Final report for GNE21-260
Project Information
This project aimed to help Maine's wild blueberry growers manage the invasive insect pest Spotted wing drosophila (SWD) in an economically and ecologically sound manner through the implementation of integrated pest management strategies. This was done in two main ways: intensive monitoring and reduced-risk management options. We found that adult captures were inconsistent across years and trap styles. We also found that phagostimulant adjuvants have the potential to aid in management of SWD, although more work is still required. Work conducted during this project was shared with growers along the entire way, be it conversations in their fields, at farm field days, or at conferences. This project was plagued by abnormally warm and dry weather, which caused abnormal population behavior of the insect pest of study.
1) Optimization of trap captures and threshold modeling.
Objective (1) will entail testing the efficiency of six different styles of SWD monitoring traps, and the incorporation of these different style trap captures into a risk-based action threshold model to help growers determine the ideal timing for management strategy implementation.
2) Exploration of reduced-risk management strategies.
Objective (2) will involve assessment of the efficacy of insecticide border applications in large scale field trials, compare the efficacy of multiple behavioral control and push-pull tactics in field cages, and a field and semi-field bioassay assessment of a phagostimulatory adjuvant.
Spotted-wing Drosophila (SWD) Drosophila suzukii (Matsumura), is a major pest of concern for fruit growers in the United States. SWD is an invasive vinegar fly native to southeast Asia. First found in the continental U.S. in 2008 [1], it rapidly expanded across the rest of the country in the next four years [2]. Unlike most other drosophila, SWD prefer ripening as opposed to overripe fruit [3]. SWD are polyphagous with a broad host range of thin-skinned crops, cultivated and uncultivated [4]. Female SWD utilize a hardened serrated ovipositor to lay eggs in ripening fruit while still in the field, leading to direct damage and fruit decay from larval feeding, as well as opening the fruit up to secondary infection by other insects and pathogens [5, 6]. SWD was first found in Maine in October 2011 [7]. During the 2012 growing season, SWD caused an estimated $1.38 million crop loss in Maine wild blueberries, with actual losses likely exceeding that number [8]. Wild blueberry (Vaccinium angustifolium Aiton) is a native and economically important Maine crop. Maine produces approximately 12% of all blueberries in North America, contributing over $250 million to the state's economy each year; in 2017, Maine was the third largest state in domestic blueberry production in terms of volume, producing over 30,000 metric tons of wild blueberry [9]. Since its establishment in Maine, SWD has become one of the major pests for wild blueberry growers, with economic impacts having the potential to exceed $6.8 million per year under worst-case scenarios [10].
A zero-tolerance policy regarding SWD larvae in the fresh and processed berry market has led to insecticide usage [11]. Insecticide applications have doubled in some crops since the SWD invasion. In highbush blueberry, these increases in sprays for SWD have reversed a trend of reducing the usage of broad-spectrum insecticides, with a 45% increase in the percentage area treated between 2011 and 2019 (Johnson and Fanning, unpublished data). Reliance on insecticides is dangerous as it will lead to insecticide resistance, currently seen with spinosad in SWD populations in California [12]. Managing SWD has increased farm labor and input costs and reduced profitability [2]. Insecticide costs have increased by $480-$1,200 per acre in CA raspberries [13] (Farnsworth et al. 2017). In Maine wild blueberries, the difference between an economical and uneconomical management program is one spray. Specialty crops employ 68% of all agricultural workers in the USA, therefore increased insecticide use for SWD management increases the potential for human exposure to toxic insecticides. One study investigated biological, behavioral, and preventative management specifically in Maine wild blueberries, exploring insect exclusion netting, entomopathogenic fungi, and mass trap deployment to attract and kill [14]. However, none of these strategies resulted in sufficient pest control to justify the replacement of insecticidal treatments.
To reduce insecticide inputs, environmental impacts, health risks associated with insecticides use and cost by growers associated with the management of this species, greater knowledge of SWD’s biology, attractants for monitoring and appropriate traps, and reduced-risk strategies are needed.
