Final report for GNE19-194
Project Information
Managing weeds in small-seeded organic vegetable crops can be challenging and costly. In organic carrot (Daucus carota L.), slow crop emergence and small seedling size leads to poor competitive ability against weeds and high mortality from cultivation, resulting in loss of crop yield and quality. Carrot breeding efforts for organic systems have worked to increase shoot growth to improve competition with weeds, but traits that confer tolerance to cultivation would also benefit organic carrot growers, allowing them to use more aggressive and effective cultivation. We selected nine carrot cultivars representing genotypes known to be large, average, and relatively small plants when mature. Over two years of greenhouse and field experiments we measured cultivar early growth characteristics and their responses to cultivation. Growth characteristics included shoot height, leaf area and mass, as well as root area and mass, root tips and forks, and anchorage force. In the field, cultivars were subject to either hand-weeding or cultivation with finger weeders or tine harrows, chosen to test effects of tools that differ in their uprooting and burial weed control mechanisms. Crop mortality, weed control efficacy, and marketable yield were measured. Cultivars had similar root and shoot mass at two-true leaves, but differed in their root and shoot morphology, including number of root tips and forks, and shoot height and area. Maximum anchorage force was unexpectedly not different between cultivars. Average anchorage force was different at two-true leaves but did not show a clear relationship to cultivar growth category and also had poor correlation with root mass. In the field, crop mortality was not different between cultivars for either the finger weeder or tine harrow, but mortality from tine harrows was greater than that from finger weeders suggesting that carrots may tolerate tools that act primarily by uprooting better than burial. Yields were inconsistent over the two-year study. Cultivars considered to be in our “small” category had lower marketable yield in year one but not year two. Despite some differences between cultivar early growth characteristics at two-true leaves, cultivar mortality differences were not detected in the field at the same growth stage. Current carrot cultivars are similar in their cultivation tolerance at the second true-leaf stage; therefore, management should focus on cultivation tool optimization and ecologically based weed seedbank management to permit more aggressive cultivation thereby increasing weed control efficacy.
- Examine selected carrot cultivar early growth characteristics in the greenhouse and lab, including shoot height, shoot and root area, root to shoot area ratio, root branching, and shoot and root mass using a previously purchased WinRHIZO™ program (Regent Instruments, Québec, Canada).
- Determine carrot cultivar root anchorage force in the lab using a previously purchased Alluris® FMI-B150 Force Gauge.
- Assess carrot cultivar tolerance to cultivation in the field by examining crop injury post-cultivation after use of either finger weeders, tine harrows, or hand-weeding with a stirrup hoe.
- Compare carrot root and shoot yield across tool treatments and compare to a hand-weeded control treatment.
- Perform correlation analyses between carrot early growth characteristics and field experiment results to determine “cultivation tolerance” and determine which growth traits may aid in cultivation tolerance.
The purpose of this project was to help identify early growth traits of carrot cultivars that make them more tolerant to cultivation or ‘physical weed control.’ Organic vegetable production in the Northeast is growing, but small-seeded crops such as carrot often suffer significant yield and quality losses to both weeds and cultivation because their slow early season growth and size make them less competitive and more prone to injury. Growth characteristics that may help a carrot better tolerate cultivation could direct future plant breeding efforts and help organic vegetable farmers make more informed cultivar and tool selections, while also permitting more aggressive cultivation to increase weed control.
Cooperators
Research
Site descriptions
Carrot early growth characteristics were assessed in greenhouse experiments at the University of Maine Roger Clapp Greenhouse in Orono, Maine (44°55'N, 68°40'W). Field experiments were conducted at the University of Maine Rogers Farm in Old Town, Maine (44°55'N, 68°41'W), residing in USDA Plant Hardiness Zone 4b, with an average annual high/low temperature of 20/-7°C, and average annual precipitation of 1,023 mm. The soil at the site is comprised of Nicholville fine sandy loam.
Greenhouse growth experiments (Objectives 1-2)
Seven commercial carrot cultivars were analyzed in 2019 to determine which cultivars and variables to assess in subsequent studies. With the assistance of carrot breeders, Drs. John Navazio and Phil Simon, we selected nine cultivars in 2020 (Table 1) based on their mid- to late-season root and shoot growth. Our goal was to include cultivars representing the spectrum of carrot growth profiles ranging from small to large roots and shoots. Cultivars were arranged in the greenhouse in a randomized complete block design with six replications. Average seed mass was determined for each cultivar prior to sowing. Seeds were passed through a sieve before sowing so that seed size would be relatively uniform across cultivars to reduce variability. Seeds were sown to a depth of 0.63cm in ConeTainers (Stuewe & Sons, Inc., Tangent, OR) filled with coarse pool sand and watered as necessary. Sand was selected as the growing media because it washes off roots easily, making root scans clearer and free of soil clods and organic matter. Cones were fertilized three times per week with Peter’s Professional 20-20-20 general purpose fertilizer to avoid nutrient stress in the sand media. Cone location within a rack was randomly selected for each cultivar and growth stage and labeled appropriately. To minimize effects of temperature and air movement gradients within the greenhouse, racks were rotated one turn clockwise every week within each replicate. Three growth stages were tested: one-, two-, and four-true leaves. Two plants were seeded for each harvest date, one for anchorage force testing and one for destructive harvest and analysis of early growth characteristics, described in detail below.
