Final report for GNE18-182
Project Information
It's unclear how the maple syrup industry will respond to future changes in climate and pest and disease pressures. Many farmers choose to diversify to reduce the likelihood of poor years. The maple syrup industry is based entirely on one genus (Acer) and almost entirely on one species (Acer saccharum), although syrup industries for other genera (such as Betula and Juglans) exist as well. The northeast is home to several other potential syrup-producing genera, such as Platanus, Fagus, and Ostrya. Though none of these species yield as much, or as sweet, sap as maples do, producing syrup from these novel trees has its perks. These novel syrups command a much higher price in the marketplace, some of these potential syrup-producing species are considered weedy in sugarbushes, and some offer season-extension possibilities since the timing of sap collection of some of these species does not overlap with that of maples.
Six species were considered for their syrup-producing potential. These species were London planetree (Platanus x acerifolia), American hophornbeam (Ostrya virginiana), white ash (Fraxinus americana), shagbark hickory (Carya ovata), American basswood (Ostrya virginiana), and American beech (Fagus grandifolia). This project was initially set out with 4 components, but due to the pandemic, the fourth component, a consumer preference analysis, was excluded. First, during year 1 of the study, five trees of each study species were monitored for sap flow during winter dormancy when sap harvesting and syrup production occur. Concurrent environmental measurements, such as air and soil temperature, were collected to help determine what environmental conditions bring about sap flow in these novel syrup-producing species. Second, during year 2, trees were tapped, sap was harvested, and syrup was produced from each species separately. Third, 18 to 24 mo after trees were tapped, trees were felled and the amount of functional sapwood above and below tap holes that became nonconductive was quantified to aid in the generation of tapping guidelines for these species. Preliminary results were shared with landowners, sugarmakers, and other researchers at various meetings and by the media.
Beech, planetree (sycamore), and hophornbeam proved to be good syrup-producing trees. No sap was harvested from basswoods, ashes, or hickories, but these trees should not be ruled out entirely from syrup production - with improvements in, or modifications to, the timing of tapping or to the methodology of sap extraction, syrup production from these species may be possible. Based on very preliminary nonconductive wood data, it appears that when trees are selected and tapped according to maple tapping guidelines, syrup production is sustainable, although a more in-depth look at radial growth rates and repeated annual tapping events will be necessary to generate more concrete tapping guidelines.
Many maple syrup producers may initially be resistant to syrup production from different species. Some may require new equipment, especially if the scale of production is different (for example, a producer who taps thousands of sugar maples would need smaller equipment to tap 100 beech trees) or if the sap has chemical properties that require that only plastic and stainless steel equipment should be used. Furthermore, some of these novel species have sap runs that overlap with that of maples, and it might not be feasible to produce more than one type of syrup at a time. Even though some of these novel species have sap runs that don't overlap with that of maples, producers may not have the wherewithal to extend their season. Despite these potential barriers, many of these novel syrups do offer consumers new tastes and they also offer producers an opportunity to make money from their sugarbush and with their expertise and their infrastructure in new ways. There are already a handful of birch syrup producers in the northeast, and it is not unlikely that new or existing producers will explore some of these novel tree syrups.
Objective 1. Identify the timing and environmental drivers of dormant-season sap flow in novel trees and the physiological mechanisms behind them. Knowledge of the timing of, and the environmental conditions that cause, dormant-season sap flow in novel species will help producers determine when and how long taps should be set out. A better understanding of the physiological mechanisms that drive dormant-season sap flow in novel species will help fill in scientific knowledge gaps and may further our understanding of dormant-season sap flow in maples, birches, and walnuts, the mechanisms for which are somewhat, but not completely, understood.
Objective 2. Determine optimal tapping depths of novel species. Though 4-cm commercially-available taps work well with maples, birches, and walnuts, preliminary work suggests that dormant-season sap flow depths vary among species. Shagbark hickory dormant-season sap flows at depths between 1 and 2 cm, with barely any flow deeper than 2.5 cm (Moore, unpublished data). Four-cm maple taps are not ideal for harvesting hickory sap because they are seated tightly in shallower sapwood depths and can block sap flow.
Objective 3. Determine the amount of nonconductive wood formed due to trees’ wounding responses to tapping, and whether mean annual diameter growth compensates for the loss of hydraulic conductivity. It is known that tapping sugar maples and paper birches causes the formation of a significant amount of nonconductive wood above and below tap holes, but that healthy sugar maples put on more than enough new girth growth each year to compensate for the loss of conductive wood if tapping guidelines are followed (van den Berg et al., 2012; van den Berg et al., 2017). This project would be the first to collect data towards developing such guidelines for other species. Species can differ widely in how they isolate wounds (Biggs, 1985; Dujesiefken et al., 2005). It is unknown how much nonconductive wood is formed in novel species because of tapping, and whether their annual stem growths compensate for this loss in hydraulic conductivity.
Objective 4. Determine the palatability of novel syrups. The marketability of novel syrup is directly related to how good it tastes.
