Final report for GNE16-133
Project Information
Organic farmers rely largely on cultural practices, such as crop rotation and cover cropping, and on biological control to manage pests. Winter cover crops add diversity to agroecosystems and can benefit soil conservation and health. Within the framework of a three-year experiment to investigate ecosystem services and disservices associated with winter cover crops during the transition of an agronomic cropping system to organic management, we examined the effects of crop, cover crop species and diversity, arthropods, and soil properties on the occurrence of Metarhizium (Hypocreales: Clavicipitacea), a fungal insect pathogen, in a single field site in central Pennsylvania. We also investigated the effect of M. robertsii isolated from the site on the growth of corn, Zea mays, and on the relative growth rate and feeding behavior of an agricultural pest, fall armyworm (FAW), Spodoptera frugiperda. M. robertsii was the only species of Metarhizium detected in the field experiment. Detection was lower in cover crop monocultures and mixtures containing brassica cover crops compared to those with legume cover crops. Cover crop biomass in the previous fall, the biomass of weeds in the current season (spring), percent silt, electrical conductivity, and activity-density of ground beetles were positively associated with and together explained 28.32% of the variation in M. robertsii in standing cover crops in the spring. In the cash crops that followed cover crops, soil labile C and electrical conductivity were positively associated, and activity-density of mites and percent sand were negatively associated with M. robertsii and together explained 21.92% of the variation in M. robertsii. In laboratory experiments to examine the interaction between endophytic M. robertsii on corn and on growth of fall armyworm, we recovered M. robertsii from leaf or root tissue from 68% (n=295) of the corn plants grown from M. robertsii-treated seed. Overall, the mean (±SEM) percentages of the detection of M. robertsii by tissue type were: roots only, 12.0% ±2.70; leaves only, 14.0% ±6.2; roots and leaves, 21.7% ±9.71; no recovery, 39.5% ±10.6. There was no significant difference in the height, chlorophyll content, or biomass of corn grown from seed treated with M. robertsii and control corn plants. In laboratory and greenhouse assays, there was no effect of endophytic M. robertsii on the relative growth rate (RGR) of fall armyworm larvae or feeding behavior of fall armyworm larvae. However, in two of the three replicate trials, FAW fed on a higher number of control leaf squares compared to the number of treated leaf squares, indicating a possible avoidance mechanism.
Objective 1: Determine the effects of cover crop treatments and soil characteristics on the occurrence and species diversity of endemic Metarhizium isolates using sentinel insect assays and molecular techniques. Completed by Puneet Randhawa in 2016.
Objective 2: Determine the ability of endemic Metarhizium species to establish growth in corn and selected cover crop species.
Objective 3: Measure the effects of Metarhizium-infection of corn on the growth of corn and a corn pest.
Objective 4: Develop extension products for outreach and extension events relating to the effects of cover crops on Metarhizium.
Biological control plays a critical role in keeping pest populations in check in agroecosystems. On organic farms, conserving natural enemies, including predators, parasitoids, and insect pathogens, is especially important because farmers cannot apply synthetic insecticides to control crop pests. Soil fungi in the genus Metarhizium Sorokin (Hypocreales: Clavicipitaceae) are entomopathogens found in a wide range of soils, including agricultural soils, and can infect and kill a variety of insects. The prevalence of Metarhizium is affected by soil properties and can be influenced by the presence of different plant species. Relatively recently, Metarhizium was found to be able to grow in plants as an endophyte that can promote plant growth and suppress plant-feeding insects and plant pathogens (Sasan and Bidochka, 2012; Bamisile et al., 2018). Therefore, the goal of this research was to examine the effects of winter cover crop mono- and polycultures on the prevalence of Metarhizium, and the effects of endophytic M. robertsii on the growth of corn, Zea mays, and the effect of endophytic corn on the feeding behavior and relative growth rate of an agricultural pest, the fall armyworm (FAW), Spodoptera frugiperda. We conducted this field-, greenhouse-, and laboratory-based experiments within the framework of a three-year experiment to investigate ecosystem services and disservices associated with winter cover crops during the transition of an agronomic cropping system to organic management (Finney et al., 2017).
