Final report for GNE19-198
Project Information
Apple scab, caused by the fungus Venturia inaequalis, is one of the most destructive diseases of apple worldwide. Managing apple scab is especially challenging in the Northeastern United States due to the ideal climatic conditions for infection that can lead to major yield loss. Growers primarily rely on sanitation and fungicide applications to reduce apple scab in their orchards. Availability of alternative tools that can be incorporated into farmer’s Integrated Pest Management (IPM) program is crucial for farmers to mitigate risk of pathogen resistance to fungicides and reduce dependence on synthetic fungicides. One strategy of IPM is to harness beneficial microbes (biopesticides) and natural compounds to suppress disease. Chitosan is a promising natural compound documented to have antifungal and disease suppressive effects. Chitosan has been used successfully postharvest to prevent storage rot and extend shelf life of perishable fruits and vegetables. Preharvest chitosan application has been effective in vegetable crops but there is limited research evaluating preharvest application of chitosan on tree fruits. Chitosan also has the potential to enhance activity of microbial biopesticides — as a food source and to stimulate production of antimicrobial metabolites — but this potential is understudied.
The overall goal of this research was to evaluate chitosan alone and when combined with biopesticides for suppression of apple scab. Specific objectives were to (1) evaluate compatibility of bacterial-based biopesticides with commercial chitosan products in vitro (2) investigate synergisms between chitosan and microbial biopesticides for suppression of apple scab and (3) investigate the potential of chitosan to reduce overwintering inoculum of Venturia inaequalis in orchard leaf litter. To achieve our research objectives, lab experiments were conducted at the University of New Hampshire and field experiments were conducted at Penn State University’s Fruit Research and Extension Center (FREC), and at two New Hampshire (NH) commercial orchards.
For objective 1, two commercial chitosan products, ARMOUR Zen 15 and Tidal Grow 2%, were evaluated for their effect on the viability of Bacillus subtilis QST 713 (as formulated in Serenade ASO) in a simulated tank-mix experiment ARMOUR Zen 15 had no effect on the viability of B. subtilis QST 713 compared to the Serenade ASO control. However, the Tidal Grow products (0.01% chitosan, 0.01% chitosan with pH adjusted to 5, and 0.0025% chitosan) did negatively affect B. subtilis QST 713 viability.
For objective 2, a 3-year research trial was conducted at FREC and 1-year trial was conducted at two NH commercial orchards. For FREC, treatments included a commercial chitosan product, Tidal Grow, alone and in combination with the microbial biopesticide, Serenade ASO. These treatments were compared to a water control and a grower standard to determine their efficacy in reducing apple scab on leaves and fruit. At FREC, chitosan + biopesticide and the chitosan alone treatments reduced severity and incidence of apple scab on leaves and fruit; however, these effects were inconsistent from year to year. When the chitosan + biopesticide treatment was combined with sulfur, apple scab severity on harvested fruit was reduced by 83% compared to the water control and was not different from the grower standard. When the chitosan rate was increased from 0.0025% to 0.01%, apple scab severity on harvested fruit was reduced by 54% compared to the water control. However, this was not comparable to the grower standard, which reduced apple scab severity by 99% compared to the water control. For the NH trials, treatments included a commercial chitosan product, ARMOUR Zen, alone and in combination with Serenade ASO overlayed onto a grower standard treatment. For one of the NH sites, severity of foliar apple scab was 50-70% less in the chitosan + biopesticide + grower standard treatment compared to the grower standard control. Additionally, this treatment reduced apple scab incidence on immature fruit by 60-95% compared to the grower standard. This effect was only observed on the cultivar Macoun and not on the cultivar McIntosh.
For objective 3, the urea treatment was the most effective at reducing ascospore discharge. The chitosan treatment delayed the discharge of ascospores in the first few weeks but did not reduce the overall amount of ascospores compared to the urea treatment.
This research provides important data for examining the role of chitosan in apple disease management. Results from this research suggest that chitosan product, rate, and disease severity can influence efficacy. Our on-farm trial suggests that a combination of ARMOUR Zen and Serenade ASO combined with standard grower practices, can reduce disease more than the grower standard alone. Future research should focus on best practices for the application of chitosan within an IPM program.
This research was disseminated to growers through multiple presentations, webinars, handouts, and twilight meetings. Growers expressed interest in the continuation of this research at UNH and other New England universities to provide them with innovations that improve environmental stewardship, profitability, and aid in creating a sustainable production system on New England apple farms.
The overall goal of this research was to evaluate chitosan alone and combined with biopesticides for suppression of apple scab. Reaching this goal will help farmers to reduce losses, decrease reliance on fungicides, and improve overall yield. The specific objectives are to;
1. Evaluate compatibility of bacterial-based biopesticides with commercial chitosan products in vitro.
2. Investigate synergisms between chitosan and microbial biopesticides for suppression of apple scab.
3. Investigate the potential for chitosan to reduce overwintering inoculum of Venturia inaequalis in orchard leaf litter.
Apple scab, caused by the fungus Venturia inaequalis (Cke.) Wint., is the most destructive disease of apples in the Northeast U.S. where warm, moist conditions can cause severe infections and devastating crop losses to farmers (MacHardy, 1996). Apple scab can cause up to 100% crop loss and significant reduction in fruit marketability due to consumers’ low threshold for imperfection on their apples (Vaillancourt and Hartman, 2000). Apple growers rely on multiple fungicides applications per season to manage apple scab (MacHardy, 1996). The development of fungicide resistance (the ability to survive when exposed to a chemical) by V. inaequalis is of large concern and several classes of fungicides, such as benzimidazole and demethylation inhibitors, have already lost their effectiveness (Holb, 2009; Ma and Michailides, 2005). Thus, alternative practices that can be incorporated with sanitation, fungicides, and other IPM strategies are critical to manage apple scab in New England.