The purpose of this project is to increase the resiliency and sustainability of the syrup industry by determining if sap from trees other than maples (Acer spp.), birches (Betula spp.), and walnuts (Juglans spp.) can be harvested and processed into syrup. The goal of this project is to develop a production manual that discusses all aspects of novel (meaning other than maple, birch, and walnut) syrup production from tree species that demonstrate an aptitude for syrup production. This production manual will cover all aspects of sap harvesting and syrup production, including the timing of and environmental conditions that drive dormant-season novel sap flows, optimal tapping depths, and all other relevant harvesting, storage, and processing techniques.
This project will directly benefit syrup producers in two ways. First, tapping different species offers season-extending opportunities. Preliminary work has shown that the timing of sycamore (Platanus spp.) and hickory (Carya spp.) sap flows differ from that of maple – both occur earlier in the year (Moore, unpublished data), and both are purported to yield edible sap that can be processed into syrup (Peterson, 1977). Indeed, birch (Betula spp.) sap runs later than maple sap, and one producer in . Extending the sugaring season will enable producers to benefit from their expertise and expensive infrastructure for more than just the 6- to 8-week maple sugaring season. If two species’ sugaring seasons do not overlap, the same equipment can be used for both species. Second, producing syrup from different species diversifies a producer’s portfolio and reduces exposure to poor years caused by bad weather or pest outbreaks affecting a single species. Sugar maple (Acer saccharum) is very sensitive to drought, pests, and pathogens, which have contributed to sugar maple decline (Bishop et al. 2015, Kosiba et al. 2017, Payette et al. 1996), so diversifying syrup production would be in a producer’s best interest.
This project also has implications beyond people who currently produce syrup. First, farmers are often busy with seasonal farm duties like preparing for crop and livestock production in Feb., Mar., and Apr., when maple, birch, and walnut sap flows, and thus they are often not able to produce these syrups. If novel species have sap flows that occur earlier in the dormant season (such as sycamores and hickories), they would present farmers with the possibility for syrup production that does not overlap with their late winter and early spring tasks. Second, landowners who are interested in syrup production but whose woodlots do not contain significant numbers of sugar maples, paper birches (Betula papyrifera), or walnuts may have the option to produce syrup if it is found that novel species present in their woodlots are viable for syrup production.
Each year, I receive e-mails from syrup producers wanting to make, and from people wishing to purchase, sycamore syrup. I ran a successful birch and sycamore syrup operation (The Crooked Chimney, www.crookedchimneysyrup.com) in Lee, NH, for several years before graduate school, and it is evident that there exists both a strong interest from syrup producers in producing syrup from different species and a strong consumer demand for these syrups. I began producing birch syrup commercially in 2010 as the first commercial birch syrup producer in the continental United States, and at that time, there was no demand for birch syrup because nobody in the region was familiar with birch syrup. After a couple of years of offering free samples at farmers markets, meeting with chefs, and networking with agricultural industry personnel, the media ran stories about birch syrup and our operation, and a remarkable demand for birch syrup developed which still exists today.
Birch syrup commands a much higher price per volume than maple syrup does (Farrell, 2013; Farrell, 2015) because birch sap is much more dilute than maple sap and consequently it requires more processing. It is likely that novel syrups would command a higher price too – preliminary work has shown that sycamore sap is about as dilute as birch sap (Moore, unpublished data). To our knowledge, there are no syrup producers making pure syrup from the sap of trees other than maples, birches, and walnuts. One producer (Paul Hovan, Tunkhannock, PA) taps sycamore trees and produces a blended syrup made from both sycamore and maple sap. One producer (Hickoryworks, Trafalgar, IN) makes syrup from shagbark hickories (Carya ovata) and tulip poplars (Liriodendron tulipifera), but they do not do so by harvesting and processing sap; rather, they harvest the inner bark of these trees, make an extract from it, and sweeten it with cane sugar.
To our knowledge, there is currently no literature available on harvesting sap and producing syrup from novel trees. A handful of accounts exist that discuss the possibility of producing syrup from different species (Farrell, 2013) and that mention that people have consumed sap from different species in the past (Łuczaj, 2008; Łuczaj, 2011; Łuczaj et al., 2014), but these accounts do not include methods for harvesting or processing sap. One paper discusses dormant-season sap flow in novel species (Essiamah, 1980), but this paper makes no reference to sap edibility or to syrup production.
In 2015, 3,434,000 gallons (valued at $125,890,000) of maple syrup were produced in the United States (United States Department of Agriculture, 2016), and birch syrup is an important commercial industry in Alaska and Canada (Cameron, 2001; Maher, 2006). If production methods for novel syrups were developed, it is likely that several sugarmakers would begin to produce novel syrups and that markets would quickly develop for these syrups.
Works Cited
Bishop, D., C.M. Beier, N. Pederson, G.B. Lawrence, J.C. Stella, and T.J. Sullivan. 2015. Regional growth decline of sugar maple (Acer saccharum) and its potential causes. Ecosphere. 6:1-14.