Research
Objective 1: Optimization of Trap Captures and Threshold Modeling
Experiment 1.1: Comparative Evaluation of Baited Monitoring Traps for SWD -
This trial entailed a field comparison of six different SWD monitoring traps. The six traps are as follows (Bait + Trap Type):
2021:
- Trece Lure + Trece Cup Trap
- Trece Lure + Red Panel Trap
- Unbaited Red Panel Trap
- Scentry Lure + Scentry Cup Trap
- Yeast / Sugar Water + Red Solo® Trap
- Spinosad Treated Red Solo Yeast + Sugar Water Trap
2022 (Changes were made due to low trap captures, or impracticality of traps from the 2021 trial):
- Trece Lure + Trece Cup Trap
- Trece Lure + Red Panel Trap
- Scentry Lure + Scentry Cup Trap
- Scentry Lure + Red Panel Trap
- Yeast / Sugar Water + Red Solo® Trap
- Diluted grape juice aged w/ NaCl + Red Solo® Cup Trap
In both 2021 and 2022 there were four replicates, with trap locations being randomized within each replicate each week to prevent location biases. Traps were suspended 0.5 m above the canopy along the field edge with approximately 10 m between neighboring traps and 15 m between replicates. Traps were checked weekly, with adult male and female SWD captures being recorded and new traps placed along the field edge. All Scentry and Trece lures were replaced every 4 weeks, while drowning liquids, liquid baits, and red panel traps were changed every week.
The Scentry and Trece cup traps utilized 180 ml distilled water with Seventh Generation® unscented dish soap (1-2 drops per trap, to reduce surface tension) as a drowning solution. Red Solo® cup traps were constructed using Solo® brand, 16 fl. oz, red polystyrene cups with light blocking lids, constructed by hot gluing red foam to the top of the clear plastic lid. Seven evenly spaced 3/16-inch holes were punched around the top of the cup just beneath the rim. Each yeast/sugar red Solo® cup trap was baited with 1 tsp active live yeast, 4 tsp sugar, and 120 ml distilled water. In 2021, Spinosad-treated traps were made by rinsing the inside of a red Solo® cup trap with the field rate equivalent (6 oz/acre) of Entrust® and drying overnight before deploying. New spinosad-treated red Solo® cup traps were made weekly, so the residues never aged more than seven days. In 2022, the grape juice bait was made weekly (1 concord grape juice : 3 distilled water, with 2% wt:vol NaCl, 120 ml total solution per trap) and allowed to age in a controlled environment (25°C, 75% RH, 16:8 photoperiod) for one week prior to being used in the field. For all styles of red Solo® cup trap the bait also served as a drowning solution.
All Scentry and Trece products were purchased from Great Lakes IPM.
Statistical Analyses: In 2021 data for overall trap captures and cumulative male captures failed to meet the assumptions of normality and equally distributed variances, so comparisons were run using a Kruskal-Wallis test. In 2022 a Log(x+1) transformation corrected for normality in overall trap captures and an ANOVA with a post-hoc Tukey test was used to compare captures. Cumulative male captures in 2022 were unable to be corrected for normality and unequal variances and were analyzed using a Kruskal-Wallis.
Experiment 1.2: Threshold Modeling -
This experiment utilized trap capture and larval infestation data from 2019-2022, and built upon data collected from 2012-2017. Each year, 10-15 fields were monitored weekly for larval infestation and adult SWD presence. Each field had three red Solo® cup traps (identical to trap 5 of Experiment 1.1) deployed along the field edges. Traps were replaced with new ones weekly. All trap contents were returned to the lab and all male and female D. suzukii captures were recorded. At each field site visit one 6oz by volume fruit sample was collected from near each trap location (three fruit samples per field). All fruit samples were weighed and then processed via the salt extraction method to determine presence of SWD larval infestation.