One way that farmers are reducing their use of chemical pesticides is by utilizing naturally occurring compounds that can promote plant health and suppress disease. One strategy is to use beneficial microbes (biopesticides) to suppress disease. Many growers have incorporated biopesticides into their spray programs with mixed success. These inconsistencies in performance remain a barrier to broad adoption. However, biopesticide may be more effective at reducing disease through synergisms with natural compounds. A promising natural compound, chitosan, a deacetylated derivative of chitin, has been documented to have antifungal properties and promote plant growth (Zhang et al., 2011). Other benefits include enhanced photosynthesis, resistance to abiotic and biotic stress, and increased plant growth and yield (Reddy et al., 2000). Chitosan is an approved food additive in the U.S. and has been used effectively as a postharvest application to prevent disease and extend shelf life of perishable fruits and vegetables (Pichyangkura and Chadchawan, 2015; Romanazzi et al., 2018). There are limited examples of research evaluating preharvest application of chitosan on tree fruits. However, success in vegetable crops to promote plant health and suppress disease suggests that chitosan may have broader applications in agriculture (El-ghaouth et al., 2000).
Most fungi, including V. inaequalis, contain cell walls composed of chitin (Jerome, 1965). Chitinase is an enzyme that denatures cell walls, effectively degrading the fungi (Boller, 1993). El-Ghaoth et al. (1994) found that chitosan amended soil controlled Pythium root rot on cucumbers and triggered the production of chitinase and other anti-fungal compounds by the host. An application of chitosan acts as a microbial food source and can stimulate production of chitinase enzymes. Researchers have investigated potential synergisms between beneficial microbes and natural products and the implications for disease management. For example, Kokalis-burelle et al. (Kokalis-burelle et al., 1992) reported a 60% reduction in early leafspot of peanut when plants were treated with Bacillus cereus and chitin. It was hypothesized that the chitin stimulated production of anti-fungal enzymes and helped the beneficial microbe persist long enough to compete with the pathogen. These studies suggest that finding synergisms between biopesticides and chitosan has great potential to improve biopesticide efficacy in suppressing diseases. Although there is significant research on chitosan in the literature documenting its potential (Zhang et al., 2011), research is needed to bring best practices to growers and facilitate adoption. The overall goal of this research was to improve biopesticide efficacy in suppressing apple scab in northeastern orchards through finding synergism with the natural product, chitosan. Reaching this goal will help farmers to reduce losses, decrease reliance on fungicides, and improve overall yields.
References:
Boller, T., 1993. Antimicrobial functions of the plant hydrolases, chitinases and 1,3 glucanases, in: Fritig, B., Legrand, M. (Eds.), Mechanisms of Plant Defense Responses. Kluwer Academic Press, Dordrecht, Netherlands, pp. 391–400.
El-Ghaouth, A., Arul, J., Grenier, J., Benhamou, N., Asselin, A., Belanger, R.R., 1994. Effect of chitosan on cucumber plants: Suppression of Pythium aphanidermatun and induction of defense reactions. Phytopathology 84, 313–320.
El-Ghaouth, A., Smilanick, J.L., Wilson, C.L., 2000. Enhancement of the performance of Candida saitoana by the addition of glycolchitosan for the control of postharvest decay of apple and citrus fruit. Post 19, 103–110.
Holb, I.J., 2009. Fungal Disease Management in Environmentally Friendly Apple Production – A Review. Sustain Agric. Rev. 2, 219–293.
Jerome, M., 1965. The cell wall, in: Ainsworth, G., Sussman, A. (Eds.), The Fungi: An Advanced Treatise. Academic Press, New York, pp. 49–76.
Kokalis-burelle, N., Backman, P.A., Rodriguez-kabana, R., Ploper, L.D., 1992. Potential for Biological Control of Early Leafspot of Peanut Using Bacillus cereus and Chitin as Foliar Amendments ’. Biol. Control 2, 321–328.
Ma, Z., Michailides, T.J., 2005. Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop Prot. 24, 853–863. https://doi.org/10.1016/j.cropro.2005.01.011
MacHardy, W.E., 1996. Apple Scab: Biology, Epidemilogy and Management. American Phytopathological Society Press, St. Paul, MN.
Pichyangkura, R., Chadchawan, S., 2015. Biostimulant activity of chitosan in horticulture. Sci. Hortic. (Amsterdam). 196, 49–65. https://doi.org/10.1016/j.scienta.2015.09.031
Reddy, M.V.B., Belkacemi, K., Corcuff, R., Arul, J., 2000. Effect of pre-harvest chitosan sprays on post-harvest infection by Botrytis cinerea and quality of strawberry fruit. Postharvest Biol. Technol. 20, 39–51.
Romanazzi, G., Feliziani, E., Sivakumar, D., 2018. Chitosan, a Biopolymer With Triple Action on Postharvest Decay of Fruit and Vegetables: Eliciting, Antimicrobial and Film-Forming Properties. Front. Microbiol. 9, 1–9. https://doi.org/10.3389/fmicb.2018.02745
Vaillancourt, L.J., Hartman, J.R., 2000. Apple scab. Plant Heal. Instr. https://doi.org/10.1094/PHI-I-2000-1005-01
Zhang, H., Li, R., Liu, W., 2011. Effects of Chitin and Its Derivative Chitosan on Postharvest Decay of Fruits: A Review. Int. J. Mol. Sci. 12, 917–934. https://doi.org/10.3390/ijms12020917
Cooperators
- (Educator and Researcher)
- (Educator and Researcher)
- (Educator and Researcher)
- (Researcher)
Research
Products:
Commercial chitosan products ARMOUR-Zen (15% chitosan) was obtained from Botry-Zen Ltd (Dunedin, New Zealand) and Tidal Grow (high molecular weight 2%) was obtained from Tidal Vision Inc. (Bellingham, WA). The biopesticide Serenade ASO, active ingredient Bacillus subtilis strain QST 713, was obtained from Bayer AG (Leverkusen, Germany).
