Final report for GNE21-252
Project Information
The oocyte expresses certain genes during folliculogenesis to regulate the acquisition of oocyte competence. Oocyte competence, or oocyte quality, is directly related to the ability of the oocyte to result in a successful pregnancy following fertilization. Over the past few decades, the development and optimization of assisted reproductive technologies, particularly in vitro fertilization, have enabled the beef and dairy industries to advance cattle genetics and productivity substantially. However, only approximately 40% of bovine embryos will develop to the blastocyst stage in vitro. In addition, bovine embryos produced in vitro are developmentally inferior compared to in vivo derived embryos due to the lack of optimization of the oocyte and embryo culture conditions in vitro. Characterization of factors regulating these processes is crucial to improve the efficiency of bovine in vitro embryo production.
RNA Sequencing data obtained by our laboratory demonstrated that the secreted protein, agouti-signaling protein (ASIP), is highly abundant in the bovine oocyte. Agouti-signaling protein (ASIP) has a characterized role in the distribution of melanin pigment in some mammalian species, including mice. In adipose tissue, ASIP expression is associated with insulin resistance and obesity. Recently, it was demonstrated that ASIP is crucial in regulating mammary epithelial cell lipid metabolism in cattle. However, the role of ASIP in the bovine oocyte and early embryo has not been previously elucidated. In this research, we aimed to characterize the ASIP spatiotemporal expression profile in the ovary and throughout early embryonic development. Further, objectives included revealing the effects of supplementation of ASIP during in vitro oocyte maturation and embryo culture on subsequent embryonic development.
In addition to oocyte expression, ASIP was detected in granulosa, cumulus, and theca cells isolated from antral follicles. ASIP mRNA was found to decline with oocyte maturation, which may suggest a prospective role for ASIP in the achievement of oocyte competence. Microinjection of presumptive zygotes using small interfering RNAs targeting ASIP led to a 13% reduction in the rate of development to the blastocyst stage. Additionally, we examined potential ASIP signaling mechanisms through which ASIP may function to establish oocyte developmental competence.
Cumulus-oocyte complexes or presumptive zygotes were placed in culture medium containing either 0, 1, 10, or 100 ng/mL of recombinant ASIP, and effects on subsequent development were examined. Supplementation of ASIP during oocyte maturation was found to improve the blastocyst development rate. Meanwhile, supplementation of ASIP during embryo culture was not found to affect blastocyst rates.
These results support a functional role for ASIP in promoting oocyte maturation and subsequent embryonic development, potentially through signaling mechanisms involving cortisol. Additionally, these data further support the role of ASIP in the acquisition of oocyte competence and suggest that the supplementation of ASIP during oocyte maturation may lead to increased production of blastocysts. Future prospective applications of this work include the optimization of bovine oocyte or embryo culture conditions to emulate better the in vivo maternal environment. Further, future research should explore the utilization of ASIP in developing improved cryopreservation techniques for bovine embryos.
- Determine the expression profile and spatial distribution of bovine ASIP mRNA and protein in bovine ovarian tissue, GV and MII oocytes, follicular cells, and early embryos
- Characterize the effect of exogenous ASIP supplementation on oocyte maturation and early embryogenesis in cattle
- Investigate the possible role(s) of bovine oocyte derived ASIP in cumulus cell expansion, oocyte maturation, and early embryonic development to determine if ASIP is an indicator of oocyte competence
The purpose of this project is to elucidate the role of ASIP in folliculogenesis, oocyte maturation, and early embryonic development in cattle. The leading contributing factor to the annual $1.2 billion loss experienced by the beef cow/calf industry is early embryonic loss [1]. The utilization of reproductive biotechnologies and specifically, in vitro fertilization (IVF), over the past few decades have enabled the beef and dairy industries to substantially improve the genetics and productivity of cattle. Natural breeding only allows a cow to usually produce a single calf per year and approximately 10 calves throughout her lifetime. The use of IVF and embryo transfer can vastly increase the number of offspring a genetically valuable cow can produce throughout her lifetime. In 2017, it was reported that, for the first time in history, a larger number of bovine embryos were produced in vitro and transferred worldwide rather than in vivo which further emphasizes the importance of IVF to the beef and dairy industries [2]. However, the rate of transferable embryos produced from cumulus-oocyte complexes (COC) in vitro ranges from 30–40% [3]. Embryos produced in vitro result in fewer pregnancies than embryos flushed from donor cows and transferred to recipients [4]. The average number of oocytes collected per oocyte pick up (OPU) for Bos taurus beef cattle is on average, 21 oocytes per session and 19 for dairy cattle [5]. Of the oocytes collected during OPU, approximately 4.25 develop into quality embryos which can be transferred into a recipient [5].