Cooperators
Research
OBJECTIVE 1: Effect of Crops and Soil Characteristics on Metarhizium
This experiment was conducted at a single site established on approximately 11 ha at the Pennsylvania State University’s Russell E. Larson Research and Education Center (RELREC) near Rock Springs, Pennsylvania (40° 43'N, 77° 55'W, 350 m elevation). The site was managed in accordance with the USDA National Organic Standards (USDA NOP, nd). No pest control or synthetic fertility materials were applied during the experiment. The field experiment was established as a full-entry, stripped, randomized complete block design field experiment at RELREC in 2013. The main cash crops were corn (Zea mays var. Master's Choice 4050, organic), soybean (Glycine max var. HS 21C40, untreated), and wheat (Triticum aestivum var. Malabar, untreated). The main plot (24 m x 348 m) in which each cash crop was planted was a strip and within each strip were subplots (24 m x 29 m) in which twelve cover crop treatments were planted in randomized order. The twelve cover crop treatments were comprised of six fall-planted cover crop species grown in monoculture, five cover crop mixture treatments, and a fallow treatment embedded in a rotation of winter wheat-cover crops-corn-cover crops-soybean-cover crops. The cover crop monocultures include Austrian winter pea (Pisum sativum var. Arvika), cereal rye (Secale cereale var. Aroostook), winter canola (Brassica napus var. Wichita), red clover (Trifolium pratense var. common), forage radish (Raphanus sativus var. Tillage Radish) and oats (Avena sativa var. Jerry). The five cover crop mixtures included: a three species mixture designed to balance nitrogen supply and retention (3SppN; Austrian winter pea, red clover and cereal rye); a three species mixture designed to suppress weeds (3SppW; red clover, oat and cereal rye), a four species mixture designed to support beneficial insects (4Spp; red clover, winter canola, cereal rye and Austrian winter pea), a six species mixture comprised of all monoculture species tested (6Spp); and a commercially-available mixture of 7 species (7Spp) comprised of annual ryegrass (Lolium multiflorum), crimson clover, red clover, sweet clover (Melilotus officinalis), oat, forage radish and sunflower (Helianthis annuus). All phases of the rotation were planted in each year and treatments were each replicated four times (Murrell et al., 2017).
We collected soil samples, comprised of 11, 20 cm deep x 2.5 cm diameter soil cores, from each of the four replicates of the 12 treatment plots in the corn and soybean phase of the rotation twice in each year of the experiment, in standing cover crops in May, and in the cash crops following the cover crops in July. Each soil sample was split into sub-samples of approximately 250 ml each for chemical and biological analyses (Randhawa et al., 2018).
OBJECTIVE 2: Ability of Metarhizium robertsii to grow as an endophyte of corn
Among five replicate trials, I grew a total of 295 corn plants from seed exposed to M. robertsii isolated from soil from canola plots in the field experiment, and 205 untreated control plants. To determine the ability of M. robertsii to grow as an endophyte in corn, seed was surface sterilized, then placed in an aqueous suspension of M. robertsii conidiospores (1×108 conidiospores/mL in 0.05% Triton X-100 solution) for 2 hours. The control seeds were soaked in the 0.05% Triton X-100 solution without conidiospores for 2 hours. I planted the corn seeds in sterile 15 cm diameter (2.5 L volume) Dillon containers containing steam pasteurized potting medium consisting of a 1:1 ratio by volume of field soil to organic potting medium (Vigoro, Syacauga, AL) at a depth of 2.5 cm. The potting medium was steamed twice at 121 °C to reduce microbial growth and allowed to rest for 48 hours prior to planting. To confirm endophytic colonization by M. robertsii in corn grown from treated seed, I collected the 2nd or 4th true leaves and two roots from each plant, surface sterilized them, and plated the tissue sections onto CTC medium (Fernandez et al., 2010). Endophytic infection of a plant was considered positive if M. robertsii grew from any of the tissue sections. When M. robertsii was not recovered, the plant was considered exposed but not endophytically colonized. My hypotheses were as follows:
Ho: Corn plants grown from seed exposed to Metarhizium will not support endophytic root or leaf colonization.
HA: Corn plants grown from seed exposed to Metarhizium will support endophytic root or leaf colonization.