Objective 1: Evaluate compatibility of bacterial-based biopesticides with commercial chitosan products in vitro.
An in-vitro experiment was conducted to simulate tank-mixing of products and to evaluate the effect of commercial chitosan on viability of the bacterial-based biocontrol agent. The two commercial chitosan products (Tidal Grow and ARMOUR-Zen) and one biopesticide (Serenade ASO) were tested in this experiment. To simulate tank-mixing products, each chitosan product was mixed (through inversion) separately with Serenade ASO and sterile reverse osmosis (RO) water (Table 1). A positive control of Serenade ASO and sterile RO water was also prepared (Table 1). These dilutions are equivalent to rates of application applied during trials at FREC and at the NH on-farm trials. Additionally, a treatment was included in which Tidal Grow’s pH was adjusted from ~3.5 to ~5.0 (using NaOH).
Twenty-five µl of the undiluted solution and ten 10-fold dilutions were plated in triplicate on tryptic soy agar in petri dishes (60 mm x 15 mm). Plates were incubated at 20°C for 48 h and enumerated for colony forming units (CFUs). This experiment was replicated three times. Data was analyzed with an analysis of variance (ANOVA) using partial (type II) sums of squares (‘car’ package) in Rstudio. Post hoc Dunnett’s tests were conducted using treatment means to determine if there is a significant difference in Log CFU/mL between the Serenade ASO control plates and the mixtures of chitosan and Serenade ASO.
Table 1. Treatment mixture rates for in-vitro assay to evaluate compatibility of products.
|
Treatment |
Serenade ASO |
Chitosan |
Water |
Percent Chitosan |
|
Serenade ASO Control |
10 μl |
- |
990 μl |
- |
|
FREC 2021 Rate |
10 μl |
1.25 μl Tidal Grow |
988.75 μl |
0.0025% |
|
FREC 2022 Rate |
10 μl |
10 μl Tidal Grow |
980 μl |
0.01% |
|
FREC 2022 Rate - pH adjusted |
10 μl |
10 μl Tidal Grow – pH adjusted to 5 |
980 μl |
0.01% |
|
NH on-farm Rate |
10 μl |
10 μl ARMOUR-Zen 15 |
980 μl |
0.15% |
Objective 2: Investigate synergisms between chitosan and microbial biopesticides for suppression of apple scab.
Controlled environment evaluations.
Research has shown that chitosan efficacy is influenced by dose (% chitosan). However, higher doses can cause phytotoxicity. To determine a dose range for growth room and field trials, preliminary experiments were conducted to determine doses that can be applied to apple leaves without causing tissue damage. These experiments were conducted in a growth room using 'Macintosh' and ‘Golden Delicious’ seeds. Surface sterilized seeds were stratified in sterile sand at 4°C for 3 months. When germinated, the seedlings were transplanted into Ray Leach cone-tainers filled with peat (ProMix Bx) and placed in a walk-in growth room (22°C, 75% relative humidity, and a 12-hour photoperiod). While these seeds are genetically different due to pollination, this is an inexpensive way to develop methods prior to using grafted apple trees.
This experiment consisted of 7 treatments (Table 2) applied to 7 replicated plants. Apple seedlings were arranged in a completely randomized design. Once the apple seedlings had 6 true leaves, the chitosan treatments were sprayed onto plants until glisten. Plant height and SPAD measurements (leaf chlorophyll content) were measured prior to the chitosan application (day 0), 8 days, and 16 days post application to determine the effect of chitosan concentration on plant growth and photosynthesis.
Table 2. Chitosan treatments applied to apple seedlings in preliminary experiments to optimize chitosan dose.
|
Treatment |
Application |
|
1 |
Chitosan 1% |
|
2 |
Chitosan 0.5% |
|
3 |
Chitosan 0.25% |
|
4 |
Chitosan 0.01% |
|
5 |
Acetic Acid 1% |
|
6 |
Milli Q Control |
|
7 |
High Tide (0.5%) |
Field evaluation of disease suppression – Research Orchard.
Chitosan and microbial biopesticide treatments were evaluated at FREC in a 0.8-acre research block on semi-dwarf apple cv. ‘Law Rome’ grafted on M.7 rootstock plant in 2015 over three seasons (2020-2021). In the 2020 season, treatments (Table 3) were applied to 4 replicate trees arranged in a randomized complete block design. Treatments were applied using a boom sprayer at 400 psi, delivering 100 gallon per acre. Treatment applications were made on 7-15 days intervals starting when trees reached tight cluster (mid-April).
Table 2. Treatment sprays for Penn State’s FREC Research Orchard, 2020
|
Treatment |
Product |
Rate per Acre |
|
Water Control |
Water Spray |
-- |
|
Grower Standard |
Manzate Pro-Stick Captan Gold |
3 lb 3 lb |
|
Grower Standard + Chitosan |
Manzate Pro-Stick Captan Gold Tidal Grow |
3 lb 3 lb 150 mL* |
|
Reduced Risk |
Micothiol Disperss 10 lb (or 5lb) Regalia Nu-film P |
10 lb (or 5lb) 2 qt 8 fl oz |
|
Reduced Risk + Chitosan |
Microthiol Disperss Regalia Nu-film P Tidal Vision |
10 lb (or 5 lb) 2 qt 8 fl oz 150 mL* |
*Label rate for Tidal Grow 2% on fruit trees in 2020
Trees were assessed in mid-June for foliar apple scab incidence. Disease incidence was determined by randomly selecting 10 terminal shoots per tree and counting the number of leaves with apple scab lesions. For apple scab severity, the number of scab lesions were counted on each leaf. Additionally, twenty-five apples per treatment per rep were evaluated for apple scab incidence.