A limiting factor of the success of bovine IVF is the knowledge of mRNAs and proteins imperative to oocyte competence and early embryonic development. The quality of the oocyte limits its ability to resume meiosis, cleave following fertilization, promote embryonic development and establishment of the pregnancy, and result in a full-term, healthy pregnancy, therefore, limiting reproductive success. The identification of mRNAs and proteins driving oocyte maturation and early embryonic development is crucial to improving the efficacy of bovine IVF technologies and reproductive success. The characterization of the transcriptome and proteome of oviductal fluid will enable better determination of the needs of the oocyte and embryo during fertilization and early embryonic development. Supplementation of culture media with the proteins found in the oviductal fluid may lead to the development of enhanced culture media with higher blastocyst rates. Analysis of RNA-Sequencing (RNA-Seq) data of bovine germinal vesicle (GV) stage oocytes obtained by our laboratory revealed the ASIP mRNA transcript was over 1000-fold more abundant in the oocyte than in other tissues including the brain, heart, lung, liver, spleen, colon, and kidney. Further investigation of ASIP may unveil a critical role of ASIP during oocyte maturation and early embryonic development in cattle. Data obtained from this research could lead to the better optimization of culture media used in the IVF process to increase the number of transferable embryos obtained by better imitating the in vivo follicle and oviduct conditions.
Research
- Determine the expression profile of bovine ASIP mRNA in bovine ovarian tissue, GV and MII oocytes, follicular cells, and early embryo.
To characterize ASIP expression in follicular cells including cumulus, granulosa, and theca cells, and both GV and MII oocytes, quantitative PCR (RT-qPCR) was conducted. Luteal-stage ovaries from Bos taurus cows were obtained at an abattoir (JBS Beef Plant, Souderton, PA) and transported to the laboratory in 0.9% saline solution. Ovaries were either utilized for follicular cell collection or cumulus-oocyte complex (COC) aspiration. Upon return, ovaries were washed in 0.9% saline, and COCs were aspirated from 2-7 mm visible follicles using an 18-gauge needle. The follicular aspirate was then washed 3 using Boviplus oocyte wash medium containing BSA (Minitube USA, Inc., Verona, WI). After sedimentation, the COCs with more than four compact layers of cumulus cells and homogeneous cytoplasm were individually selected and washed. For germinal vesicle (GV) stage oocyte samples, cumulus cells were removed via hyaluronidase (0.1%) digestion and were vortexed for 5 min. Denuded GV oocytes were then stored with minimal volume at -80°C. For MII oocyte samples, COCs were matured in groups of 50 in BO-IVM medium (IVF Bioscience, Falmouth, United Kingdom) for 21-24 h at 38.5°C in 5% CO2 in humidified air. Following IVM, additional oocytes underwent in vitro fertilization (IVF) to generate embryo samples. Bovine spermatozoa from a frozen-thawed semen straw were washed twice using 4 mL of BO-Semen Prep medium (IVF Bioscience), centrifuged at 328 g for 5 minutes, and resuspended in approximately 350 μL of BO-Semen Prep. Expanded COCs were washed in 50 μL drop of BO-IVF medium and transferred to 4 well plates containing BO-IVF medium. Matured COCs and sperm (2.0 x 106 sperm/mL) were co-incubated for 12 h in wells containing 500 μL of BO-IVF medium at 38.5°C in 6.5% CO2 in humidified air. Following 12 h post insemination, presumptive zygotes were then denuded as stated previously and placed in groups of 50 in 500 μL of BO-IVC medium. Embryo culture was performed in humidified air at 38.5°C in 5% CO2 and 5% O2. Stages of embryonic development were collected at the following times: 2-cell embryos were collected 33 h post insemination (hpi), 4-cell embryos 44 h hpi, 8-cell embryos 52 h hpi, 16-cell embryos 72 hpi, morula 5 days pi, and blastocyst-stage embryos 8 days pi. All embryo samples for gene expression analysis were stored at -80°C in minimal volume until analysis.