Greenhouse data collection
Prior to sowing, seed weight was determined by weighing four samples each of 25 seeds to get a representative seed weight for each cultivar. At each harvest date, plant shoot height was recorded, and plants were removed from cones, carefully washed, and placed in a tray for scanning using a WinRHIZO™ system (Regent Instruments, Québec, Canada) to quantify root and shoot area. Roots and shoots were separated, dried at 65ºC for at least three days in a lab drying oven, and weighed. Additional plants were subjected to anchorage force testing using an Alluris® FMI-B150 Force Gauge (Alluris GmbH & Co., Germany). A metal clip was attached to the carrot stem at the soil level and attached to the force gauge. Plants were pulled straight up, while continuously recording force at the clip, until all roots had been removed from the soil (Figure 1). The maximum and average force (Newtons) required to remove a plant from the soil were recorded.
Field experiments (Objectives 3-5)
Carrot cultivars’ tolerance to cultivation was assessed in field experiments in a 6 x 3 factorial, completely random design with four replications. Fertility was supplied based on soil test results using OMRI-approved materials. A phosphate deficiency was detected in year one (2020), and bone char (FedCo Seeds, Clinton, ME, USA) was applied at 200 kg ha-1 plant-available phosphorus. Nutri-wave™ 4-3-2 (Northeast Agricultural Sales, Inc., Detroit, ME, USA) granular fertilizer was applied prior to planting in both years at 50 kg ha-1 plant-available nitrogen. Pre-plant fertilizer was incorporated with a Perfecta® field cultivator. Feathermeal was sidedressed by hand at 33 kg ha-1 plant-available nitrogen four and six weeks after planting to meet N requirements. N applications were split to prevent carrot root forking.
Treatments consisted of six cultivars (Table 1) and three weed control treatments; finger weeder, tine harrow, and a hand-weeded, weed-free control. After pre-plant fertilizer application and seedbed preparation, soil was firmed with a cultipacker. Carrots were sown using a Jang six-row tractor-mounted seeder using recommended seed rollers and calibrations. Plots (3 m x 0.5 m) consisted of two rows of carrots on 50-cm rows sown at a depth of 0.63cm with 1.2cm in-row spacing. Six days after planting in both years, prior to crop emergence, all carrot beds were flame-weeded using a Pyroweeder® (Farmers Friend, Williamsport, TN). The flame weeder was centered over each bed and operated at 20 psi at a speed of 1.6 km h-1, with burners adjusted to a 45° angle. Carrot stands were thinned at the cotyledon growth stage to a 2.5cm intra-row spacing.
When carrots reached the two to three true-leaf stage, they were subject to physical weed control by either the finger weeder or tine-harrow. Cultivars Bolero, Yellowstone, and Dragon were cultivated four weeks after planting, while cultivars SFF, NB8483, and NB8524 were cultivated five weeks after planting due to their slower emergence. Finger weeders (HAK Schoffeltechniek, Bleiswijk, Holland) were selected to represent a tool that acts primarily with an uprooting mode of action and were belly-mounted on a HAK cultivating tractor. Fingers were adjusted to touch tip-to-tip and operated at 3.2 km h-1. A tine harrow (Tiny Treffler, Man@Machine, Grijpskerke, Netherlands) was selected to represent a tool that acts primarily with a burial mode of action. Tines were adjusted to a 62˚ angle relative to the soil and operated at a forward speed of 3.2 km h-1.
Rainfall was supplemented with irrigation to provide 2.5 cm of precipitation per week. Raintowers (Irrigation King, Tualatin, OR) were centered at each replication and sprinkler heads adjusted to cover the entire replication. Soil moisture was measured the day of cultivation to assess the uniformity of precipitation across the experimental area. Three random measurements were made in each plot to a depth of 10 cm using a Delta-T HH2 Moisture Meter equipped with a Theta Probe (Delta-T Devices, Burwell, United Kingdom). Soil moisture data were non-significant across all treatments; therefore, data are not presented.
Field data collection
Prior to cultivation, carrot density was measured in the central 2.4 m of each row in each plot. Ambient weed density was also measured within the 10-cm intra-row zone centered on the crop row in each 2.4 m section. Approximately 24 h after cultivation, crop and ambient weed densities were again measured to assess post-cultivation effects.
Weed-free control treatments were hand-weeded every 10-14 d using stirrup hoes and hand pulling. The time to hand weed was recorded for each plot. In plots receiving cultivation treatments, timed hand-weeding was performed once 14 days after cultivation using the same method described above to give an estimate of labor requirements following a cultivation event.
Cultivar categories were harvested as they reached maturity. All carrots in each of the two rows were harvested by hand using a broad fork, briefly washed to remove soil, and separated into “marketable” and “unmarketable” based on USDA grading standards. Within each category the number of carrots was recorded, total weight determined, and root weight after removing shoots.