In October, twenty-five fruit per tree per treatment were evaluated for apple scab incidence and severity. Apple scab incidence was measured by counting the number of fruits with apple scab and apple scab severity was measured by counting the number of lesions per fruit.
In the 2021 season, treatments (Table 4) were applied to 6 replicate trees arranged in a randomized complete block design. Treatments were applied using a boom sprayer at 400 psi, delivering 100 gallon per acre. Treatment applications were made on 7-15 days intervals starting when trees reached tight cluster (mid-April).
Table 4. Treatment sprays for Penn State’s FREC Research Orchard, Season 2021
|
Treatment |
Product |
Rate per Acre |
|
Water Control |
Water Spray |
-- |
|
Grower Standard |
Manzate Pro-Stick Captan Gold Luna Sensation Inspire Supre LI 700 |
3 lb 2.5 lb 5 fl oz 12 fl oz 1 pt |
|
Chitosan |
Tidal Grow 2% |
473 mL |
|
Reduced Risk |
Micothiol Disperss Serenade ASO |
10 lb 4 qt |
|
Reduced Risk + Chitosan |
Microthiol Disperss Serenade ASO Tidal Vision 2% |
10 lb 4 qt 473 mL |
Trees were assessed in mid-June for foliar and fruit apple scab incidence. Disease incidence was determined as described for 2020. In October, twenty-five fruit per tree per treatment were evaluated for apple scab incidence and severity using a 0-to-6 rating scale as described by Poleatewich et al. (2012) (Figure 1).
Figure 1. Example apples for the 0-6 ratings of apple scab severity as described in Poleatewich et al. (2012). Image created by L. DeGenring.
In the 2022 season, treatments (Table 5) were applied to 5 replicate trees arranged in a randomized complete block design. Treatments were applied using a boom sprayer at 400 psi, delivering 100 gallon per acre. Treatment applications were made on 7-15 days intervals starting when trees reached tight cluster (mid-April).
Table 5. Treatment sprays for Penn State’s FREC Research Orchard, Season 2022
|
Treatment |
Product |
Rate per Acre |
|
Water Control |
Water Spray |
-- |
|
Grower Standard |
Manzate Pro-Stick Captan Gold Inspire Supre Miravis |
3 lb 2.5 lb 12 fl oz 3.42 fl oz |
|
Chitosan |
Tidal Grow 2% |
1893 mL |
|
Reduced Risk |
Serenade ASO |
4 qt |
|
Reduced Risk + Chitosan |
Serenade ASO Tidal Vision 2% |
4 qt 1893 mL |
Trees were assessed in mid-June for foliar and fruit apple scab incidence. Disease incidence was determined as described for 2020. In October, twenty-five fruit per tree per treatment were evaluated for apple scab incidence and severity as described for 2021.
For all three years, disease severity and incidence on leaves and fruit and fruit quality were analyzed for statistical significance using ANOVA in R studio. Statistical significance was assessed at p < 0.05 and a Tukey HSD Post-hoc test was used to separate the means.
Field evaluation of disease suppression – NH On-farm trials
Two experiments were conducted in 2022 on two commercial orchards in NH. These farms had a history of apple scab (personal communication with farmer) and thus this research relied on natural inoculum. The NEWA’s apple scab models were used to collect weather data and to predict infection periods and inoculum load using the weather station located at each farm.
On NH on-farm #1, this trial was conducted on a commercial farm in a 3.0-acre orchard with semi-dwarf cultivars ‘McIntosh’ and ‘Macoun’. Treatments (Table 6) were applied to 7 replicate ‘McIntosh’ trees and 8 replicate ‘Macoun’ trees arranged in a randomized complete block design. The grower standard treatment was applied using the grower’s equipment. The grower standard + chitosan and grower standard + chitosan + biopesticide treatments were applied using two Dramm backpack BP-4Li sprayers at 150 psi, delivering 24 gallon per acre. Treatment applications were made on ~10 day intervals starting on 5-May for primary V. inaequalis infection and ~30 day intervals for secondary infection. The final treatment application was made on 8-September at harvest.
Trees were assessed for leaf and immature fruit disease incidence bi-weekly starting on 7-June and 22-June (respectively), at symptom onset as described for the FREC trials. In September, twenty-five fruit per tree per treatment were evaluated for apple scab incidence and severity as described for the FREC trials.
On NH on-farm #2, this trial was conducted on a commercial farm in a 0.9-acre orchard with semi-dwarf cultivars ‘Kingston Black’, ‘Dabinett’, and ‘Wickson’. Treatments (Table 6) were applied to 4 replicate trees arranged in a randomized complete block design. The grower standard treatment was applied using the grower’s equipment. The grower standard + chitosan and grower standard + chitosan + biopesticide treatments were applied using two Dramm backpack BP-4Li sprayers at 150 psi, delivering 24 gallon per acre. Treatment applications were made on ~10 day intervals starting on 6-May for primary V. inaequalis infection and ~30 day intervals for secondary infection. The final treatment application was made on 4-October at harvest.
Trees were assessed for leaf and immature fruit disease incidence bi-weekly starting on 10-June and 19-June (respectively), at symptom onset as described for the FREC trials. In October, twenty-five fruit per tree per treatment were evaluated for apple scab incidence and severity as described for the FREC trials.
For both NH on-farm trials, disease severity on leaves and fruit and fruit quality were analyzed for statistical significance using ANOVA in R studio. Statistical significance was assessed at p < 0.05 and a Tukey HSD Post-hoc test was used to separate the means.
Table 6. Treatment sprays for NH On-farm trials, Season 2022
|
Treatment |
Product |
Rate per Acre |
|
Grower Standard |
Varied* |
-- |
|
Grower Standard + Chitosan |
Varied* Armour Zen 15% |
-- 4 qts |
|
Grower Standard + Chitosan + Biopesticide |
Varied* Armour Zen 15% Serenade ASO |
-- 4 qts 4 qts |
*Full spray records for each on-farm trial can be requested from Liza DeGenring.