Cameron, M. 2001. Establishing an Alaskan birch syrup industry: Birch syrup – It’s the un-maple!TM. In: Davidson-Hunt, I.; Duchesne, L.C., and Zasada, J.C., editors, Forest communities in the third millennium: Linking research, business, and policy toward a sustainable non-timber forest product sector. Proceedings of the meeting, Kenora, Ontario, Canada, 1-4 Oct. 1999. General Technical Report NC-217, United States Department of Agriculture, Forest Service, North Central Research Station, St. Paul, MN. p. 135-139.
Essiamah, S.K. 1980. Spring sap of trees. Ber. Deutsch. Bot. Ges. Bd. 93:257-267.
Farrell, M. 2013. The Sugarmaker’s Companion: An Integrated Approach to Producing Syrup From Maple, Birch, and Walnut Trees. Chelsea Green Publishing. White River Junction, VT.
Farrell, M. 2015. Weighing the pros and cons of producing birch syrup. Cornell Small Farms Program. Cornell University. Viewed online 17 Apr 2018. <https://smallfarms.cornell.edu/2015/04/06/weighing-the-pros/>.
Kosiba, A.M., P.G. Schaberg, S.A. Rayback, and G.J. Hawley. 2017. Comparative growth trends of five northern hardwood and montane tree species reveal divergent trajectories and response to climate. Can. J. For. Res. 47:743-754.
Łuczaj, Ł. 2008. Archival data on wild food plants used in Poland in 1948. J. Ethnobiol. Ethnomed. 4:4.
Łuczaj, Ł. 2011. Edible wild plants used in Poland from the mid-nineteenth century to nowadays. Ethnobiologia Polska. 1:57:125.
Łuczaj, Ł, M. Bilek, and K. Stawarczyk. 2014. Sugar content in the sap of birches, hornbeams and maples in southeastern Poland. Cent. Eur. J. Biol. 9:410-416.
Maher, K.A.C. 2006. Factors influencing birch sap production in Alaskan birch (Betula neoalaskana Sarg.). University of Alaska-Fairbanks School of Natural Resources and Agricultural Sciences. Viewed online 2 May 2018. <https://www.uaf.edu/files/ces/aknfc/resources/workshops/06FactorInfluenceBirchSap.pdf>.
Payette, S., M.-J. Fortin, and C. Morneau. 1996. The recent sugar maple decline in southern Quebec: Probable causes deduced from tree rings. Can. J. For. Res. 26:1069-1078.
Peterson, L.A. 1977. Edible Wild Plants: Eastern/Central North America. Peterson Field Guides®. Houghton Mifflin Company. New York, New York.
United States Department of Agriculture. 2016. Northeast Maple Syrup Production. United States Department of Agriculture. <https://www.nass.usda.gov/Statistics_by_State/New_England_includes/Publications/Current_News_Release/2016/Maple.pdf>.
Research
During the fall of 2018, sensors for measuring sap flow were built in preparation for the first season of data collection (early Jan. of 2019 through early Apr. of 2019). These sensors were constructed following the protocol of Burgess et al. (2001). In the original proposal for this project, it was stated that sap flow would be monitored at 0.5-cm intervals throughout the entire sapwood in each tree. Since this would require many more sensors than were budgeted for – for example, a tree with a sapwood depth of 8 cm would require at least 5 sensors (3 depths are measured per sensor) – we realized that this original plan was too ambitious due to time and money constraints. Thus, sap flow was instead monitored at 6 different sapwood depths (0.5, 1.0, 1.5, 2.5, 4.0, and 6.0 cm beneath the cambium), which is still much more intensive that most sap flow studies. The sapwood depths at which sap flow will be measured were carefully chosen to provide high resolution at shallower depths while still being able to generate accurate sap flow radial profiles to a depth notably greater than the depth of taps used in the syruping industry (tap holes are generally made to depths of between 3.8 and 5.1 cm).
During the fall of 2018, 30 study trees (5 trees of each species) were identified in Lee, NH. The 6 species included in the study are London planetree (Platanus x acerifolia), shagbark hickory (Carya ovata), American basswood (Tilia americana), quaking aspen (Populus tremuloides), American beech (Fagus grandifolia), and American hophornbeam (Ostrya virginiana). Healthy trees with full live crowns and whose diameters at breast height are at least 20 cm (for hophornbeams, which do not grow very large, the minimum diameter is 12 cm) were selected. Sap flow sensors were installed in these selected sample trees in Jan. and Feb. of 2019 and were removed after buds broke in Mar. or Apr. of 2019. Two sap flow sensors were installed in each study tree. A shorter sensor measured sap flow at shallower depths (0.5, 1.0, and 1.5 cm beneath the cambium), and a longer sensor measured sap flow at deeper depths (2.5, 4.0, and 6.0 cm beneath the cambium). Sap flux densities were calculated from the data (Marshall, 1958).