Statistical analysis
Early growth characteristics and anchorage force were analyzed using goodness-of-fit Shapiro-Wilk tests and analysis of variance in JMP 16.0 (SAS Institute Inc., Cary, NC, USA). Cultivar, growth stage, and their interaction were tested, and year and block were used as random effects. Based on ANOVA results, Tukey-Kramer mean separations were performed at a = 0.05.
Crop mortality, weed control efficacy, hand weeding time, and crop yield were analyzed using goodness-of-fit Shapiro-Wilk tests and analysis of variance in JMP 16.0. Cultivar, weed treatment, and their interaction were tested. Tukey-Kramer mean separations were performed at a = 0.05. Orthogonal contrasts of cultivar category were performed to test responses to weed control treatments. Correlation analyses were also performed between early growth characteristics from the greenhouse and field trial response variables.
Objective 1. Examine selected carrot cultivar early growth characteristics in the greenhouse and lab, including shoot height, shoot and root area, root to shoot area ratio, root branching, and shoot and root dry weights.
As true leaf growth stages were reached, plant shoot height (cm) was recorded and plants were destructively harvested, carefully washed, and placed in a tray for scanning using the WinRHIZO™ system (Regent Instruments, Québec, Canada) to quantify root and shoot area. Roots and shoots were separated, dried at 65ºC for at least three days in a lab drying oven, and weighed to determine dry root and shoot mass. Root and shoot area data were pulled from WinRHIZO-generated scan files for analysis.
Objective 2. Determine carrot cultivar root anchorage force.
Additional plants were subjected to anchorage force testing using an Alluris® FMI-B150 Force Gauge. Plants were attached at the soil level to the force gauge hook using a metal clip and pulled straight up at a constant speed until all roots had been removed from the soil. The maximum and average force (Newtons) required to remove a plant from the soil were recorded and data was pulled from the Alluris-generated Excel files for analysis.
Objective 3. Assess carrot cultivar tolerance to cultivation by examining crop injury post-cultivation.
We measured crop mortality from cultivation with either finger weeders or tine harrows by recording the number of carrots pre- and post-cultivation in each of the two rows per plot. Post-cultivation data collection was performed approximately 24 hours after cultivation to ensure crop desiccation. Crop density was also recorded for hand-weeded treatments to determine if hand-weeding resulted in any crop mortality.
Objective 4. Compare carrot root and shoot yield across tool treatments and compare to a hand-weeded control treatment.
Carrots were harvested by hand digging all carrots in each row. Carrots were removed from the soil using broad forks, briefly washed in water to remove soil, and brought to the farm lab for processing. Carrots were divided into ‘marketable’ versus ‘unmarketable’ (very small or having major defects). Within each category, the number of carrots was recorded, as well as total fresh weight in grams. Carrot tops were removed using garden clippers, and carrot root fresh weight was recorded (grams). Carrot yields were analyzed in JMP 16 Pro and contrasts used to compare cultivated treatments to a hand-weeded control treatment to determine any differences.
Objective 5. Determine whether carrot early growth characteristics are related to “cultivation tolerance” and determine which growth traits may aid in cultivation tolerance.
Using JMP 16 Pro, early growth data including root and shoot biomass, root and shoot area, and shoot height were correlated with crop mortality and carrot yield data from the field trials. This analysis was used to determine if meaningful relationships exist between root and shoot morphology and crop mortality from cultivation.
Table 1. Selected carrot cultivars, assigned by size category, and respective suppliers for greenhouse and field trials.
|
Categorya |
Cultivar |
Supplier |
Greenhouse Experiments |
Field Experiments |
|
Large |
Bolero |
Johnny’s Selected Seeds |
Yes |
Yes |
|
Large |
Red Cored Chantenay |
John Navaziob |
Yes |
No |
|
Large |
Yellowstone |
Johnny’s Selected Seeds |
Yes |
Yes |
|
Average |
Dragon |
John Navazio |
Yes |
Yes |
|
Average |
Napoli |
Johnny’s Selected Seeds |
Yes |
No |
|
Average |
SFF |
Phil Simonc |
Yes |
Yes |
|
Small |
Mokum |
Johnny’s Selected Seeds |
Yes |
No |
|
Small |
NB 8483 |
Phil Simon |
Yes |
Yes |
|
Small |
NB 8524 |
Phil Simon |
Yes |
Yes |
a Category represents breeders’ expert opinions regarding root and shoot sizes.
b Carrot breeder, Johnny’s Selected Seeds
c Carrot breeder, University of Wisconsin-Madison
Objectives 1-2 (greenhouse)
Early growth characteristics – root and shoot mass
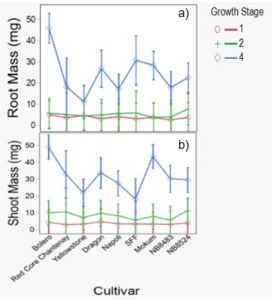
Root mass was surprisingly not different between cultivars at two-true leaves when cultivation occurred (Table 2, Table 3), and did not increase until four-true leaves (Figure 2). Mass was different between years at two-true leaves, averaging 3.1 mg (±0.3) and 7.2 mg (±0.5) across cultivars in 2020 and 2021, respectively. Root mass was highly correlated with the number of root tips and forks, indicating that factors such as root diameter (thickness) may vary between cultivars.