The effect of follicle size on ASIP expression was also examined using cells and oocytes collected from small (3-5 mm) and large (8-18 mm) antral follicles. Ovaries were obtained from a local abattoir and small (n = 6) and large follicles (n = 6) were dissected for granulosa and theca cell isolation. Cumulus-oocyte complexes (COCs) were aspirated from small and large follicles and GV oocytes (pools of 10) were denuded and collected for RNA isolation. Additional COCs from small and large follicles underwent in vitro maturation (IVM) for 22-24 h to obtain MII oocytes (pools of 10) and expanded cumulus cells. RNA was isolated from all cells and oocytes, reverse transcribed into cDNA, and examined for ASIP expression via RT-qPCR. Conditions for RT-qPCR reactions will follow methods as previously published [18]. ASIP expression was normalized to the expression of RPL19 and relative expression values were calculated using the standard curve method. Differences in ASIP expression were determined by two-way ANOVA using JMP statistical software testing for the effect of follicle size and cell type. Individual mean comparisons were performed using Tukey’s HSD.
- Characterize the effect of exogenous ASIP supplementation on oocyte maturation and early embryogenesis in cattle
The effect of recombinant ASIP (rASIP) supplementation during IVM on early embryonic development was examined. As it was commercially available, recombinant human ASIP protein (R&D Systems, Minneapolis, MN; cat. # 9094-AG) was supplemented to COCs at the beginning of IVM in a preliminary experiment at the concentrations of 0, 1, 10, 100, 500, or 1000 ng/mL (10-30 COCs/well, n = 4 replicates/treatment) to determine the optimal concentrations of ASIP for blastocyst development. Further replications were performed using 0, 1, 10, and 100 ng/mL to assess the effect of rASIP supplementation during IVM on blastocyst development. Differences in gene expression were determined using either a Student’s t-test or One-way ANOVA using JMP statistical software version 15.2 (SAS Institute, Cary, NC). Individual mean comparisons were performed using Tukey’s HSD. Statistical analysis of ASIP supplementation blastocyst rate data was conducted using Dunnett’s test to compare treatment groups to the control. Differences in means will be determined using Tukey-Kramer. Statistical significance will be designated as P < 0.05.
- Investigate the possible role(s) of bovine oocyte derived ASIP in cumulus cell expansion, oocyte maturation, and early embryonic development to determine if ASIP is an indicator of oocyte competence
Microinjection of ASIP siRNA into zygotes and subsequent embryo culture was conducted using procedures described previously using (Lee et al., 2014). The custom dicer-substrate siRNA (DsiRNA) design tool (Integrated DNA Technologies, Coralville, IA) was used to design a siRNA specie targeting the ORF of bovine ASIP mRNA. The siRNA was interrogated by BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) search against the bovine transcriptome and genomic database to rule out homology to any bovine sequences. The ASIP siRNA species was generated commercially (Integrated DNA Technologies, Coralville, IA). Presumptive zygotes (n = 30-38/treatment) were collected 12-16 h p.i. for microinjection, which was performed using M2 medium (Medium 199 containing HEPES supplemented with 2% FBS). Zygotes were injected individually with approximately 15 pl of either ASIP siRNA (50 µM), negative control siRNA (50 µM, universal control species 1; Ambion Inc., Austin, TX), or remained as noninjected controls. The percent development of zygotes reaching the blastocyst stage was determined on day 8. To validate ASIP knockdown, 4-cell embryos (n = 4 pools of 10) were collected was determined via RT-qPCR. Differences in gene expression were determined using either a Student’s t-test or One-way ANOVA using JMP statistical software version 15.2 (SAS Institute, Cary, NC). Individual mean comparisons were performed using Tukey’s HSD. Differences were considered statistically significant at a probability value of P < 0.05. Statistical analysis of microinjection data was conducted using a contrast to analyze the difference between blastocyst development.
Detection of ASIP in the bovine oocyte
A total of 85 million raw reads were generated from the sequencing of a bovine oocyte library. After quality control, 78 million clean reads were obtained. All clean reads were further mapped to the bovine genome (UMD3.1) using TopHat2[62]. The transcriptome was reconstructed using ab initio assembly software Scripture [63] and Cufflinks [64]. Transcripts reconstructed by these two assemblers were merged into a combined set of transcripts, resulting in the assembly of a total number of 42,396 transcripts from 37,678 genomic loci. All assembled transcripts were categorized using the bovine genome annotation obtained from UCSC and Ensembl genome browser. Approximately 40% of the transcripts correspond to already annotated transcripts.