OBJECTIVE 3A: Effect of Metarhizium robertsii on corn growth
In four replicate trials, I measured the above-ground height and chlorophyll content of 421 corn plants (260 grown from M. robertsii-treated seed and 161 control plants) at the V1 stage through the V4 stage. The chlorophyll content per unit leaf area of the second true leaf was measured using a SPAD-502 Plus chlorophyll meter (Ling et al., 2011) and is reported as a mean based on three readings per leaf. To determine the presence of endophytic M. robertsii, I collected the second true leaf from each plant in the first trial and the fourth true leaf in all subsequent trials, in addition to two roots from each plant. The leaf and root sections were surface-sterilized and plated onto CTC medium. I determined the dry aboveground biomass of 185 corn plants (114 grown from M. robertsii-treated seed and 71 control plants) that had been dried in an oven at 55-60 °C for 72 hours. Therefore, my hypotheses were as follows:
Ho: Corn plants grown from seed exposed to Metarhizium will not differ in height, chlorophyll content, or biomass from corn plants grown from seed that was not exposed to Metarhizium.
HA: Corn plants grown from seed exposed to Metarhizium will differ in height, chlorophyll content, or biomass from corn plants grown from seed that was not exposed to Metarhizium.
OBJECTIVE 3B: Effect of endophytic Metarhizium robertsii on relative growth rate of fall armyworm
Lab Assay: Neonate FAW larvae of known weight were placed individually into 1-oz. food service cups prepared with 3 mL of 3% Bacto Agar in the bottom to provide humidity and a 9-cm section of the fourth true leaf from treated or control V4 corn plants. After 4 days, the Relative Growth Rate (RGR) of each larva was calculated using the formula:
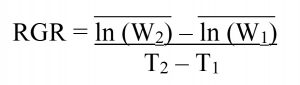
where the mean natural log (ln) of the initial masses (W1) of larvae fed control leaf sections was subtracted from the mean natural log (ln) of the final masses (W2) of larvae fed control leaf sections, and this difference was divided by the initial time (T1) subtracted from the final time (T2) in days. This was repeated for larvae fed corn leaves from plants grown from treated seed. Across three trial replicates, the RGR was calculated for 133 larvae fed leaves from plants grown from M. robertsii-treated seed, and 91 larvae fed leaf sections from control plants. Sub-samples of each leaf used in the assay, and two root pieces from each plant used in the assay were plated onto CTC medium to confirm endophytic colonization by M. robertsii.
Greenhouse Assay:
Across two replicate trials, the Relative Growth Rate (RGR) of sixth instar fall armyworm larvae was calculated six days after placement on control corn plants or plants grown from M. robertsii-treated seed. In a greenhouse, individual pre-weighed larvae reared on artificial diet were placed on corn plants covered by mesh bags to prevent larval escape. The larvae were left on the plants for 6 days (144 hours), then weighed. Over the two trials, the RGR was calculated for 84 larvae (36 larvae from control plants and 48 larvae from treated plants). The RGR was calculated with the formula used in the lab RGR assay. My hypotheses were as follows:
Ho: Relative Growth Rate (RGR) of fall armyworm larvae fed on corn plants grown from seed exposed to M. robertsii will not differ from the RGR of fall armyworm fed on corn plants grown from seed that was not exposed to M. robertsii.
HA: Relative Growth Rate (RGR) of fall armyworm larvae fed on corn plants grown from seed exposed to M. robertsii will differ from the RGR of fall armyworm fed on corn plants grown from seed that was not exposed to M. robertsii.
OBJECTIVE 3C: Effects of endophytic Metarhizium robertsii on feeding preference of fall armyworm
We conducted a feeding assay with three treatments: 1) no-choice with corn leaf tissue from plants grown from M. robertsii-exposed seed; 2) no-choice with corn leaf tissue from plants grown from untreated control seed; 3) choice tests with corn leaf tissue from each type of plant. For each treatment, third instar fall armyworm were placed individually in petri dishes containing 6 corn leaf squares measuring 1.1 x 1.1 cm positioned around the perimeters of the dishes (Fig. 3A-1). Across three replicate trials, 29 larvae were placed in petri dishes that contained 6 leaf squares from control plants, 51 larvae were placed in petri dishes with 6 leaf squares from M. robertsii-exposed plants, and 75 larvae were placed in petri dishes that contained 3 leaf squares from control plants and 3 leaf squares from exposed plants placed in an alternating pattern near the edge of the petri dish. The orientation of the leaf squares for the choice arenas was recorded to account for effect of external stimuli, such as light, which could influence the movement of the fall armyworm. The location of the larvae and feeding events were recorded every 30 minutes for 2 hours. To determine endophytic colonization of plants used in the assay, we collected, surface sterilized, and plated residual leaf squares and two root sections from each plant on CTC medium. The number of leaf squares fed on in each treatment was counted and the proportion consumed per leaf square was measured using ImageJ. My hypotheses were as follows:
Ho: Fall armyworm will not preferentially feed on corn leaf tissue grown from M. robertsii-treated seed.