Shoot mass was also unexpectedly not different between cultivars at two-true leaves at the time carrots were cultivated (Table 2) and did not increase until four-true leaves (Figure 2). Shoot mass averaged 9.1 mg (±0.7) and 7.7 mg (±0.7) across cultivars in 2020 and 2021, respectively. Shoot growth characteristics such as area may not translate completely to mass. This may be due to differences in shoot morphology that contribute to mass, like shorter leaves, or leaves and stems with greater thickness, which could be important factors to consider for decreasing crop mortality from cultivation tools that act primarily by a burial mechanism. In field settings, differences in shoot mass may arise due to intra-specific competition; however, carrots in the greenhouse were grown individually to remove the effect of competition.
Early growth characteristics – root morphology
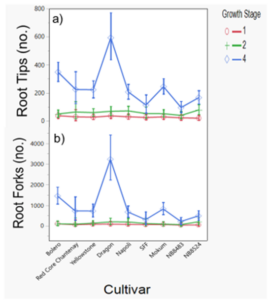
The number of root tips and forks were different between cultivars and cultivar interaction with year (Table 2, Table 3). Number of root tips was similar at the time of cultivation, but root forks were different between cultivars at two-true leaves, and both increased at four-true leaves (Figure 3). Ranked means of the number of root forks unexpectedly did not always correspond to designated cultivar categories, suggesting that other factors can influence root branching, such as the growing media or cultivar uptake and response to fertilization.
In other crops, seed mass can influence early growth characteristics. In canola, wheat, and sunflower, greater seed mass can result in quicker emergence, taller and thicker shoots, and greater early-season crop biomass and may be attributed, in part, to greater nutrient reserves (Ambika et al. 2014; Harker et al. 2015). Although seeds in this study were sieved to achieve uniformity, seed mass could be an important factor in future breeding efforts (Simon 2010). Embryo length may also be important, as greater embryo length results in quicker seedling emergence (Gray and Steckel 1983). Breeding for this trait could shorten carrot emergence time, thereby increasing competitive ability with weeds and allowing farmers to cultivate earlier.
Early growth characteristics – shoot morphology
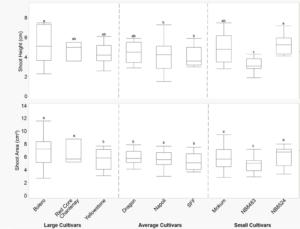
Shoot height was different between cultivars at two-true leaves when carrots were cultivated in the field (Figure 4). At two-true leaves, cultivars in the “large” category (Bolero, Red Core Chantenay, Yellowstone) had similar shoot height to each other, but surprisingly did not have greater shoot height than some cultivars in the “average” and “small” categories (Figure 4). Contrasts of cultivar category were examined and found to be non-significant (data not shown). Genetic variability of shoot height can exist within carrot cultivars (Turner et al. 2018) and can also be influenced by the angle at which the petiole grows (Benjamin 1984), although this angle was not measured in our study. Shoots that prioritize height earlier in the season may be a desirable trait to select for to permit earlier and more aggressive cultivation with tools that throw soil and bury plants.
Shoot area was different between cultivars at two-true leaves (Table 2, Figure 4). At two-true leaves, when carrots were cultivated in the field, shoot area tended to be higher for cultivars in the “large” category, but cultivars in the “small” category, such as Mokum and NB8524, had comparable shoot area to Bolero and Red Core Chantenay at the second true-leaf (Figure 4). In crops such as potato and soybean, greater leaf area has shown increased crop competitiveness with weeds (Mohler, 2001). However, in carrots, selection of cultivars with greater shoot area may not be as important as previously thought for tools that act by a burial mechanism.
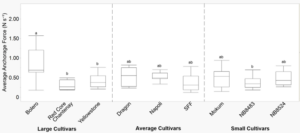
Anchorage force
Average anchorage force was different between cultivars at two-true leaves but not different between years (Table 2). At two-true leaves, Bolero had higher average anchorage force, while NB8483 and SFF had a lower average anchorage force (Figure 5), but contrasts of cultivar category were found to be non-significant (data not shown). Further examination found that root area, tips, and forks were highly correlated with one another, but these variables showed weak (<0.20) correlations with anchorage force. While root morphology does play a role in anchorage force, other factors can influence anchorage force such as the growing media and soil moisture (Ennos 1990), and potentially the environmental conditions at the time of data collection (i.e., daily temperature and humidity fluctuations).
Table 2. Effects of year, cultivar, growth stage, and their interactions on carrot early growth characteristics.