Each week the total number of males captured per field was averaged among the traps, and a cumulative total per field was kept. The cumulative average total of males captured the week prior to first larval detection was was then fit to a gamma distribution. This was then compared to the original threshold model for Maine wild blueberry using data from 2012-2017.
Objective 2: Exploration of Reduced Risk Management Strategies
Experiment 2.1: Efficacy of Border Insecticide Applications -
This trial took place on two fields in the Downeast region of Maine. Fields were monitored around the field edge, with both adult traps (identical to trap 5 in experiment 1.1) and fruit samples for larval infestation being used. Management for one field was based on findings from the adult traps, and management decisions for the second field was based on results of fruit sampling for larval infestation. Fields would use a perimeter spray or full field spray depending on SWD density revealed by the trapping.
Experiment 2.2: Cage Trial Comparison of Behavioral Control Tactics -
This experiment took place in Jonesboro, ME, and followed a randomized block design with six treatments, each treatment being replicated four times. Treatments are as follows:
2021:
- ACTTRA SWD® + Delegate WG® (ACTTRA SWD® mixed with Delegate WG® at 0.25% a.i. (v/v), applied at a rate of 1. 5 L of formulation/acre1. (6.90 mL formulation per cage of 200 sq. ft).
- Food-grade gum (one hemp fiber pad carrying 6gm Decoy disc per cage placed in one side of the cage in a shaded area with a water source).
- Combi-Protec® + Delegate WG® as a full cover spray, full rate (14 fl. oz. combi-protec, 50 gal water and 6 oz. Delegate WG® per acre).
- Combi-Protec® + Delegate WG® as full cover spray, half rate (14 fl. oz. combi-protec, 50 gal water and 3 oz. Delegate WG® per acre)
- Delegate WG® + Induce spray at the highest field recommended rate for SWD control in blueberries (6 oz/acre of Delegate WG® in 50-gal water/acre + 0.12% Induce as surfactant)
- Untreated control
2022:
- ACTTRA SWD® + Delegate WG® (ACTTRA SWD® mixed with Delegate WG® at 0.25% a.i. (v/v), applied at a rate of 1. 5 L of formulation/acre1. (6.90 mL formulation per cage of 200 sq. ft).
- Combi-Protec® + Delegate WG® as a full cover spray, full rate (14 fl. oz. combi-protec, 50 gal water and 6 oz. Delegate WG® per acre).
- Combi-Protec® + Delegate WG® as full cover spray, half rate (14 fl. oz. combi-protec, 50 gal water and 3 oz. Delegate WG® per acre)
- Delegate WG® + Induce spray at the highest field recommended rate for SWD control in blueberries (6 oz/acre of Delegate WG® in 50-gal water/acre + 0.12% Induce as surfactant)
- Delegate WG® + Induce as full cover spray, half rate(3 oz/acre of Delegate WG® in 50-gal water/acre + 0.12% Induce as surfactant)
- Untreated control
2022 saw the addition of a replicate treated with Delegate+Induce full cover spray at half rate, to determine if any differences seen in full vs half rate sprays are due to the presence of Combi-protec.
Twelve 10ft x 12ft insect exclusion cages were used to keep out ambient D. suzukii and retain introduced D. suzukii. Cages were spaced at least 10m from each other to prevent any crossover effects. Treatment 1 was applied as three, 2ml droplets equally spaced along the center of the cage on blueberry bush shoots. Treatments 3-5 were applied in 35 gallons of water-mixture per acre with a CO2-propelled, 80-inch boom sprayer (76-inch swath) equipped with four, flat-spray, 8002VS TeeJet® nozzles operating at 35 psi and at a slow walking speed, regulated with a metronome. 100 D. suzukii adults (at a ratio of 50 male:50 female) were added to each cage approximately 8 hours after application of treatments.