During the fall of 2019, additional species were identified in which sap flow would be monitored during the subsequent winter dormant period. These species were American hornbeam (Carpinus caroliniana), smooth alder (Alnus serrulata), weeping willow (Salix babylonica), hardy kiwi (Actinidia arguta), sassafras (Sassafras albidum), and tulip poplar (Liriodendron tulipifera). Additionally, it was decided that London planetree should be monitored for another dormant season as well. Five individuals of each of these species were selected that meet the criteria above for monitoring with the following exception: the minimum diameters at breast height for kiwiberry, smooth alder, and American hornbeam were 2 in. These three small study species only received one sap flow sensor per tree - only the short sensor was used because their diameters were not great enough to warrant using the longer sensors. Sap flow sensors were installed in these selected sample trees in Jan. and Feb. of 2020 and were removed after buds broke in Mar. or Apr. of 2020. Sap flux densities were calculated from the data (Marshall, 1958). These additional species were not tapped for syrup production nor used in any nonconductive wood studies.
In Feb. of 2020, the shagbark hickory, American basswood, quaking aspen, American beech, and American hophornbeam trees that were monitored in 2019 were tapped for syrup production. Trees were tapped to a depth of 1.5 in with modified (shortened) 0.3125-in nylon taps. Sap tubing was used to tie all five tapped trees within a species together so that the sap of each species could be collected in one bucket. One vacuum pump (Pentair, Minneapolis, MN) was used for each species to increase sap yields. Sap was harvested daily when it was running unless temperatures were below freezing, which would prevent it from spoiling.
For species that yielded enough sap to warrant syrup production, syrup was produced by using heat to evaporate water from the raw sap. Only stainless steel and plastic equipment were used for collecting, transporting, storing, and processing sap to avoid metallic off-flavors from being imparted on the sap, and relatively low temperatures (temperatures near the boiling point of sap) were used to evaporate the water from the sap to prevent scorching. Raw sap was filtered once prior to evaporation and again when it was finished syrup. Reverse osmosis was not used during this process. Sap and syrup samples were collected, filtered, and frozen so that they can be used in the future for chemical analyses and for consumer preference analyses.
Between Jan. or Feb. and Mar. or Apr. of 2021, 2 more species were monitored for sap flow. These species were American elm (Ulmus americana) and black locust (Robinia pseudoacacia). The same methods used for collected sap flow data in 2019 and 2020 were followed in these species in 2021. These additional species were not tapped for syrup production nor used in any nonconductive wood studies.
Three of the five study species (American basswood, quaking aspen, and American hophornbeam) were felled between Jul. and Dec. of 2021 for nonconductive wood column quantification. Nonconductive wood columns are darker regions of wood that extend vertically above and below old tap holes. Although this step was originally planned for the fall of 2020, Dr. Tim Perkins from the University of Vermont's Proctor Maple Research Center in Underhill, VT, suggested waiting 12 to 18 mo after tap holes were installed to quantify nonconductive wood volumes because the nonconductive wood columns would be darker in color and easier to use with digital image processing software. Between Jul. and Dec. of 2021, these trees were felled, and cookies were taken orthogonally to the axis of tree growth every 2 in above and below the tap hole until the columns of nonconductive wood disappeared. ImageJ software will be used to determine the area of nonconductive wood for each cookie, and then an estimation of the total volume of nonconductive wood can be obtained using the fact that cookies were 2 in apart.
Although a consumer preference test was planned as part of this study to evaluate novel syrup marketability, the pandemic precluded it from happening.
During the fall of 2022, five different shagbark hickories and five different American beeches tapped for syrup production in 2021 will be felled and analyzed for nonconductive wood to complete this study.
Works Cited
Burgess, S.S.O., M.A. Adams, N.C. Turner, C.R. Beverly, C.K. Ong, A.A.H. Khan, and T.M. Bleby. 2001. An improved heat pulse method to measure low and reverse rates of sap flow in woody plants. Tree Physiol. 21:589-598.
Marshall, D.C. 1958. Measurement of sap flow in conifers by heat transport. Plant Physiol. 33:385-396.
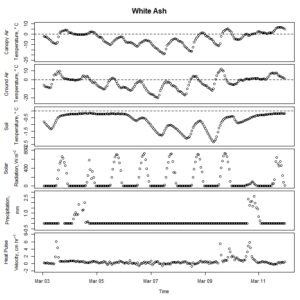
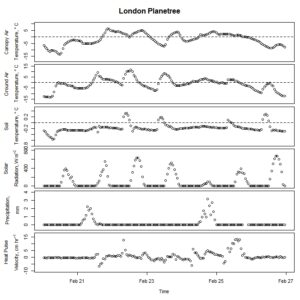
Winter-dormant-season sap flow was observed in all seven study species between February of 2019 and leaf-out. In general, winter-dormant-season sap flow appeared to occur mainly during the day, and temperature (and, perhaps to a lesser extent, solar radiation) may be the primary driver of this sap flow in most of the species. One species, the London planetree (Platanus x acerifolia), appeared to be driven by precipitation (Fig. 6).