Table 3. Mean (±SE) root mass, tips, forks, and area at two-true leaves, and main effects of year, growth stage, and cultivar. a
|
Category |
Cultivar |
Root mass |
Root tips |
Root forks |
Root area |
||||
|
|
|
2020 |
2021 |
2020 |
2021 |
2020 |
2021 |
2020 |
2021 |
|
|
|
--------- mg --------- |
------------------------- No. ------------------------- |
-------- cm2 -------- |
|||||
|
Large |
Bolero |
3±1 |
9±1 |
36±15 b |
85±28 |
66±44 b |
216±96 ab |
7±1 |
8±1 |
|
Large |
Red Core Chantenay |
2±1 |
7±1 |
20±10 c |
125±7 |
61±10 ab |
259±20 ab |
6±1 |
7±1 |
|
Large |
Yellowstone |
2±1 |
6±1 |
50±17 a |
81±16 |
108±54 a |
200±48 ab |
4±1 |
9±1 |
|
Average |
Dragon |
3±1 |
7±2 |
50±20 a |
110±25 |
146±64 a |
353±152 a |
5±1 |
7±1 |
|
Average |
Napoli |
3±1 |
8±1 |
55±15 a |
105±29 |
112±30 a |
352±113 a |
5±1 |
9±1 |
|
Average |
SFF |
4±1 |
9±2 |
45±8 ab |
65±11 |
98±26 ab |
149±10 b |
4±1 |
6±1 |
|
Small |
Mokum |
3±1 |
5±1 |
53±21 a |
60±7 |
81±37 ab |
123±12 b |
6±1 |
8±1 |
|
Small |
NB8483 |
2±1 |
5±1 |
31±8 bc |
55±9 |
34±10 c |
110±20 c |
4±1 |
6±1 |
|
Small |
NB8524 |
4±1 |
11±2 |
66±12 a |
98±3 |
168±51 a |
253±32 a |
5±1 |
7±1 |
|
|
|
|
|
|
|
|
|
|
|
|
Main Effects: |
|
|
|
|
|
|
|
|
|
|
Year |
2020 |
11±2 |
107±16 |
465±112 |
6±1 |
||||
|
|
2021 |
10±1 |
111±9 |
314±35 |
8±1 |
||||
|
|
|
|
|
|
|
|
|
|
|
|
Growth Stage |
|
|
|
|
|
|
|
|
|
|
|
1-leaf |
4±1 B |
40±2 C |
77±5 C |
5±1 B |
||||
|
|
2-leaf |
5±1 B |
70±5 B |
164±18 B |
6±1 B |
||||
|
|
4-leaf |
25±3 A |
247±22 A |
998±170 A |
8±1 A |
||||
|
|
|
|
|
|
|
|
|
|
|
|
Cultivar |
|
|
|
|
|
|
|
|
|
|
|
Bolero |
18±5 A |
160±33 A |
679±243 A |
7±1 A |
||||
|
|
Red Core Chantenay |
8±2 B |
130±49 A |
427±214 A |
7±1 A |
||||
|
|
Yellowstone |
6±1 B |
100±21 AB |
310±78 AB |
7±1 A |
||||
|
|
Dragon |
10±3 AB |
142±40 A |
594±274 A |
6±1 AB |
||||
|
|
Napoli |
9±1 B |
110±18 AB |
352±72 AB |
7±1 A |
||||
|
|
SFF |
10±3 AB |
53±10 C |
134±32 C |
5±1 B |
||||
|
|
Mokum |
12±3 AB |
122±28 AB |
472±220 A |
7±1 A |
||||
|
|
NB8483 |
8±2 B |
61±13 C |
140±50 C |
5±1 B |
||||
|
|
NB8524 |
11±2 AB |
94±17 B |
280±75 B |
6±1 AB |
||||
a Within column sections, means not followed by the same letter are significantly different (p<0.05).
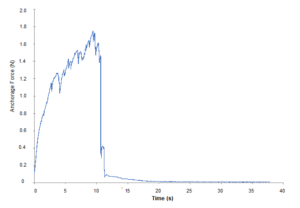
Figure 1. Anchorage force profile of a two-true leaf Bolero seedling as it is uprooted. Force tended to increase until primary roots broke; a sharp decline is then observed.
Figure 2. Root mass (a) and shoot mass (b) by carrot cultivar and growth stage. Data were pooled across years. Error bars represent standard error of the mean.
Figure 3. Number of root tips (a) and root forks (b) by carrot cultivar and growth stage. Data were pooled across years. Error bars represent standard error of the mean.
Figure 4. Shoot height (cm) and shoot area (cm2) by carrot cultivar at two-true leaves. Data were pooled across years. Error bars represent standard error of the mean. Different letters indicate significant differences in the main effect of cultivar at a=0.05.
Figure 5. Average anchorage force (N s-1) by carrot cultivar at two-true leaves. Data were pooled across years. Error bars represent standard error of the mean. Different letters indicate significant differences in the main effect of cultivar at a=0.05.
Objectives 3-5 (field)
Cultivar mortality from cultivation
Despite carrot cultivar differences in root branching, anchorage force, and shoot morphology at two-true leaves, and contrary to our hypothesis motivating this work, mortality from cultivation was not different between carrot cultivars in either year (Table 4, Table 5), suggesting that early growth characteristic differences at two-true leaves may not be as important for cultivation tolerance as previously thought. Carrot mortality from cultivation was affected by the interaction of year, cultivar, and weed control treatment, but this was largely driven by year-to-year variability (Table 5). Mortality from finger weeders was not different between years, averaging 9% (±1) and 10% (±1) across cultivars in 2020 and 2021, respectively (Table 5). Mortality from tine harrows was higher in 2020 compared to 2021. Carrot mortality averaged 21% (±2) and 9% (±1) across cultivars in 2020 and 2021, respectively (Table 5). Mortality from tine harrows was greater than finger weeders in 2020 but was similar between the two cultivation tools in 2021. In comparison, Hitchcock-Tilton (2017) observed an average mortality of 16% from finger weeders and 17% from flextine harrows; however, the author notes evidence that carrot mortality of 10% can result in yield losses of 5-9% due to carrot density loss. Crop mortality of 10% or less is generally acceptable, supporting the aggressiveness of the tools used in our study.