For fruit sampling purposes, each cage was split into quadrants, with four fruit samples being taken per cage and collected in a 6 oz cup. All samples were then weighed to account for differences in sample weights. Samples were collected twice: once before treatments were applied to determine baseline infestation, and then again seven days after the addition of D. suzukii to look for treatment effects. All twelve cages were then moved to new patches of wild blueberry and the trial was repeated for replicates 3 and 4. In 2021, replicates 1 and 2 were run from 27 July to 3 August and replicates 3 and 4 from 17 August to 23 August. In 2022, replicates 1 and 2 ran from 26 July to 2 August, and replicates 3 and 4 ran from 12 August to 23 August. Larval infestation was determined through the salt extraction method [16] for all fruit samples.
Statistical Analyses: In 2021 splitting the replicates between two different dates displayed no effect on larval infestation, so all replicates were grouped for analysis. Due to non-normality, data were analyzed using a Kruskal-Wallis test. In 2022 there was little to no infestation in the first two replicates, and no statistics were run on the third and fourth replicates from 2022.
Experiment 2.3: Field and Semi-field Evaluation of Efficacy of Combi-protec -
There were eight treatments as follows:
2021:
- Delegate WG®
- Delegate WG® + Combi-Protec (14 oz/50 gal)
- Delegate WG (50% rate) + Combi-Protec (14 oz/50 gal)
- Assail 70WP®
- Assail 70WP + Combi-Protec (14 oz/50 gal)
- Assail 70WP (50% rate) + Combi-Protec (14 oz/50 gal)
- Combi-Protec only, control treatment (14 oz/50 gal)
- Untreated control
2022 (Treatments were altered in 2022 to only test one chemical, instead looking at different rates and coverages of that chemical with and without Combi-protec):
- Delegate WG® (6 oz/acre; full rate)
- Delegate WG® (6 oz/acre) + Combi-Protec (14 oz/50 gal)
- Delegate WG® (3 oz/acre; 50% rate)
- Delegate WG® (3 oz/acre; 50% rate) + Combi-Protec (14 oz/50 gal)
- Delegate WG® (6 oz/acre) at 50% coverage – 17.5 gal/acre
- Delegate WG® (6 oz/acre) + Combi-Protec (14oz/50 gal) at 50% coverage – 17.5 gal/acre
- Combi-Protec only, control treatment (14 oz/50 gal)
- Untreated control
Treatments were applied in a complete block randomized design in Jonesboro, ME. There were four applications of each treatment. In 2021, sprays were applied on 3, 10, 17, and 25 August, and in 2022, sprays were applied on 3, 10, 19, and 25 August. All materials were applied in 17.5 (50% coverage only) or 35 gallons of water-mixture per acre with a CO2-propelled, 80-inch boom sprayer (76-inch swath) equipped with four, flat-spray, 8002VS TeeJet® nozzles operating at 30 psi and at a slow walking speed. Walking speed for each application was regulated using a metronome.
Experiment 2.3a: Field Evaluation -
For each plot, a 6 oz berry sample was collected weekly six to seven days post application. Fruit samples were weighed, and then evaluated for larval infestation using the salt extraction method [16].
Statistical Analyses: In 2021 there was little to no infestation at the first two sampling dates (9 and 16 August), so they were not included in any analyses. Due to non-normality data were analyzed using a Kruskal-Wallis test. Data were adjusted for sample weight prior to analysis. In 2022 the first three weeks of the trial had little to no infestation, so only the final week (1 September) was included for statistical analysis. Due to non-normality and heteroscedasticity data were analyzed with a Kruskal-Wallis test.
Experiment 2.3b: Semi-field Bioassay -
This experiment ran in conjunction with 2.3a.
Each bioassay container consisted of a 32oz deli cup, a water pick, a wire-mesh container to hold loose berries, a fabric-mesh lid, and a small amount of fly diet. At one- and three-days post treatment, leaf terminals with 3-4 leaves were clipped and placed in a water pick (filled with water) in a bioassay container. Thirteen berries were collected from each plot and placed in the wire mesh containers. Ten 5-7 day old SWD adults (5 male and 5 female) were then added to each bioassay arena. Containers were placed in an environmental chamber (22°C; 70% RH) for 6 days. Adult fly mortality was assessed at 24 and 48 hours after addition of adult SWD. On day 6, berries were removed from the bioassay containers and placed in a rearing cup in an environmental chamber (22°C; 70% RH) for 20 days to allow for adult emergence, which was then quantified.