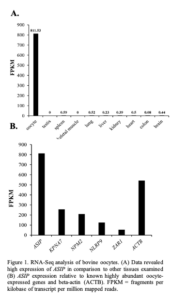
The expression of the annotated transcripts in oocyte vs other bovine tissues was compared using RNA-Seq data sets of 9 bovine tissues downloaded from the NCBI SRA database (Accession number SRR594491- SRR594499). Expression of ASIP was found to be much higher in the oocyte in comparison to the other tissues (oocyte FPKM: 811.53; other tissues <0.6 FPKM; Figure 1A). Based on FPKM values, the level of ASIP expression appears to be similar to the expression levels of several known oocyte-specific genes (Figure 1B) including KPNA7 [8].
Characterization of ASIP expression in the ovarian follicle and early embryos
To characterize ASIP expression within the ovarian follicle, cumulus, granulosa, and theca cell samples were collected and transcript abundance was analyzed via RT-qPCR. Results indicate low cumulus cell expression.
granulosa and theca cells isolated from antral follicles (Figure 2A; n = 12-16). To characterize ASIP expression throughout early embryonic development, pools of 20 oocytes (GV and MII) and embryos ranging from the 2-cell stage to the blastocyst stage of early embryonic development were collected. Mature oocytes and embryo samples were generated via IVM and IVP, respectively. Data validated the RNA-Seq results as the GV and MII oocyte was found to express ASIP. During early embryonic development, ASIP transcript abundance remains stable throughout the early cell divisions while increasing following EGA (Figure 2B; n = 4 pools of 20), and blastocysts displayed very high levels of ASIP transcript.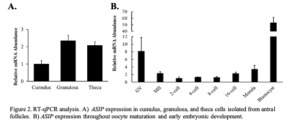
The effect of recombinant ASIP supplementation during IVM
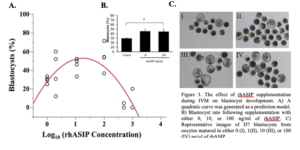
As ASIP was detected in the bovine oocyte, surrounding follicular cells, and the early embryo, the effect of recombinant human ASIP (rhASIP) supplementation during IVM on the rate of early embryonic development was examined. rhASIP was utilized as it is commercially available. rhASIP protein was supplemented to cumulus-oocyte complexes (COCs) during in vitro maturation (IVM) at the concentrations of 0, 1, 10, 100, 500, or 1000 ng/ml (10-30 COCs/well, n = 4 replicates/treatment). The control (0 ng/ml rhASIP) blastocyst rate was 29% ± 1.8. Data from this experiment the optimal concentration of rhASIP for blastocyst development lies between 10 and 100 ng/ml as determined by the peak in Figure 3A. The addition of rhASIP to the IVM medium displayed embryotropic effects at the concentrations of 10 and 100 ng/ml as an increase in day 7 blastocyst rates (45% ± 4.2 at 10 ng/ml and 45% ± 5.5 at 100 ng/ml) was observed (Figure 3B). The 100 ng/ml treatment led to a significantly higher blastocyst rate in comparison to the control (P = 0.0113; Figure 3B) as revealed by Dunnett’s test comparing both treatment groups to the control. Meanwhile, the addition of rhASIP at the concentrations of 500 and 100 ng/ml proved to be detrimental to preimplantation embryonic development as blastocyst rates were 5% ± 2.4 and 20% ± 10, respectively.
