grasp, especially for children, this outreach activity successfully portrayed this point and it engaged children of all ages. Furthermore, we created and distributed an informational handout which provides ways to help support our native pollinators (Figure 8). This activity involved participation of undergraduate researchers on this project, thus providing valuable outreach training experiences.
How to Help PA Bumble Bees Handout
Figure 8: Informational handout designed to display how to support Pennsylvania bumble bees.
Publications:
The results of this study will be submitted for publication to a peer-reviewed journal in 2019. In addition, an article describing a classroom activity used during the Upward Bound course will also be published in a peer-reviewed journal in 2019.
In addition, a description of this project has been posted on the Hines Lab webpage which will be updated with an additional blog post regarding the results in 2019.
Project Outcomes
This research provides additional support for the need of multi-faceted longitudinal monitoring projects that measure the cumulative pressures applied to bee communities over time to better understand both the cyclic seasonal patterns and yearly deviances that occur. The results from this project have emphasized that pathogens and parasites vary in response to other stressors as well as their host infection strategies resulting in varying temporal and spatial structure at a local scale. Additionally, we found that bee taxa differ dramatically in how pathogens and parasites influence their population dynamics and in their role as potential pathogen and parasite reservoirs. Our research suggests that honey bees may maintain pathogen and parasite populations during the winter, thus, re-introducing pathogens and parasites to native bee populations year after year.
Overall, our research has sparked interest among scientists, beekeepers, and the general public. Through integrating information on pathogen and parasite prevalence temporally (seasonal and yearly) and spatially at a local scale across different bee taxa, we have improved our understanding of the patterns of pathogen epidemiology and transmission. Furthermore, our research advances our understanding of the threat that these pathogens pose to pollinator communities as a whole. Together, this improved understanding of bee pathogen epidemiology and transmission will help sustain pollinator populations which in turn will maintain high productivity of crops.
- We have provided data towards establishing models for predicting the seasonal prevalence of DWV and BQCV in honey bees and bumble bees throughout the active season. Thus, this research improves understanding of pivotal time periods which should be targeted for management of bee health. In the future, this model could be improved through the addition of further explanatory variables to associate with DWV and BQCV seasonal prevalence and comparative understanding of other bee pathogens.
- This project formed a substantial part of my doctoral training and graduate thesis. Not only did this project improve and require me to learn new techniques, but through this project I gained project and personnel management experience and skills.
- This project supported training for six undergraduate research assistants and a laboratory technician. These individuals learned how to collect and identify bumble bees and honey bees, dissect bumble bees and honey bees, conduct molecular assays to screen for viruses including RNA extractions, cDNA synthesis, RT-PCR, and gel electrophoresis, assess for microsporidian and nematode infections using phase-contrast microscopy, and improved scientific literacy through journal clubs and bi-annual research presentations. In addition, one of these students procured an Apes Valentes Undergraduate Award to conduct summer research to explore the seasonal prevalence of pathogens detected in bumble bee and honey bee corbicular loads, how pathogens found in corbicular loads relates to those found in the foraging bee, and determine if certain plants are hotspots for bee pathogens as an extension of this project.
- Through observations made in the field and interest in building upon results from this project. 3 grants of been applied for and 2 have been received. These grants include an Apes Valentes Undergraduate Research Awards, a USDA-FFAR grant, and an application for a USDA NIFA postdoctoral fellowship. The results of this project directly influenced the experimental design for bumble bee collection for the USDA-FFAR project examining landscape effects on pathogen prevalence throughout Pennsylvania. It was determined that all the collection events should occur in a single year to prevent year to year variability when trying to assess landscape impacts on pathogen prevalence. In addition, the results of this project spurred an application for a USDA NIFA postdoctoral fellowship aiming to improve understanding of how viral and temperature stressors impact diapausing bumble bee gynes. The goal of this project is to determine if the loss in pathogen prevalence we observed in bumble bees from fall to spring is occurring because gynes that are infected die or if gynes have strong immune responses that allow them to recover from illness during this overwintering period.
Information Products
- How to Help Pennsylvania Bumble Bees (Fact Sheet)