Weed control efficacy and selectivity
Intra-row weed control efficacy was different between cultivars, and also between years for certain cultivars and between years for weed control treatments (Table 4). Efficacy was higher in 2020 compared to 2021 for both finger weeders and tine harrows (Table 5), which may be attributed to the heavy rain following cultivation in 2021 that could have resulted in increased weed survival. Plots with Bolero, Yellowstone, and Dragon had comparable efficacy to each other, and higher efficacy compared to SFF, NB8483, and NB8524 (Table 5). This may be partially due to differences in cultivation timing, which would have affected weed size at cultivation. All cultivars were hand weeded at the cotyledon stage, but SFF, NB8483, and NB8524 were cultivated approximately one week later than the other cultivars due to their slower growth. While the timing of cultivation was appropriate for the carrot growth stage, weeds were likely larger, resulting in reduced efficacy. At the time of cultivation (two-true leaves), few differences in early growth characteristics were detected, which is a departure from Colquhoun et al. (2017) who observed quicker canopy development and larger shoots in Bolero compared to SFF. Factors other than crop competition due to morphological traits may influence efficacy, such as the present weed species and their growth traits; for example, weed species that have high leaf area or who grow by rhizomes.
Selectivity, calculated as percent crop survival divided by percent weed survival, showed a similar trend to efficacy, where selectivity was different between cultivars and cultivar interaction with year (Table 4). Bolero, Yellowstone, and Dragon had comparable selectivity to each other, and higher selectivity than SFF, NB8483, and NB8524 in both years (Table 5). Selectivity did not differ between finger weeders and tine harrows (p=0.201). Selectivity averaged 3.20 in 2020 and 2.25 in 2021 across Bolero, Yellowstone, and Dragon, showing a decrease in efficacy between years. Selectivity averaged 1.47 in 2020 and 1.68 in 2021 across SFF, NB8483, and NB8524. Cultivar traits that would confer greater tolerance to cultivation may not be larger roots and shoots, as shown by few differences in early growth characteristics despite differences in efficacy and selectivity. Examining different traits, such as root length and width, petiole angle, or embryo length may provide more insight into improving selectivity. Varying tool choice and aggressiveness may also help improve efficacy and selectivity given the lack of cultivar differences at the time of cultivation.
Hand-weeding time
Total time spent hand weeding was different between weed control treatments but unexpectedly was not different between cultivars despite differences in efficacy and selectivity (Table 4). Hand-weeding time was higher in 2021 compared to 2020 (Table 5), likely caused by heavy rainfall in 2021 that delayed hand weeding, resulting in larger weeds that took more time to remove. In both years, time to hand weed averaged 240 h ha-1 (±13) in the hand-weeded control and was higher than the finger weeder and tine harrow treatments, which was expected due to the number of hand weeding events that took place in the control treatment to keep it weed-free. Hand-weeding time was not different between the finger weeder and tine harrow treatments in both years (Table 5). The finger weeder and tine harrow treatments reduced hand-weeding time by 36% across years compared to hand-weeding only. Labor requirements for the hand-weeded control are consistent with those of Sørensen et al. (2005) and Van Der Weide et al. (2008), who reported average hand-weeding time for carrots to be 100-300 h ha-1, and tools like finger weeders or torsion weeders can reduce hand-weeding labor by 40-70% (Bleeker et al. 2002; Van Der Weide et al. 2008).
Cultivar yield
Marketable root fresh weight was different between cultivars in 2020 but not in 2021, and also higher across cultivars in 2020 compared to 2021 (Table 4, Table 6). Marketable shoot fresh weight was different across cultivars, but not years or weed control treatments (Table 4, Table 6). Root and shoot fresh weight ranked means did not show a direct relationship with cultivar category. This suggests that yield differences may be attributed to several factors other than larger roots and shoots, such as cultivar responses to fertilization, precipitation, and weed competition.
Due to the unexpected differences in marketable root yield between cultivars in 2020, we hypothesized that cultivars may show biomass losses to cultivation earlier in the season but grow out of this injury by harvest. To test this, we planted a second plot for each cultivar and weed control treatment in 2021 where we measured carrot root and shoot dry biomass 14 days post-cultivation. However, no differences between cultivars, weed control treatments, or their interaction were detected for either root or shoot biomass (data not shown). Root area and number of root tips and forks were positively correlated with carrot marketable yield, suggesting that these traits are important for early season growth so that yield will not suffer even if some root damage occurs during cultivation.