Objective 3: Investigate the potential for chitosan to reduce viability of overwintering spores of Venturia inaequalis in leaf litter.
The apple scab pathogen, V. inaequalis survives from year-to-year on infected leaves that have fallen to the ground as leaf litter. New spores (ascospores) are released in the spring during periods of rain and high humidity. An important component of apple scab management is to reduce the amount of primary inoculum (ascospores) in an orchard (MacHardy, 1996). In this study we will investigate the effect of chitosan applications to the leaf litter on potential ascospore dose (PAD) and spore viability. These treatments will be compared to an application of urea, which is a common strategy to decrease primary inoculum of apple scab.
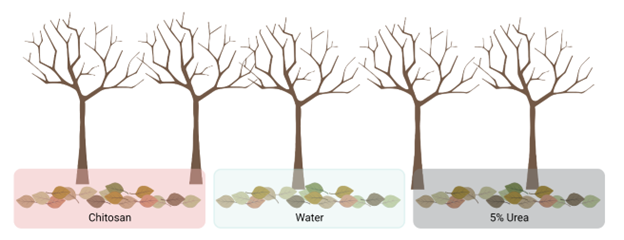
Field study to determine the potential of chitosan to reduce spore production under natural conditions. These field trials were performed at Penn State’s FREC in the winter of 2021 and 2022. Apple leaves infected with V. inaequalis were collected from the research orchard at the end of each season. The collected leaves were placed into 9 replicate sachets per treatment and then sprayed with the treatments. This experiment consisted of three treatments (Tidal Grow, 5% urea solution (42 lb/gallon), and a water control) (Figure 2). For winter 2020/2021 trials a rate of 3 mL per gallon was used for Tidal Grow. For the 2020/2021 trials a rate of 30 mL per gallon was used for Tidal Grow. After treatment application, leaves were overwintered fixed to the research orchard floor. Starting at the end of March, three sachets per treatment were analyzed for ascospore production weekly. Sachets were soaked in sterile water for 1 minute to induce spore release. The sachets were then placed on a vacuum to pull the spores onto a microscope cover slide. The cover slide was placed onto a slide bottom with a drop of Lactophenol (blue dye). The number of ascospores were counted under a compound microscope. For each sachet, the area under the disease progress curve was calculated and analyzed using a One-Way ANOVA in R studio. Statistical significance was assessed at p < 0.05 and a Tukey HSD Post hoc test was used to separate the means.
Figure 2. Overwintering trials at PSU FREC. Treatments were applied to sachets filled with scabbed leaves and overwintered on the orchard floor. Image created by L. DeGenring in Biorender.
References:
MacHardy, W.E., 1996. Apple Scab: Biology, Epidemilogy and Management. American Phytopathological Society Press, St. Paul, MN.
Poleatewich, A.M., Ngugi, H.K., Backman, P.A., 2012. Assessment of Application Timing of Bacillus spp. to Suppress Pre- and Postharvest Diseases of Apple. Plant Dis. 96, 211–220. https://doi.org/10.1094/PDIS-05-11-0383
Objective 1: Evaluate compatibility of bacterial-based biopesticides with commercial chitosan products in vitro.
ARMOUR Zen prepared at 0.15% chitosan (v/v) did not inhibit or enhance the viability (expressed as CFU/mL) of B. subtilis QST 713 in the simulated tank-mix when plated at 106 dilution (p = 0.9877). Tidal Grow at 0.0025% chitosan (v/v) and Tidal Grow at 0.01% chitosan (v/v) slightly reduced the viability of B. subtilis in the simulated tank-mix at 106 dilution (p ≤ 0.0203). We included a pH adjusted treatment to determine if the low pH of Tidal Grow (~3.5) could inhibit B. subtilis QST 713 viability. The pH adjusted to ~5.0 Tidal Grow 0.01% reduced the viability of B. subtilis in the simulated tank-mix at 106 dilution (p = 0.0065). Results from this experiment suggest that some chitosan products are compatible with Serenade ASO but some may reduce viability. Additional research is needed to determine how reduction in B. subtilis QST 713 viability influences efficacy.
Objective 2: Investigate synergisms between chitosan and microbial biopesticides for suppression of apple scab in laboratory and on-farm studies.
Controlled environment evaluations
In the preliminary growth room experiments, the apple seedlings did not exhibit phytotoxicity after being exposed to the chitosan treatments up to a concentration of 1% (w/v). Chitosan treatments did not have an effect on plant height or SPAD. These results suggest that chitosan, applied at the rates tested, does not cause phytotoxicity on apple leaves or fruit (as opposed to other plants that do not have a waxy leaf (DeGenring et al., 2022)).
Field evaluations of disease suppression – Research Trials
In 2020, all treatments significantly reduced apple scab incidence on leaves (p < 0.001) and fruit (p < 0.005) compared to the water control. However, there was no difference between the reduced risk + chitosan and reduced risk without chitosan. We did not include a standalone chitosan treatment in 2020. Thus, the reduction in apple scab compared to the water control could have been solely due to the efficacy of the reduced risk treatments. Further, the Tidal Grow label rate of 150 mL per acre equals a chitosan dose of 0.000798% (v/v). Based on results published in the literature and our own observations, it is unlikely that this dose is effective. The research trial conducted at Penn State’s FREC during the 2020 season gave us insight on how to improve our experimental design for the 2021 season. We decided to increase the rate in the 2021 trials to 0.0025% chitosan. Finally, for the 2021 trials, we decided to change the biopesticide from Regalia to Serenade ASO. This is because Serenade ASO’s active ingredient is Bacillus subtilis QST 713, which is more likely to have a synergistic effect with chitosan than Regalia, which is a plant-extract based bioproduct (Benhamou and Theriault, 1992; Kokalis-burelle et al., 1992).