HA: Fall armyworm will preferentially feed on corn leaf tissue grown from M. robertsii-exposed seed.
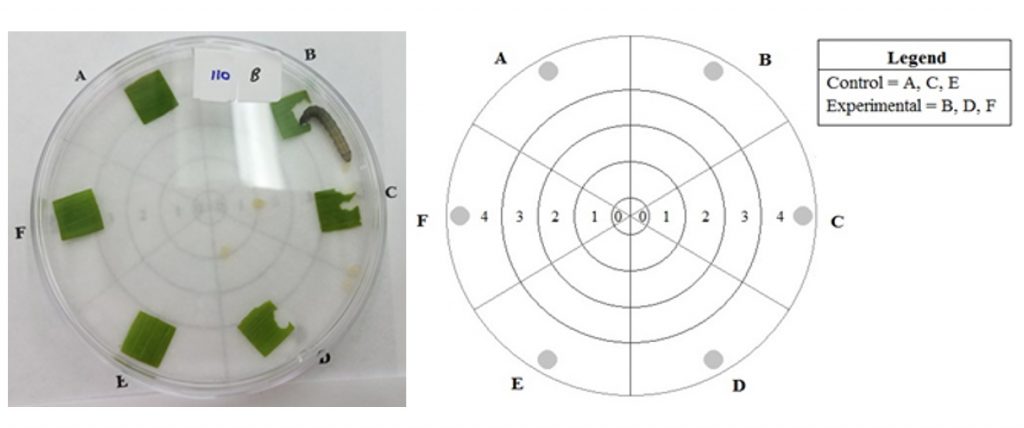
Fig. 3C-1: Choice Assay Diagram. An individual third instar fall armyworm was placed in the center of the petri dish at Zone 0. The numbered zones 1-4 show how far from the center of the petri dish the larva has moved. Leaf squares were placed at locations A, B, C, D, E, and F where the shaded circles are present. In the choice assay, the control leaf squares were placed at A, C, and E and the leaf squares from M. robertsii-treated plants were placed at B, D, and F. For the no-choice assays, only control leaf squares or treated leaf squares were arranged at locations A-F.
OBJECTIVE 1 - RESULTS:
In the field experiment, neither cover crop treatment nor level of diversity affected the percentage of G. mellonella killed by M. robertsii (Randhawa et al., 2018). The lack of species diversity in Metarhizium in our study may have several causes. Perhaps the belowground food web in this agroecosystem is redundant at the species level and mainly driven by the functional attributes of the dominant species (Wardle, 1999).
Percentage infection was different (F1,282=33.13; p < 0.0001) in soil samples collected pre- vs. post- cover crop termination. The mean percentage infection of sentinel G. mellonella by M. robertsii was 15.52% (±0.01) and 8.73%(±0.008) in standing cover crop (pre-termination) samples and in post-cover crop termination samples, respectively. The functional group of the cover crop affected M. robertsii (F4, 274=2.582; p = 0.0365), and the percentage of G. mellonella killed by M. robertsii was significantly lower (p = 0.0146) in treatment plots with brassica cover crops compared to plots with legume cover crops. The proportion of forage radish in the fall biomass sample (p = 0.0247) and the proportion of cereal rye (p = 0.0006) in the spring sample were negatively related to the pre-termination detection of M. robertsii. These two cover crop variables explained 18.75% of the variation in the detection of M. robertsii in the pre-termination samples. This finding is consistent with existing literature on the role of brassicas and legumes in influencing soil microorganisms (Vukicevich et al., 2016). Root exudates may serve as a selective agent through which a plant is able to regulate the fungal community in the surrounding rhizosphere. The more frequent detection of M. robertsii associated with some legumes may be due to the quantity or quality of root exudates, as well as secondary metabolites (Biate et al., 2015). Similarly, low detection in brassicas and cereal rye could be due to the inhibitory effects of glucosinolates or other secondary metabolites (Uren et al., 2000; Vierheilig et al, 2000). Soil moisture and S concentration were positively associated, and CEC was negatively associated with the detection of M. robertsii. Although high soil moisture can negatively affect the survival of conidia, sufficient moisture is essential for germination of conidia, mycelial growth, and sporulation (Inglis et al., 2012).