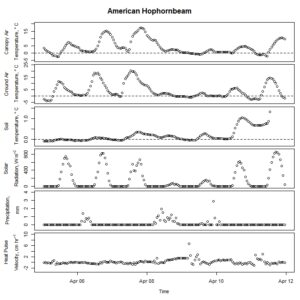
Figure 1. Winter-dormant-season sap flow (expressed as corrected heat pulse velocities) in an American hophornbeam (Ostrya virginiana) tree in Lee, New Hampshire, from April 5th to April 11th, 2019. Environmental variables (canopy air temperature, ground air temperature, soil temperature at a depth of 10 cm, solar radiation, and precipitation) monitored nearby are included as well. Dashed lines on graphs depicting temperatures represent the freezing point of water (0 ° C).
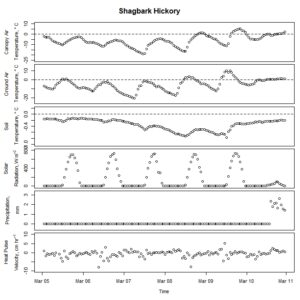
Figure 2. Winter-dormant-season sap flow (expressed as corrected heat pulse velocities) in a shagbark hickory (Carya ovata) tree in Lee, New Hampshire, from March 4th to March 10th, 2019. Environmental variables (canopy air temperature, ground air temperature, soil temperature at a depth of 10 cm, solar radiation, and precipitation) monitored nearby are included as well. Dashed lines on graphs depicting temperatures represent the freezing point of water (0 ° C).
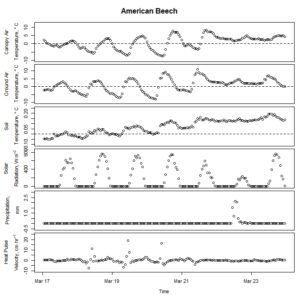
Figure 3. Winter-dormant-season sap flow (expressed as corrected heat pulse velocities) in an American beech (Fagus grandifolia) tree in Lee, New Hampshire, from March 17th to March 23rd, 2019. Environmental variables (canopy air temperature, ground air temperature, soil temperature at a depth of 10 cm, solar radiation, and precipitation) monitored nearby are included as well. Dashed lines on graphs depicting temperatures represent the freezing point of water (0 ° C).
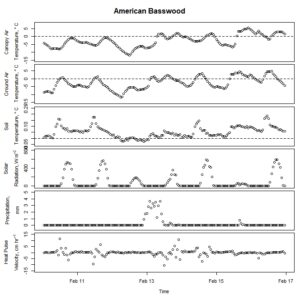
Figure 4. Winter-dormant-season sap flow (expressed as corrected heat pulse velocities) in an American basswood (Tilia americana) tree in Lee, New Hampshire, from February 10th to February 16th, 2019. Environmental variables (canopy air temperature, ground air temperature, soil temperature at a depth of 10 cm, solar radiation, and precipitation) monitored nearby are included as well. Dashed lines on graphs depicting temperatures represent the freezing point of water (0 ° C).
Figure 5. Winter-dormant-season sap flow (expressed as corrected heat pulse velocities) in a white ash (Fraxinus americana) tree in Lee, New Hampshire, from March 3rd to March 11th, 2019. Environmental variables (canopy air temperature, ground air temperature, soil temperature at a depth of 10 cm, solar radiation, and precipitation) monitored nearby are included as well. Dashed lines on graphs depicting temperatures represent the freezing point of water (0 ° C).
Figure 6. Winter-dormant-season sap flow (expressed as corrected heat pulse velocities) in a London planetree (Platanus x acerifolia) tree in Lee, New Hampshire, from April 5th to April 11th, 2019. Environmental variables (canopy air temperature, ground air temperature, soil temperature at a depth of 10 cm, solar radiation, and precipitation) monitored nearby are included as well. Dashed lines on graphs depicting temperatures represent the freezing point of water (0 ° C).
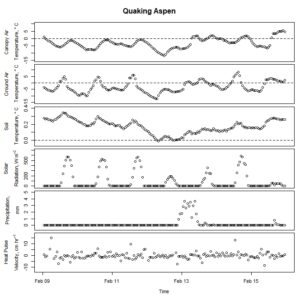
Figure 7. Winter-dormant-season sap flow (expressed as corrected heat pulse velocities) in a quaking aspen (Populus tremuloides) tree in Lee, New Hampshire, from February 9th to February 15th, 2019. Environmental variables (canopy air temperature, ground air temperature, soil temperature at a depth of 10 cm, solar radiation, and precipitation) monitored nearby are included as well. Dashed lines on graphs depicting temperatures represent the freezing point of water (0 ° C).
Though none of the species exhibited winter-dormant-season sap flow anywhere near the magnitude of maples (Acer spp.), birches (Betula spp.), or walnuts (Juglans spp.), clear patterns that often followed diel cycles were nonetheless evident in all study species except for aspen (Fig. 7). Aspen sap flow data was more noisy than sap flow data from other species, despite equipment being in good condition. It is speculated that something to do with aspen wood anatomy precludes the sensor's heat pulse from generating as crisp of a signal as was found in other species.