In 2021, the reduced risk and the reduced risk + chitosan treatments reduced incidence of apple scab on leaves, immature fruit, and harvested fruit compared to the water control (Figure 3). On leaves, apple scab incidence was reduced by 50% and 53% for the reduced risk and reduced risk + chitosan treatments respectively compared to the water control (p ≤ 0.0253). On immature fruit, apple scab incidence was reduced by 100% and 82% for the reduced risk and reduced risk + chitosan treatments respectively compared to the water control (p ≤ 0.05). For harvested fruit, apple scab incidence was reduced by 88% and 80% for the reduced risk and reduced risk + chitosan treatments respectively compared to the water control (p < 0.001). Additionally, reduced risk and reduced risk + chitosan reduced number of apple scab lesions by 96% and 90% respectively (p ≤ 0.0015) and apple scab severity by 92% and 83% respectively (p ≤ 0.001) on harvested apples compared to the water control. Furthermore, reduction in disease on reduced risk + chitosan treated trees was statistically equivalent to the reduction seen in the grower standard treatments. This suggests that the reduced risk and reduced risk + chitosan treatments are as effective as the grower standard at reducing apple scab incidence.
Figure 3. Example harvested apples for each treatment from the 2021 FREC research trials.
While chitosan alone did not significantly reduce incidence of apple scab, the chitosan treatment did significantly reduce number of apple scab lesions on harvested fruit by 61% compared to the water control (p = 0.0189). All harvested apples scored low on the apple scab severity rating with the average rating between 0-1. While not significant, chitosan did reduce apple scab severity by 40% on harvested fruit compared to the control.
Based on our findings in 2021, adjustments were made to the experimental design for the 2022 season. The rate of chitosan was increased to 0.01%. Additionally, the sulfur component of the reduced risk treatment was removed to better determine the effects of chitosan on efficacy of Serenade ASO.
In 2022, apple scab incidence in June was low (0-10%) and there were no significant differences between treatments for leaf incidence (p > 0.213). On immature fruit (p = 0.0213) and harvested fruit (p < 0.001), only the grower standard treatment significantly reduced apple scab incidence compared to the control. On harvested fruit, the apple scab incidence and severity were also low (0-16% for apple scab incidence and 0-1.5 for apple scab severity rating). This could have been due to the increased presence of marssonina leaf blight (Marssonina brunnea) on the apple trees at FREC. M. brunnea could have competed with V. inaequalis, reducing the severity of apple scab. Chitosan, although not significant, reduced apple scab incidence and apple scab severity on harvested fruit by 46% and 54% respectively compared to the water control. While this reduction is promising, it is not comparable to the grower standard that reduced apple scab incidence and severity on harvested fruit by 97% and 99% compared to the water control.
Surprisingly, the reduced risk treatment was not as effective in 2022 as it was in 2021. The reduced risk treatment reduced apple scab incidence and severity on harvested fruit by 16% and 41% respectively compared to the water control. Additionally, the reduced risk + chitosan treatment was not effective. The lack of apple scab control in 2022, may be related to low disease pressure in the orchard.
Overall, apple scab severity is a better indicator of efficacy for biopesticides and natural products, which often work at reducing the size of the lesions but not completely eliminating disease. Results from the field trials suggest that chitosan can reduce severity of apple scab, however, inconsistent results from year to year make it difficult to draw conclusions at this stage.
Field evaluations of disease suppression – NH On-Farm Trials
For NH on-farm #1, the grower standard + chitosan + biopesticide was the most effective at reducing disease on cv Macoun and there was a reduction of 50-70% apple scab severity on leaves compared to the grower standard treatment. The grower standard + chitosan treatment reduced disease but only by 35-45% compared to the grower standard. For cv McIntosh, there was no effect of treatment on apple scab severity on leaves. Interestingly, both cv Macoun and McIntosh are highly susceptible to apple scab (Beckerman, 2006), however, cv McIntosh had overall higher apple scab severity on leaves (60-70% more than cv Macoun). It is possible that greater apple scab severity on McIntosh leaves, prevented the chitosan and the chitosan + biopesticide treatments from being as effective as observed on Macoun.
On immature fruit (classified as fruit on the tree that is not ready for harvest), grower standard + chitosan + biopesticide reduced apple scab incidence by 60-95% compared to the grower standard on cv Macoun over the course of the season. There was no effect of the grower standard + chitosan treatment. Additionally, on cv McIntosh, there was no effect of treatment on apple scab incidence on immature fruit.
While not significant, the grower standard + chitosan + biopesticide treatment on harvested McIntosh fruit reduced apple scab incidence by 35% compared to the grower standard treatment (p = 0.0879). Additionally, on McIntosh fruit, the grower standard + chitosan + biopesticide treatment reduced apple scab severity by 49%, although this was not significant (p = 0.1786). The lack of significance could be due to low apple scab severity score (0-1) because determining statistical significance is harder at smaller ranges.
Throughout this data, there is a trend that the chitosan + biopesticide combined treatment overlayed onto the grower standard is effective at reducing disease.
For NH on-farm #2, there was no apple scab present in the orchard. The farmer had suggested that this area of the orchard would have apple scab disease on the fruit but either there was not enough inoculum in the orchard, the grower standard was too effective, or the cultivars were too resistant. While great for the farmer, this resulted in no apple scab data. However, we were able to collect data on other fungal diseases, such as powdery mildew, frog eye, and marssonina leaf blight. This data will not be included in this reported but will be included in the journal article published with the apple scab data.
Objective 3: Investigate the potential for chitosan to reduce viability of overwintering spores of Venturia inaequalis in leaf litter.