At any particular site, agricultural practices will influence the complex interaction of multiple biotic and abiotic characteristics that shape the observed soil community. Even though Metarhizium is among the most studied entomopathogens, especially in light of the multifunctionality of this fungus, further research is needed to develop an understanding of how to use production system and practices to influence multivariate interactions to foster and conserve biological control in the soil.
OBJECTIVE 2 – RESULTS:
Although Metarhizium is reported to primarily be a root endophyte, I recovered M. robertsii from leaf tissue in a greater percentage of corn plants than from root tissue. I have not found any currently published papers that have found this to be the case. Across five replicate trials, I recovered M. robertsii from leaf or root tissue of 201 of the 295 corn plants grown from treated seed. Overall, the mean (± SEM) percentages of recovery of M. robertsii by tissue type were: roots, 12.0% ±2.70; leaves, 14.0% ±6.2; both roots and leaves, 21.7% ±9.71; and no recovery, 39.5% ±10.6 (Fig. 2-1).
Fig. 2-1: Overall detection of Metarhizium robertsii in corn leaf and root tissue
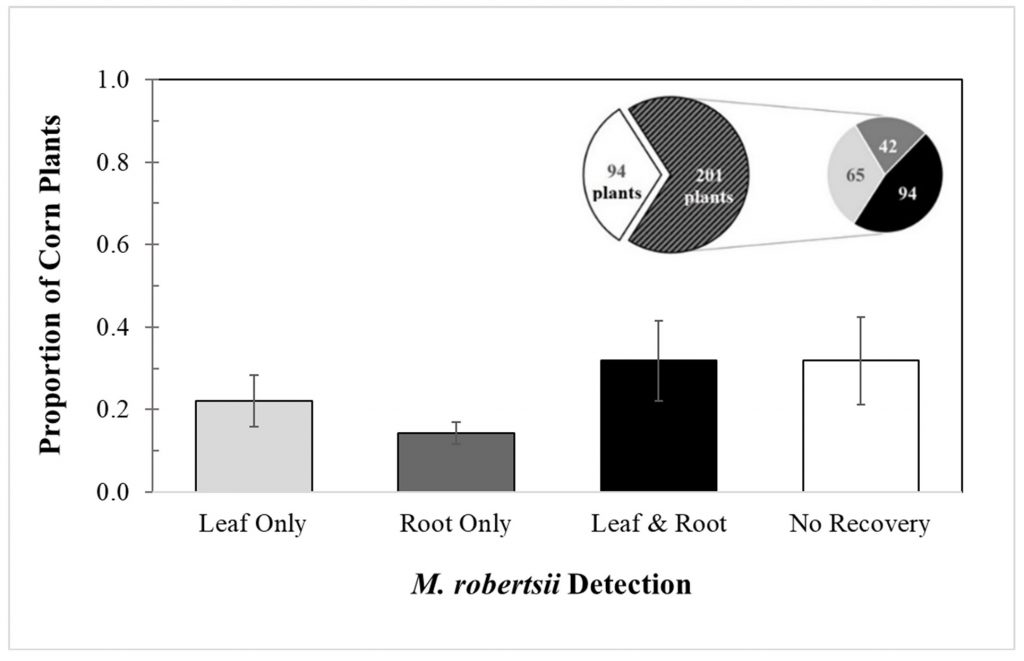
The left pie chart represents the proportion of treated corn plants with no M. robertsii detection (white) and M. robertsii detection in at least one of their tissues (black pattern), and the right pie chart represents the numbers of plants according to tissue from each plant where M. robertsii was detected.
OBJECTIVE 3A – RESULTS:
Although Metarhizium has been reported to promote plant growth, I did not find any effect of seed treatment with M. robertsii on plant growth. Across four replicate trials there was no significant difference in height, chlorophyll content, or dry aboveground biomass of control corn plants and corn plants grown from M. robertsii-treated seed. Because there may be variation in isolates of M. robertsii in their growth-promoting effects, it could be that the particular isolate in my study is not as effective in aiding plant growth, or corn does not respond similarly to plants where plant growth promotion has been observed, including wheat, common bean, tomato, soybeans, coffee, opium poppy, cassava, sorghum, cotton (Bamsile et al., 2018). Tests using M. robertsii are needed to confirm this.