Table 4. Effects of year, cultivar, weed control treatment, and their interactions on carrot mortality, intra-row weed control efficacy, selectivity ratio, hand-weeding time, and carrot marketable yield.
|
Source |
Carrot Mortality |
Intra-row Weed Control Efficacy |
Selectivity Ratiob |
Hand- Weeding Time |
Marketable Root Wt. |
Marketable Shoot Wt. |
|
|
--------------------------------------------- p-value --------------------------------------------- |
|||||
|
Year (Y)a |
<0.001 |
0.556 |
0.526 |
0.053 |
0.594 |
0.958 |
|
Cultivar (C) |
0.995 |
<0.001 |
<0.001 |
0.352 |
<0.001 |
0.002 |
|
Weed Control (W) |
<0.001 |
0.856 |
0.201 |
<0.001 |
0.067 |
0.386 |
|
C x W |
0.287 |
0.710 |
0.117 |
0.493 |
0.322 |
0.987 |
|
Y x C |
0.983 |
<0.001 |
0.002 |
0.401 |
0.002 |
0.311 |
|
Y x W |
0.009 |
<0.001 |
0.303 |
0.388 |
0.137 |
0.669 |
|
Y x C x W |
0.005 |
0.593 |
0.159 |
0.182 |
0.484 |
0.849 |
a Year was included as a random effect.
b Calculated by dividing percent crop survival by percent weed survival.
Table 5. Mean (±SE) carrot mortality when cultivated at the two-true leaf stage, intra-row weed control efficacy, selectivity, hand-weeding time, and main effects of year, weed control tool, and cultivar. a
|
Category |
Cultivar |
Carrot Mortality (%) |
Intra-row Weed Control Efficacy (%) |
Selectivity Ratio b |
Hand-Weeding Time (h ha-1) |
||||||||||||||
|
|
|
Finger Weeder |
Tine Harrow |
Finger Weeder |
Tine Harrow |
Finger Weeder |
Tine Harrow |
Finger Weeder |
Tine Harrow |
||||||||||
|
|
|
2020 |
2021 |
2020 |
2021 |
2020 |
2021 |
2020 |
2021 |
2020 |
2021 |
2020 |
2021 |
2020 |
2021 |
2020 |
2021 |
||
|
Large |
Bolero |
4±2 |
10±3 |
17±4 |
7±3 |
65±6 |
64±2 |
72±9 |
55±8 |
2.98 |
2.52 |
3.75 |
2.25 |
109±4 |
137±20 |
104±6 |
183±65 |
||
|
Large |
Yellowstone |
11±4 |
8±1 |
36±5 |
6±1 |
78±2 |
59±4 |
78±1 |
51±6 |
4.34 |
2.34 |
2.85 |
2.00 |
97±5 |
157±13 |
125±17 |
177±13 |
||
|
Average |
Dragon |
14±3 |
14±5 |
20±3 |
13±4 |
62±8 |
63±4 |
69±3 |
55±6 |
2.61 |
2.32 |
2.70 |
2.08 |
111±5 |
178±37 |
104±16 |
188±9 |
||
|
Average |
SFF |
9±1 |
9±3 |
13±3 |
12±1 |
34±6 |
53±7 |
43±6 |
42±7 |
1.41 |
2.12 |
1.60 |
1.58 |
185±32 |
162±21 |
134±13 |
196±16 |
||
|
Small |
NB8483 |
7±2 |
10±2 |
18±2 |
10±4 |
31±5 |
40±6 |
40±4 |
41±5 |
1.37 |
1.58 |
1.38 |
1.56 |
141±5 |
222±30 |
127±11 |
240±28 |
||
|
Small |
NB8524 |
10±1 |
11±3 |
24±7 |
8±2 |
36±5 |
47±6 |
54±5 |
36±6 |
1.42 |
1.77 |
1.70 |
1.50 |
132±9 |
183±19 |
128±7 |
185±14 |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
Main Effects: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
Year |
2020 |
|
15±2 A |
|
|
55±3 |
|
|
2.34 |
|
|
125±5 B |
|
||||||
|
|
2021 |
|
10±1 B |
|
|
51±2 |
|
|
1.97 |
|
|
184±7 A |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
Weed Control |
Hand-Weeded |
|
- |
|
|
- |
|
|
- |
|
|
240±13 A |
|
||||||
|
|
Finger Weeder |
|
10±1 B |
|
|
53±3 |
|
|
2.23 |
|
|
151±7 B |
|
||||||
|
|
Tine Harrow |
|
15±2 A |
|
|
53±2 |
|
|
2.08 |
|
|
157±7 B |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
Cultivar |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
Bolero |
|
9±2 |
|
|
64±3 A |
|
|
2.88 A |
|
|
133±12 |
|
||||||
|
|
Yellowstone |
|
15±3 |
|
|
67±4 A |
|
|
2.89 A |
|
|
139±10 |
|
||||||
|
|
Dragon |
|
15±2 |
|
|
62±3 A |
|
|
2.42 A |
|
|
145±14 |
|
||||||
|
|
SFF |
|
11±1 |
|
|
43±4 B |
|
|
1.68 B |
|
|
169±11 |
|
||||||
|
|
NB8483 |
|
11±2 |
|
|
38±2 B |
|
|
1.47 B |
|
|
182±16 |
|
||||||
|
|
NB8524 |
|
13±2 |
|
|
43±3 B |
|
|
1.59 B |
|
|
157±9 |
|
||||||
a Within column sections, means not followed by the same letter are significantly different (p<0.05).
b Calculated by dividing percent crop survival by percent weed survival.