In 2020/2021 overwintering experiment there was no significant difference in number of ascospores on leaves treated with chitosan application compared to the water control. The urea treatment resulted in significantly lower ascospore production than the chitosan and water treatments. However, the rate used for the chitosan application was the label rate of Tidal Grow of 3 mL per gallon which is 0.0015% chitosan. We hypothesize that this concentration is too low to have an effect. We decided that the rate needed to be higher for the 2021/2022 winter trials. Although the results were not what we expected, as the chitosan industry is still new and developing, narrowing in on the proper rates is crucial.
In 2021/2022 overwintering experiment, chitosan (Tidal Grow) delayed the discharge of ascospores in the first few weeks of spring but did not reduce total ascospore production compared to urea. Tidal Grow application to the overwintering leaf litter could still be beneficial as the delay in spore production could allow farmers time to get their first protective spray on in the early spring. During this season, ascospore production was much lower than the previous year (the highest ascospore count in 2020/2021 season we 13200 where in 2021/2022 it was only 2000. This low ascospore production could be influencing our results. Future replications of this trial should be done during both high and low ascospore production years to determine if efficacy varies with changes in disease pressure. Additionally, testing other commercial products, such as ARMOUR Zen, would give a better picture of how chitosan products compare to urea with respect to reducing ascospore production in the orchard.
References:
Beckerman, J. 2006. Disease susceptibility of common apple cultivars. Purdue University Extension. Fruit Diseases, BP-132-W.
Benhamou, N., Theriault, G., 1992. Treatment with chitosan enhances resistance of tomato plants to the crown and root rot pathogen Fusarium oxysporum f. sp. radicis-lycopersici. Physiol. Mol. Plant Pathol. 41, 33–52.
DeGenring, L.M., Dickson, R.W., Poleatewich, A.M., 2022. Inhibition of Botrytis cinerea growth and suppression of gray mold on petunia leaves using chitosan. Plant Dis. https:// 10.1094/PDIS-07-22-1628-RE
Kokalis-burelle, N., Backman, P.A., Rodriguez-kabana, R., Ploper, L.D., 1992. Potential for Biological Control of Early Leafspot of Peanut Using Bacillus cereus and Chitin as Foliar Amendments ’. Biol. Control 2, 321–328.
Overall, commercial chitosan products may be a viable option for apple scab management, but more research is needed. Our research examining the role of chitosan concentration and application rate in reducing diseases has highlighted that there is still more to be done in optimizing these commercial products.
Additionally, while these products can be effective, it is likely that they will be most effective as part of an IPM strategy. In this regard, chitosan products could be used in rotation with conventional fungicides or used when disease pressure is low. Our research is exciting in that it highlights that an ARMOUR Zen + Serenade ASO treatment overlayed onto a grower standard could reduce apple scab. Future research should continue to focus on the combined application of chitosan and microbial biopesticides for suppression of disease.
From preliminary studies evaluating chitosan phytotoxicity, it was found that chitosan can be applied at high levels (1% v/v) on apple leaves without causing damage. This is promising as other studies have shown that chitosan can cause phytotoxicity on some crop plant species (DeGenring et al., 2022). Results from this research suggest that there is a synergistic effect of combining the commercial chitosan product, ARMOUR Zen, and Serenade ASO, in reducing apple scab when applied as part of an IPM program. Furthermore, ARMOUR Zen is promising as it did not reduce Bacillus subtilis QST 713 viability in vitro. Future research should examine the role of ARMOUR Zen and Serenade ASO applied together without being integrated into a grower standard. This was not possible at the commercial orchard test sites as it was not an economically sound decision for the farmer to not spray their orchard with a conventional fungicide treatment.
The Tidal Grow product seems to have some efficacy in reducing apple scab, although not comparable to a grower standard. Our research suggests that there is an optimum rate of chitosan needed to see effective disease control. For ARMOUR Zen trials, we applied 0.15% chitosan, while at the highest level, Tidal Grow was only applied at 0.01%. This was largely due to economic costs; suggesting that Tidal Grow may still be too expensive to be used for disease management. Additionally, it appears that Tidal Grow may be reducing B. subtilis QST 713 in vitro, which may be why the chitosan + Serenade ASO treatment at FREC was not as effective in reducing disease.
Furthermore, it appears that chitosan could reduce ascospore discharge if applied in the fall on overwintering leaves but not as effectively as a urea spray. Again, the largest issue is the cost of Tidal Grow; it was not economical to spray the overwintering leaves at higher rates of chitosan, which may have improved the efficacy of the product in reducing ascospore discharge.
Education & Outreach Activities and Participation Summary
Participation Summary:
Factsheet: A factsheet was provided to growers at the July UNH extension twilight meeting. 42 growers attended this meeting.
Journal Articles: A manuscript is in preparation to be submitted to the journal Crop Protection in January 2023.
Published Press Articles: This research was featured in a UNH Today article, a Good Fruit Grower article, and as an article in Inspired, a report by the UNH Agriculture Experiment Station.
Webinars, talks, and presentations: The research was disseminated through the following sources:
- UNH graduate student Liza gave a brief introduction to her work with chitosan during the Winter Webinar Series hosted by Northeast Extension Fruit Consortium in March 2022. There were 135 participants from around the country on zoom. Additionally, the video was recorded and has 90 views on Youtube.
- Liza presented this research to New Hampshire tree fruit growers at the annual NH Fruit Growers Association Meeting in April 2022. There were 67 growers in attendance.
- Anissa Poleatewich gave a presentation on the research during the 2022 New England Fruit and Vegetable Conference to at least 50 growers (final numbers for the talk have not been calculated at the time of submission).
- Liza gave a poster presentation during the 2022 New England Fruit and Vegetable Conference. At least 16 attendees stopped and discussed the research during the poster session, however, there could have been many more growers reached as the poster was available to read for the entirety of the conference (1500 attendees were registered for the conference).