OBJECTIVE 3B – RESULTS:
Across three replicate trials there was no differences between the RGR of fall armyworm in M. robertsii-treated and control plants. Over the two replicate trials there was no difference in RGR of fall armyworm larvae in the greenhouse assay in which the RGR was calculated on whole corn plants grown from treated seed and on control plants. As growth suppressive effects of endophytic Metarhizium have been observed for other insects, there is clearly variability in insect species' ability to tolerate the direct or indirect effects of Metarhizium, or there may be variability among fungal strains in their effects on FAW. Further research is needed to understand the mechanism of tolerance FAW has to other isolates of Metarhizium.
OBJECTIVE 3C – RESULTS:
There have been several studies in which endophytic Metarhizium deters plant-feeding insects from feeding on crops, but the relative growth rate of fall armyworm in my study was not different in treated and control plants. Across three replicate trials, the proportion consumed per control leaf squares and leaf squares from corn treated with M. robertsii were similar.
LITERATURE CITED
Bamisile, B.S., Dash, C.K., Akutse, K.S., Keppanan, R., Wang, L., 2018. Fungal endophytes: Beyond herbivore management. Front. Microbiol. 23(9): 544.
Biate, D.L., Kumari, A., Annapurna, K., Kumar, L.V., Ramadoss, D., Reddy, K.K., Naik, S., 2015. Legume root exudates: Their role in symbiotic interactions, in: Arora, N.K. (ed). Plant Microbes Symbiosis: Applied Facets. Springer, India. pp. 259–271.
Fernandes, É.K., Keyser, C.A., Rangel, D.E., Foster, R.N., Roberts, D.W., 2010. CTC medium: A novel dodine-free selective medium for isolating entomopathogenic fungi, especially Metarhizium acridum, from soil. Biol. Control 54, 197–205.
Finney, D.M., Murrell, E.G., White, C.M., Baraibar, B., Barbercheck, M.E., Bradley, B.A., Cornelisse, S., Hunter, M.C., Kaye, J.P., Mortensen, D.A., 2017. Ecosystem services and disservices are bundled in simple and diverse cover cropping systems. Agric. Environ. Lett. 2, https://doi.org/10.2134/ael2017.09.0033 (Accessed 11 December 2018)
Inglis, G.D., Enkerli, J., Goettel, M.S., 2012. Laboratory techniques used for entomopathogenic fungi: Hypocreales, in: Lacey, L. (Ed.), Manual of Techniques in Invertebrate Pathology. Academic Press, NY, pp. 189–253.
Klingen, I., Eilenberg, J., Meadow, R., 2002. Effects of farming system, field margins and bait insect on the occurrence of insect pathogenic fungi in soils. Agric. Ecosyst. Environ. 91, 191–198.
Ling, Q., Huang, W., Jarvis, P., 2011. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth. Res. 107(2): 209-214.
Murrell, E.G., Schipanski, M.E., Finney, D.M., Hunter, M.C., Burgess, M., LaChance, J.C., Baraibar, B., White, C.M., Mortensen, D.A., Kaye, J.P., 2017. Achieving diverse cover crop mixtures: effects of planting date and seeding rate. Agron. J. 109, 259–271.
Randhawa, P. K., Mullen, C., Barbercheck, M. 2018. Plant identity, but not diversity, and agroecosystem characteristics affect the occurrence of M. robertsii in an organic cropping system. Biological Control 124:18-29. https://doi.org/10.1016/j.biocontrol.2018.06.001
Sasan, R.K., Bidochka, M.J., 2012. The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae) is also an endophyte that stimulates plant root development. Am. J. Bot. 99: 101–107.
Schulz, M., Marocco, A., Tabaglio, V., Macias, F.A., Molinillo, J.M., 2013. Benzoxazinoids in rye allelopathy-from discovery to application in sustainable weed control and organic farming. J. Chem. Ecol. 39, 154–174.
Uren, N. C., 2000. Types, amounts, and possible functions of compounds released into the rhizosphere by soil-grown plants, in: Pinton, R., Varanini, Z., Nannipieri, P., (Eds.). The Rhizosphere: Biochemistry and Organic Substances at the Soil–Plant Interface. Marcel Dekker, New York, pp. 19 – 40.