Figure 8. Heat-ratio-method sap flow sensors in an American basswood (Tilia americana) tree in Lee, New Hampshire. This photo was taken by David Moore at the conclusion of the study (after leaf-out occurred), which is why green foliage is evident.
Sap flow did not decrease with sapwood depth as expected. During 2017 and 2018, winter-dormant-season sap flow was observed in freshly-cut shagbark hickory and white ash logs in January and February in only the outermost sapwood depths (i.e., within 2.5 cm from the cambium; Moore, unpublished data). We thus expected to see sap flow radial profiles depicting the greatest sap flows occurring at shallow sapwood depths, but this was not the case (Figs. 9 and 10). The heat-ratio-method sap flow sensors used in this study were likely the source of this unexpected result. Upper and lower sap flow sensor probes, which each contain three thermocouples to measure temperatures are three different sap flow depths, are measured by the datalogger in single-ended mode and not in differential mode. The three copper wires in each upper and lower sap flow sensor needle are all connected to the same constantan wire, and constantan wires from all sensors connected to the same datalogger are connected to the same ground. Thus, the signal received by the datalogger from the copper wire contains relatively accurate information about how the sap is flowing in the sapwood, but the signal received by the datalogger from the constantan wire is actually an average of all of the information received from all of the constantan wires measured by that datalogger. Thus, relative differences in sap flow signals should still be evident, but absolute values will not be correct as signal differences between trees and between sapwood depths will be reduced, making it more difficult to determine statistically when and where different signals occur.
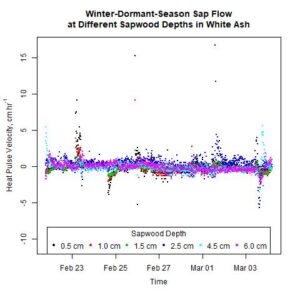
Figure 9. Winter-dormant-season sap flows (expressed as heat pulse velocities) in a white ash (Fraxinus americana) tree at six different sapwood depths (0.5, 1.0, 1.5, 2.5, 4.0, and 6.0 cm below the cambium) in Lee, New Hampshire, from February 21st to March 4th, 2019.
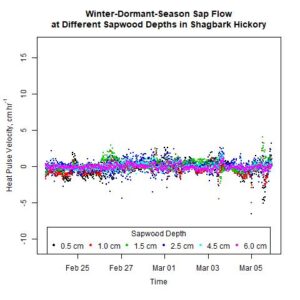
Figure 10. Winter-dormant-season sap flows (expressed as heat pulse velocities) in a shagbark hickory (Carya ovata) tree at six different sapwood depths (0.5, 1.0, 1.5, 2.5, 4.0, and 6.0 cm below the cambium) in Lee, New Hampshire, from February 23rd to March 5th, 2019.
It is evident that sap flow can occur at different sapwood depths independently. For example, in the white ash tree depicted in Fig. 9, on February 21st, significant sap flow occurred at the 4.5-cm sapwood depth but not at other depths. Two days later, significant sap flow occurred at the 0.5- and the 1.0-cm sapwood depths, but not at any other depths (Fig. 9). Furthermore, on March 3rd, sap flow occurred in different directions at different depths simultaneously, and sap flow abruptly changed direction at the 4.5-cm depth (Fig. 9). Other trees, species, and times showed similar inconsistent sap flow patterns between sapwood depths as well (data not shown). These observations illustrate how complex the processes governing winter-dormant-season sap flow are. Since nothing is currently known on the mechanisms driving winter-dormant-season sap flow in these novel species, any attempts to explain these observations are purely speculative. These sap flow measurements were taken at a single point on a tree's bole, but to fully understand the nature of sap flow dynamics, it is important to remember that plant vascular systems are a complex network of xylem cells that run from fine roots to small twigs. In sugar maples, winter-dormant-season sap flow dynamics are primarily driven by freeze-thaw cycles where gas bubbles in the xylem tissue contract as temperatures decrease, and water potential gradients change as water changes from a liquid to a solid (Graf et al., 2015). Since an entire tree does not freeze or thaw instantaneously, freezing or thawing fronts can move across woody tissue a number of different ways and cause sap flow to occur dynamically. In sugar maples, gas bubbles in the xylem change in volume as temperature changes and cause sap to flow during the dormant season. If certain sapwood depths contain lots of gas bubbles, or if the xylem tissue at a certain sapwood depth is connected via a network of xylem cells to xylem tissue containing lots of gas bubbles, sap flow would presumably occur more at those depths. Gas abundance in xylem tissue is dynamic and depends on a host of factors, including temperatures; thus, different temperatures (and potentially other environmental conditions) could cause sap flow to occur differently at different sapwood depths. Though these mechanisms pertain to sugar maples, it is possible for similar mechanisms to occur in other species as well. Starch hydrolysis leading to increases in sugar concentrations in certain parts of the tree driven by certain environmental conditions may play a role as well: osmotic gradients are an important part of the mechanism causing winter-dormant-season sap flow in birches (Westhoff et al., 2008).