Other educational activities: The research was presented at the July 2022 UNH extension twilight meeting to 42 growers.
Project Outcomes
This project has provided much needed data on the pre-harvest application of chitosan alone and in combination with a microbial biopesticide to reduce apple scab. These data are essential to integrating chitosan into a disease management program in apple orchards.
Economic sustainability
For this research, our chitosan rates for the commercial products were based on efficacy and cost per acre. The cost per acre for ARMOUR Zen at 0.15% chitosan (v/v) and Tidal Grow at 0.01% chitosan (v/v) were comparable to the cost per acre for other biopesticides. However, as Tidal Grow at the rate of 0.01% was not completely effective, the cost of the product must go down for it to be a feasible option for farmers. Moreover, the combination of ARMOUR Zen + Serenade ASO would be more expensive than these products alone. Thus, to be cost effective, these products would need to increase the revenue from treated apples to offset the product cost. While an economic analysis was not a part of this study, we have collected data that could be used in future economic studies. For example, this research determined the percent reduction in apple scab severity on harvested fruit for our treatments compared to the grower standard. An economic analysis could determine the increase in revenue from the decrease of apple scab severity on the marketed fruit. Additionally, this research highlights the need for commercial chitosan producers to reduce their costs to be an economically viable option for growers. An economic analysis could also be done to do a cost benefit analysis of integrating chitosan into a disease management program.
Environmental sustainability
Through this research, there is a potential that chitosan and a microbial biopesticide could be applied during low infection periods as a rotation to a conventional fungicide application. This would reduce the amount of fungicide applications being applied to the orchard throughout the season. While not included in this research, other researchers have found that chitosan is significantly safer for wildlife, pollinators, and water sources, than conventional fungicides. Adding this product to a spray regime could help reduce overall pollutants that are entering the environment. Furthermore, chitosan and microbial biopesticides’ modes of action are different from those of conventional fungicides. Thus, adding these products to an IPM program would reduce the risk of fungicide resistance. Additionally, the use of chitosan could reduce the amount of fungicide residues found on apples. While not included in this report, our research included examining the effect of pre-harvest sprays on post-harvest rots. In this research, chitosan reduced post-harvest rots, meaning that it could be a replacement for the final spray at the end of the season. This would reduce the amount of fungicide residues found on apples and thus how much consumers are being exposed to the chemical.
Farmer outreach
Information gained in this project was disseminated to stakeholders in the form of presentations, hand-outs, webinars, and twilight meeting events. Specifically, we reached 67 growers at the New Hampshire Fruit Grower’s Annual Meeting in April 2022 and 42 growers at the July 2022 UNH extension twilight meeting. Additionally, we reached growers and researchers at the New England Fruit and Vegetable Conference, that had 1500 attendees, in which at least 50 attended a presentation on this research and 16 discussed the research during a poster session. This outreach stimulated conversation with both farmers and researchers about the future direction of this research. For example, farmers are interested in chitosan as a soil additive and researchers at the University of Massachusetts want to collaborate on the potential to add chitosan to a management system for apple pests. Additionally, we have shared our research with the commercial chitosan companies in hopes to make their product more effective. These communications have led to connections with other researchers from around the world who are also researching chitosan.
Through countless discussions with farmers and other researchers, it is clear that to create a sustainable agricultural system, farmers need to have access to a diversity of crop protection products. Farmers are under extreme pressure to produce high quality crops while trying to reduce their environmental impact and risk of fungicide resistance. Thus, farmers need a toolbox full of options for an integrated approach to disease management.
Additionally, during our research at FREC, there was a shift in disease pressure in which marssonina leaf blight infections and incidence became more severe. In 2022, Marssonina leaf blight caused by the fungus Marssonina brunnea, was so severe at FREC, that entire trees were almost completely defoliated. While not as severe, one of the NH on-farm trial sites also had a large amount of marssonina leaf blight on one of the cultivars, Dabinett. We added marssonina leaf severity ratings (0-6) to our data collection protocol and by the end of the season leaves were rating as high as 6 (completely covered in M. brunnea). This data was not included in this report but will be included in our journal publication. This change in disease pressure was an alarming display of how quickly the challenges facing farmers can shift. During our conversations with Dr. Kari Peter, she spoke of her concern that marssonina leaf blight might be the new apple scab in terms of disease pressure and importance for New England farmers. Compared to V. inaequalis, much less is known about M. brunnea, and currently in the United States, there are no fungicides labelled for control of marssonina blotch of apple (Li, 2019). This highlights the importance of continuing our research to find other tools for growers to use for disease management. Additionally, this shift in disease pressure highlights the importance of working with farmers to identify challenges that they are facing in a changing climate.
Our future work will continue to examine natural products and biopesticides for disease management. Through discussions with commercial chitosan producers, growers, and other researchers, it is clear that there is more work to be done on how chitosan can be integrated into a disease management program. Thus, our future research will continue to examine the role of chitosan to reduce diseases alone and in combination with microbial biopesticides. Additional work will focus on other diseases and cropping systems. Some research has already been done to examine the effect of chitosan on reducing Botrytis cinerea in greenhouse productions (DeGenring et al., 2022). Research examining the combined use of chitosan and microbial biopesticides to reduce B. cinerea in greenhouse production is underway on an American Floriculture Endowment grant.
References:
DeGenring, L.M., Dickson, R.W., Poleatewich, A.M., 2022. Inhibition of Botrytis cinerea growth and suppression of gray mold on petunia leaves using chitosan. Plant Dis. https:// 10.1094/PDIS-07-22-1628-RE
Li, Y.H., 2019. Marssonina blotch of apple. The Connecticut Agricultural Experiment Station.
Information Products
- Using seafood byproduct chitosan as a natural disease suppressant for apples (Article/Newsletter/Blog)