Vierheilig, H., Bennett, R., Kiddle, G., Kaldorf, M., Ludwig-Muller, J., 2000. Differences in glucosinolate patterns and arbuscular mycorrhizal status of glucosinolate-containing plant species. New Phytol. 146, 343–352.
Vukicevich, E., Lowery, T., Bowen, P., Úrbez-Torres, J.R., Hart, M., 2016. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agronomy for Sustainable Development 36, 48.
Wardle, D.A., Bonner, K.I., Barker, G.M., Yeates, G.W., Nicholson, K.S., Bardgett, R.D., Watson, R.N., Ghani, A., 1999. Plant removals in perennial grassland: vegetation dynamics, decomposers, soil biodiversity, and ecosystem properties. Ecol. Monogr. 69, 535–568.
I was successful in endophytically colonizing corn plants with an isolate of M. robertsii and I found that M. robertsii can establish endophytically from seed treatment in corn leaves and roots. I found that there may be species or strain variability in the ability of endophytic M. robertsii to promote plant growth, as I did not observe an effect of the tested fungal isolate on corn plant height, chlorophyll content, or dry aboveground biomass. Likewise, there appears to be variability among insects in their susceptibility to endophytic M. robertsii as the fungal isolate that I tested did not affect fall armyworm relative growth rate (RGR), or their feeding preference.
Education & Outreach Activities and Participation Summary
Participation Summary:
In 2018, I participated in two field days at the Russell E. Larson Agricultural Research Center in Rock Springs, PA. The first field event involved presenting to a delegation of eleven Argentinian farmers to teach them about pest arthropods in organic Pennsylvania farms, survey methods for these pests, and ways to control these pests. The second field event involved teaching 30 Pennsylvania State University student summer workers about my research with Metarhizium robertsii and the potential relationships it has with corn and fall armyworm. At both field days, we disseminated information about cover crop research, fostered information sharing and co-learning among farmers about cover crop mixture design, and discussed possible ecosystem services provided by conservation of Metarhizium. Additionally, two study circles convened to discuss organic production topics, including cover crop mixtures and associated ecosystem services. Furthermore, I presented the initial findings of my research at three conferences including: the 21st Penn State Plant Biology Symposium: Wild and Tamed Phytobiomes in State College, PA, the Penn State Life Science Symposium in State College, PA, and the Eastern Branch Entomological Society of America Conference in Annapolis, MD. I also contributed to spring and fall 2018 project newsletters and presented material in an extension event for agricultural professionals.
Poster Presented at the 21st Penn State Plant Biology Symposium: Wild and Tamed Phytobiomes, Penn State Life Science Symposium, and the Eastern Branch Entomological Society of America Conference
Information Disseminated in Field Events

Presentation for Argentinian Delegation on Pest Insect Field Surveys (Posters created by Karly Regan)

Project Outcomes
My research involved exploring the relationship between three organisms important to agriculture: the beneficial soil fungus, Metarhizium robertsii, corn, Zea mays, and fall armyworm, Spodoptera frugiperda. Although much is known about these three organisms individually, there is no current research on how their associations with one another could impact crop and insect pest performance. I did not find any effect of endophytic M. robertsii on corn or fall armyworm growth, which could be due to the history these organisms have occurring together in the same environment. Furthermore, endophytic M. robertsii did not deter or attract fall armyworm larvae to corn plants, which could mean the fall armyworm cannot detect the fungus in the plant tissue, or that it does not impact them. More research is needed to determine how conservation of M. robertsii could benefit organic farms by becoming an alternative to chemical pesticide application and potentially increasing crop yield.
During my research, I learned that M. robertsii can successfully colonize corn plant tissue. By continuing research with endophytic Metarhizium in crops, we will discover the variability in its influence on the crop pests' development, feeding behavior, and mortality. Seed treatment to create endophytic M. robertsii-colonized crops could allow farmers to use less chemical pesticides and help organic farmers and farmers who want to reduce pesticide to manage insect pests. This project helped me gain further insight into sustainable and organic agriculture, soil biological health, and biological processes in agricultural ecosystems. In the future, I hope to obtain a career in sustainable agriculture using biological control agents as alternatives against crop pests.