The effect of ASIP siRNA-mediated knockdown on early embryonic development
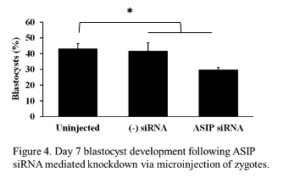
Expression of ASIP was observed throughout early embryonic development, therefore, we wanted to address the effects of ASIP knockdown on the rate of blastocyst development. Presumptive zygotes (n = 30-37 zygotes/treatment) were collected 12-16 h post-fertilization and injected with approximately 20 pl of either ASIP siRNA (25 µM), negative siRNA (25 µM), or remained as uninjected controls. The experiment was repeated 4 times. On day 7, blastocyst rates were examined. There was no difference in blastocyst rates between the uninjected (45% ± 2.98) and negative siRNA injected (45% ± 3.8) controls. Statistical analysis using a contrast revealed blastocyst development was significantly decreased by 13% in embryos injected with ASIP siRNA (29% ± 2.98) as shown in Figure 4 (P = 0.024)
In the future, additional siRNA species targeting bovine ASIP will be developed to determine if a higher rate of effectiveness in knocking down ASIP can be achieved. However, expression of ASIP during the early cleavage stages of embryonic development has been shown to be relatively low, with notably higher levels in the oocyte and the blastocyst. Manipulation of ASIP expression during the process of oocyte maturation may be more informative into the role of ASIP in oocyte competence and early embryonic development. A study by Lee et al. (2014) exhibited that authors were able to successfully microinject cumulus-enclosed GV oocytes with siRNA to knock down gene expression during oocyte maturation. Following the knockdown of JY-1, rates of both oocyte maturation and early embryonic development significantly declined as oocyte competence was diminished in JY-1 knockdown oocytes (Lee et al., 2014)
These results provide a basis for the full characterization of oocyte expression of ASIP. Additionally, these data further support the role of ASIP in the acquisition of oocyte competence and suggest that the supplementation of ASIP during oocyte maturation may lead to the increased production of blastocysts. Future prospective applications of this work include the optimization of bovine oocyte or embryo culture conditions to emulate better the in vivo maternal environment through normalizing lipid metabolism and, subsequently, minimizing stress.
Education & Outreach Activities and Participation Summary
Participation Summary:
Due to the molecular nature of the proposed work, outreach to fellow researchers and embryologists will grant the most benefit as they may offer productive feedback and implement findings in their embryo production practices. This research investigated whether ASIP has the potential to enhance bovine IVF through supplementation of culture media or the use of ASIP as a marker of oocyte competence. The use of IVF can be beneficial for both large and small-scale producers as a tool to enhance their herds' genetics and decrease the generation interval. Improvement of the efficiency of the bovine IVF process will allow beef and dairy producers to spend less time and funds to obtain embryos. If more transferable embryos can be produced from a single OPU session, the producer will increase the profitability of each session.
To share information acquired throughout this project, I attended and presented this research at several international and highly regarded scientific meetings. I presented data from this research project at the Society for the Study of Reproduction (SSR) Annual 2021 meeting in St. Louis, MO. I presented a poster presentation entitled "The Expression of Agouti-Signaling Protein During Folliculogenesis and Oocyte Maturation in Cattle". In 2022, I once again presented an update of my findings from this project at the annual SSR meeting in Spokane, WA with a poster presentation entitled "Characterization of Agouti-Signaling Protein and its Receptors in the Bovine Oocyte and Early Embryo".
I also presented findings a the 2023 International Congress on Animal Reproduction (ICAR) meeting. The ICAR meeting was focused on reproductive physiology and reproductive technologies with attendees from over 40 countries with attendees including academic and industry scientists and veterinarians. Here, I presented a poster presentation entitled "Agouti-Signaling Protein Impacts Blastocyst Development In Cattle".
Data obtained throughout this project are expected to result in a manuscript(s) thoroughly explaining the ASIP bovine expression pattern in the oocyte, follicular cells, and early embryo, effects of ASIP on oocyte maturation, and early embryonic development and describing pathways which ASIP mediates its effects. We will aim to submit the manuscript(s) to either Biology of Reproduction or Reproduction for publication. Both journals are frequently read by bovine embryologists, researchers, and veterinarians. All animal and human reproductive researchers will benefit from the published work as biological processes are often conserved between various species.
Project Outcomes
Overall, this project aimed to understand the functional role of ASIP in the bovine oocyte and early embryo. As assisted reproductive technologies, specifically IVF, are becoming a vital part of the beef and dairy industries, advancements in vitro embryo production and bovine IVF systems would contribute to further advancements in efficiency and sustainability in bovine reproduction. This research developed a basis for future research investigating applications for ASIP in IVF culture systems.
As a result of this project and collaboration with my local beef slaughterhouses, my labmates and I developed a bovine IVF lab at my University. This enabled us to gain immense experience in in vitro embryo production and provide numerous teaching experiences to undergraduate students at WVU, many of whom were interested in veterinary medicine. Additionally, this research required many hours of visiting large-scale commercial slaughterhouses and working with their team to collect bovine ovaries. This experience provided me with a further appreciation and understanding of the process of beef processing and sustainable agriculture in a modern beef processing facility. This grant enabled me to devote many hours to perfecting the in vitro embryo production laboratory at WVU where I developed a passion for embryology and was able to gain a large amount of advanced hands-on embryology skills. Overall, this project contributed immensely to deciding my final future career path which is working in human medicine in a clinical IVF lab as an embryologist.