Statistical Analyses: In 2021, Kruskal-Wallis tests were used to look for differences in adult mortality and emergence due to failures of the assumptions of normality and equal variances. In 2022, a Kruskal-Wallis test was used for adult mortality and emergence at the 1 D.A.T.. An ANOVA with post-hoc Tukey test was used for the 3 D.A.T. timepoint.
Experiment 1.1: Comparative Evaluation of Baited Monitoring Traps for SWD -
In 2021 traps were placed in the fields on 7 July, with first trap captures occurring during the week of 28 June. Data collected from 28 June to 9 August were used for the analysis; SWD populations after 9 August were high enough to overpower any difference in trap style. All baited traps performed significantly better than an unbaited red sticky trap (Fig. 1). The Trece lure + red panel trap significantly outperformed all the other styles of traps tested (Fig. 1). When looking at cumulative adult male capture the Trece lure + red panel trap reached conservative threshold levels of SWD quicker than any other of the trap styles (Fig. 2).
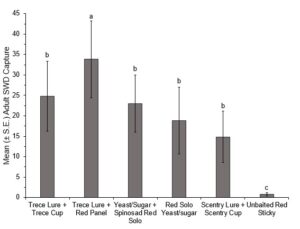
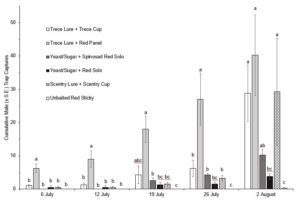
In 2022 traps were placed in the fields on 14 July, with first trap captures occurring during the week of 14 June, and for the first two weeks only the red panel traps (with both lures) captured SWD. Due to extremely low trap captures in the first five weeks of this trial, only data from 26 July to 9 August were included for analysis of overall captures. The ANOVA revealed significant differences between the treatments (F(5,66)=11.266, P=0.0001) (Fig. 3). Looking at cumulative male trap captures within each week, there was no significance between trap styles until the last two weeks (Fig. 4, Fig. 5).
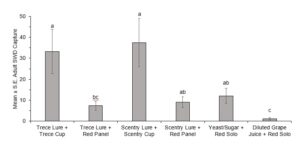
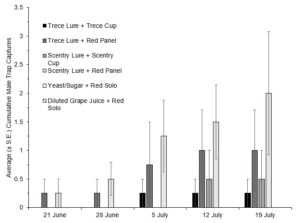
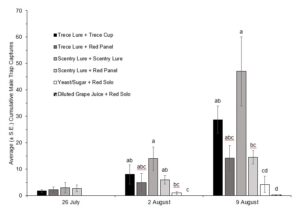
In both 2021 and 2022 baited red panel traps show promise for the earlier detection of SWD. In 2021 the baited red panel trap had a greater trap capture than all of the other traps; however, in 2022 the baited red panel traps had lower captures than the commercially available cup traps. This could be due to the differences in climate during the two seasons: 2022 was much more of a drought year with warmer temperatures and less rainfall, which might have encouraged the SWD towards the cup traps due to their moisture content. There is also a possibility that the warmer temperatures impact the rate of volatilization of the baits which could skew trap captures. Other SWD focused trapping trials have also reported inconsistent results with time of year, reproductive status of the females, host crop, and state all playing roles.
Experiment 1.2: Threshold Modeling -
Adding in four additional years worth of trap captures and larval infestation data resulted in a more conservative threshold model (i.e. fewer male captures correlated to a higher probability of larval infestation the following week). When data from 2021 and 2022 were removed, the model became slightly less conservative, and remained close to the predictive capabilities of the original threshold model. This model did not perform well for 2018, 2021, or 2022. These were all years in which first SWD capture was early, and captures remained low across the season due to higher than normal temperatures.