For the syrup production component of the study, only two species, American hophornbeam and American beech, yielded any sap. Only American beech yielded enough sap to warrant processing it into syrup. American hophornbeam sap ran during the same time of year when paper birch sap runs (at the end of, and just after, maple sugaring season), but American beech sap ran during the same time that maple sap runs. Beech sap is much more dilute than maple sap; though over 50 gal of beech sap were harvested, less than 1 pt of beech syrup was produced. This beech syrup was much darker than maple syrup likely because reverse osmosis was not used. Its taste was reminiscent of that of maple syrup, although it had its own unique flavor as well.
Other researchers and practitioners have successfully harvested American basswood sap and processed it into syrup. After speaking with two people who have had success doing so - Mike Farrell of New Leaf Tree Syrups (Lyon Mountain, New York) and Aaron Wightman of Cornell University (Ithaca, New York) - it was thought that tap holes were made too early in this study. By the time the basswood sap may have ran later in the dormant period, the tap holes may have closed up due to the tree's natural wounding response. Future work will investigate whether a later tapping time may allow for the harvesting of basswood sap. Environmental conditions may have precluded basswood sap from running as well, but this idea is unlikely because of the wide array of environmental conditions present during the winter dormant period while these trees were tapped.
For the nonconductive wood component of this study, only four of the seven initial study species were studied due to landowner concerns. In general, it was evident that quaking aspen nonconductive wood columns varied quite a bit depending on whether or not the heartwood was penetrated by the tap hole. For quaking aspens whose heartwood was not penetrated by tap holes, nonconductive wood columns were generally quite short. For those whose heartwood was penetrated by tap holes, it was unclear exactly how tall the nonconductive wood columns were because the tap hole nonconductive wood columns and the nonconductive wood of the heartwood ran together. It appeared that a bulge on the side of the heartwood's nonconductive wood column nearest to where the tap hole was represented additional nonconductive wood from the tap hole. Furthermore, although all five quaking aspens were perfectly healthy at the beginning of this study, two of the five quaking aspens tapped for syrup production developed visible signs of rot (conks) between 1 and 2 yr after tapping. It is not surprising that quaking aspens do not compartmentalize tap hole wounds efficiently - it is speculated that these early-successional species preferentially allocate carbon to axial growth at the expense of defense (M. Vadeboncoeur, personal communication, 2019). By this logic, it is surprising that quaking aspen nonconductive wood columns were relatively short when the heartwood was not penetrated by the tap hole - perhaps it is important to develop shorter taps for species with shallower sapwood to prevent exacerbating nonconductive wood formation. For maples and birches, the two most commonly-used trees for syrup production, heartwood is almost never penetrated by tap holes because these two species have deep sapwood depths. Due to difficulties discerning between heartwood nonconductive tissue and tap-hole-related nonconductive tissue, it is impossible to quantify nonconductive wood column size for quaking aspens when the heartwood was penetrated by the tap hole. When the heartwood was not penetrated by the tap hole, quaking aspen nonconductive wood columns were as short as 20 in tall.
For American basswoods, nonconductive wood columns were between 6 and 20 in tall. For American hophornbeams, nonconductive wood columns were between 24 and 34 in tall. Hophornbeam nonconductive wood columns, unlike the other three species, became very faint in color as distance above or below the tap hole increased. For white ashes, nonconductive wood columns were between 4 and 13 in tall. The ability of white ashes to compartmentalize wounds is shown in Fig. 11 - the darkened region around the wound is very short (approximately 2 in high). White ashes appeared to behave like quaking aspens in regards to their differential ability to compartmentalize wounds depending on whether or not heartwood was penetrated by tap holes.
Figure 11. White ash (Fraxinus americana) radial sections. Two short sections of a white ash from Lee, New Hampshire felled for this study that contained fence bolts were cut out of the tree and split in half using an axe. It is evident from this photo that white ash compartmentalizes wounds very effectively. Unlike tap holes, where a tap is installed for 1 to 2 mo and then removed, these two holes were never open to the atmosphere - they contained bolts the entire time they were present in the living tree. It is not known if the presence of the bolt affected nonconductive wood formation. A golf ball is included for scale, showing that nonconductive wood columns are approximately 2 in tall.
Many, but not all, of these nonconductive wood columns rapidly declined in cross-sectional area as distance above or below the tap hole increased. It is difficult to accurately determine which nonconductive wood columns are associated with the tap hole in each cookie, especially as distance above or below the tap hole increases. The images of these cookies will be processed more rigorously with ImageJ software (National Institutes of Health, Bethesda, MD) during the summer of 2022 to more accurately quantify nonconductive wood column volumes.
Works Cited
Graf, I., M. Ceseri, and J.M. Stockie. 2015. Multiscale model of a freeze-thaw process for tree sap exudation. J. R. Soc. Interface. 12:1-15.