Table 6. Mean (±SE) carrot marketable root and shoot yield, and main effects of year, weed control tool, and cultivar. a
|
Category |
Cultivar |
Marketable Root Fresh Wt. |
Marketable Shoot Fresh Wt. |
||||||
|
|
|
Finger Weeder |
Tine Harrow |
Finger Weeder |
Tine Harrow |
||||
|
|
|
2020 |
2021 |
2020 |
2021 |
2020 |
2021 |
2020 |
2021 |
|
|
|
-------------------------------------- Mg ha-1 --------------------------------- |
|||||||
|
Large |
Bolero |
17±1 |
6±1 |
12±2 |
6±2 |
4±1 |
6±2 |
5±1 |
3±1 |
|
Large |
Yellowstone |
10±1 |
8±2 |
7±2 |
8±1 |
7±1 |
7±1 |
5±2 |
6±2 |
|
Average |
Dragon |
16±2 |
9±2 |
17±3 |
7±2 |
7±1 |
8±2 |
7±2 |
5±2 |
|
Average |
SFF |
8±3 |
9±1 |
10±1 |
7±1 |
2±1 |
5±2 |
2±1 |
4±1 |
|
Small |
NB8483 |
11±1 |
8±1 |
7±1 |
4±1 |
2±1 |
4±1 |
2±1 |
2±1 |
|
Small |
NB8524 |
4±1 |
7±2 |
3±1 |
5±1 |
2±1 |
4±2 |
2±1 |
4±2 |
|
|
|
|
|
|
|
|
|
|
|
|
Main Effects: |
|
|
|
|
|
|
|
|
|
|
Year |
2020 |
|
10±1 |
|
|
4±2 |
|
||
|
|
2021 |
|
8±1 |
|
|
5±1 |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
Weed Control |
|
|
|
|
|
|
|
|
|
|
|
Hand-Weeding |
|
9±1 |
|
|
5±1 |
|
||
|
|
Finger Weeder |
|
9±1 |
|
|
5±1 |
|
||
|
|
Tine Harrow |
|
8±1 |
|
|
4±1 |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
Cultivar |
|
|
|
|
|
|
|
|
|
|
|
Bolero |
|
10±1 A |
|
|
5±1 ABC |
|
||
|
|
Yellowstone |
|
11±1 A |
|
|
7±1 AB |
|
||
|
|
Dragon |
|
13±1 AB |
|
|
8±1 A |
|
||
|
|
SFF |
|
9±1 AB |
|
|
3±1 BC |
|
||
|
|
NB8483 |
|
8±1 AB |
|
|
3±1 BC |
|
||
|
|
NB8524 |
|
5±1 B |
|
|
2±1 C |
|
||
a Within column sections, means not followed by the same letter are significantly different (p<0.05).
For many of the early growth characteristics measured in this study, differences between cultivars were not detected until after the second true-leaf stage, and often these differences did not match predetermined cultivar categories and showed that high variability can exist both within cultivars and across years. Early growth characteristics that could aid in cultivation tolerance, thereby contributing to reduced mortality and greater selectivity, may not always be the biggest roots and shoots. For cultivation tools that primarily act by uprooting, breeding efforts could focus on selecting for cultivars that have smaller, but many roots (i.e., high degree of root branching) to increase crop anchorage force at earlier growth stages. For cultivation tools that primarily act by burial, selecting for cultivars with thicker shoots at earlier growth stages may aid in greater resistance to burial when carrots are still small. Selecting for traits at earlier growth stages may also permit cultivation prior to two-true leaves, which may aid in reduced hand-weeding labor. While carrot mortality was greater from tine harrows compared to finger weeders, unexpectedly, no differences between cultivars were detected. Contrary to this, cultivars in the “large” category, as well as Dragon, resulted in greater intra-row weed control efficacy and selectivity despite few differences in early growth characteristics that may have influenced crop competitive ability; however, this was likely due to differences in cultivation timing, which could affect cultivar selection. Total hand-weeding time was also surprisingly not different between cultivars, even though most early growth characteristic differences were not detected until later growth stages when additional post-cultivation hand weeding was performed. Differences in marketable yield were not consistent with predetermined cultivar growth categories and also varied by year, pointing to differences in cultivar responses to other factors, such as fertilization and environmental conditions. Future work could focus on exploiting cultivation tool selection rather than cultivar choice, selecting additional tools and tool settings to determine if cultivar cultivation tolerance can be improved in this manner.
Education & Outreach Activities and Participation Summary
Participation Summary:
NA
Project Outcomes
NA
During the duration of this project, my knowledge about carrot production and my skills in their production drastically changed. It is one thing to read literature and talk to farmers about their experiences with growing carrots; it is another thing to be the one in charge of every aspect of the production. I was in charge of everything from purchasing seed, to seedbed prep, planting, weeding, irrigating, and harvesting. I now have a much greater appreciation for the work that goes into growing carrots, and this further reinforces why this research is so important for organic farmers. While our results were unexpected, the information gained from this project was still important. It changed the way we think of “cultivation tolerance,” and will still be of use in plant breeding and farmer cultivar and tool selection.