Westhoff, M., H. Schneider, D. Zimmermann, S. Mimietz, A. Stinzing, L.H. Wegner, W. Kaiser, G. Krohne, St. Shirley, P. Jakob, E. Bamberg, F.-W. Bentrup, and U. Zimmermann. 2008. The mechanisms of refilling of xylem conduits and bleeding of tall birch during spring. Plant Biol. Stuttg. 10:604-623.
American beech, London planetree, and American hophornbeam are viable syrup-producing trees. Though syrup was not produced from American hophornbeam in this study, it is evident that hophornbeams yield enough sap for syrup production, and if more than 5 trees had been tapped using our vacuum system, syrup production would have been possible. Sustainable tapping guidelines have not been developed for these three species, and our preliminary investigation into nonconductive wood formation may serve as a basis for these guidelines. Nonconductive wood columns should be compared to annual growth rates (annual basal area increments) to determine conclusive and precise tapping guidelines for these species - to be sustainable, trees must compensate for the amount of functional sapwood they lose to tapping each year by new radial growth. Since almost all of our study trees were never tapped prior to the study, follow-up studies should be conducted to determine how syrup yields, nonconductive wood columns, and tree health is affected by repeated annual tapping.
Education & Outreach Activities and Participation Summary
Participation Summary:
This work on harvesting sap and producing syrup from trees other than maples was featured in the media extensively in 2021. It was featured in the Union Leader, on New Hampshire Public Radio, on Science Friday, on Living on Earth, and on two radio programs. Preliminary results from this study were presented at a New Hampshire Agricultural Experiment Station field day to a small audience of stakeholders and also at a New Hampshire Maple Producers Association meeting to an audience of about 50 maple syrup producers. For a complete list of outreach activities, please visit https://mypages.unh.edu/sites/default/files/ecohydrology-lab/files/moore_cv.pdf.
Project Outcomes
This project will help improve syrup-production sustainability by providing preliminary results that can be used to generate preliminary tapping guidelines for novel syrup-producing species. This project may also help improve the sustainability of the syrup industry by offering producers more information on how to harvest sap and produce syrup from trees other than maples, birches, and walnuts.
When this project began, I already had a strong background in syrup production and a keen interest in developing novel types of syrups. My interests were mostly practical, though, and it was because of this project that I was able to begin investigating more basic-science concepts such as what environmental conditions drive sap flow in woody angiosperms during winter dormancy. Recently, my advisor and I received funding to continue this work. Our new projects investigate the anatomical and physiological mechanisms of winter-dormant-season sap flow more intensively and also include sap pressure (in addition to sap flow) as a response variable. I believe now that sap pressure is more important to monitor than sap flow - it is sap pressure and not sap flow that allows for sap extraction without vacuum, and sap flows across sap pressure gradients. In other words, it is possible for there to be no sap flow even when sap pressure is great if sap pressure is equal across the entire tree, and it is possible to have sap flow events with very little sap pressure if sap lows from a region of low pressure to a region of even lower pressure. From a practical standpoint, sap pressure may be a more important response variable than sap flow. Furthermore, our new projects allow us to do an in-depth investigation into the chemical composition of these novel saps and syrups using methods such as chromatography, nuclear magnetic resonance spectroscopy, and mass spectrometry.
Furthermore, years ago when I was a syrup producer, my work exploring novel syrups was only taken seriously by a handful of progressive syrup producers. Most wrote off birch and sycamore syrup as not worthwhile. Though I believe that none of these novel syrups will replace (or even come near replacing) maple syrup in the market, I do believe that they offer nice opportunities for season extension and for diversification for both the producer and the consumer.
In the future, I plan to continue investigating the applied- and basic-science aspects aspects of syrup production from trees other than maples, including more work in wood anatomy and physiology. I also plan to do more work on sugar maples to fill in knowledge gaps and to increase the efficiency of maple sap extraction.
I believe now that sap pressure is more important to monitor than sap flow - it is sap pressure and not sap flow that allows for sap extraction without vacuum, and sap flows across sap pressure gradients. In other words, it is possible for there to be no sap flow even when sap pressure is great if sap pressure is equal across the entire tree, and it is possible to have sap flow events with very little sap pressure if sap lows from a region of low pressure to a region of even lower pressure.
After talking with my committee, and after a conversation with John Stockie from Simon Fraser University, I also believe that capturing axial variability in wood temperature, solar radiation, wood water content, osmolality, bark transpiration, sap flow, and sap pressure will be important for determining the mechanisms that drive sap flow and sap pressurization in these trees during winter dormancy. A more in-depth look at wood anatomy and physiology (including the size and distribution of the different types of xylem cells and their connectivity) will also be important to look at alongside our time-series data of tree and environmental variables to truly understand why sap flow and sap pressurization occur during winter dormancy. Some of this work has already been done in maples and birches, and many of these methods are accessible to me; with funding from a new project, I'll be able to monitor many of these variables across axial gradients.