Experiment 2.1: Efficacy of Border Insecticide Applications -
2022 saw unusually warm and dry weather during the wild blueberry growing season. While SWD were detected early in the season, the heat and lack of rainfall kept their populations extremely low. At the fields where this trial took place, monitoring did not show a need for SWD targeted insecticide sprays, so no comparison of full field and perimeter spray was able to happen.
Experiment 2.2: Cage Trial Comparison of Behavioral Control Tactics -
In 2021, all treatments except for the Decoy disc displayed a significant reduction in SWD larvae per gram of fruit sampled compared to the untreated control cages (P = 0.0005) (Fig. 6). Also, the Combi-Protec with full rate Delegate resulted in significantly lowered infestation than the full rate Delegate treatment (P = 0.0117) (Fig. 6).
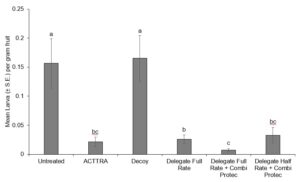
In 2022, replicates 1 and 2 had little to no infestation, most likely due to the abnormally hot and dry weather deterring the added SWD from ovipositing or causing mortality in the added SWD. Due to this we were not able to perform statistical analysis on the data from 2022.
In 2021, both ACTTRA TD and Combi-Protec combined with lowered levels of insecticides did not result in reduced control relative to a full-rate spray of Delegate. However, as there was no treatment of half rate Delegate alone, we are not able to claim that the levels of control seen in the half rate Delegate with Combi-Protec treatment are due to the addition of the phagostimulant.
Experiment 2.3a: Field Evaluation of Efficacy of Combi-protec -
Of the fruit sampled on 24 August, 2021, the only difference in larval infestation was between the Combi-Protec (CP) only and Delegate WG + CP treatments (P = 0.0304), with the CP treatment oddly having fewer SWD larvae (Fig. 7). For fruit collected on 31 August, 2021, Assail had significantly fewer larvae than the untreated control, Delegate half rate + CP, and Delegate + CP (P < 0.05) (Fig. 7).
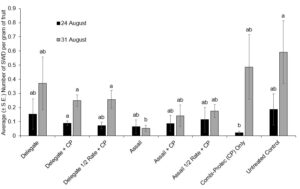
In summer 2022, no significance was found between the treatments (H(7)=13.108, p=0.0695). The results are outlined in figure 8.
Experiment 2.3b: Semi-field Bioassay of Combi-protec -
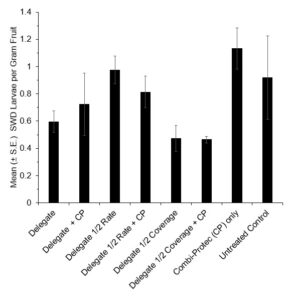
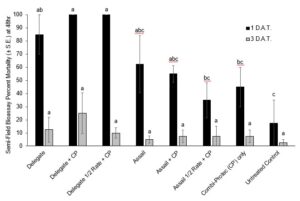
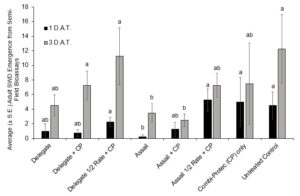
In 2021, at the 1 D.A.T. time point, Delegate, Delegate mixed with Combi-Protec, and Delegate half rate mixed with Combi-Protec all had significantly higher mortality than the untreated control (P < 0.05) (Fig. 9). There was no significant difference in mortality between any of the treatments at the 3 D.A.T. time point (Fig. 9). When it came to emergence of adult flies from berries in the bioassay containers, at the 1 D.A.T. timepoint only Assail at the full rate had significantly fewer SWD emerging than the untreated control, and at the 3 D.A.T. timepoint both Assail and Assail + Combi-protec had a significant reduction in emergence relative to the untreated control. (Fig. 10).
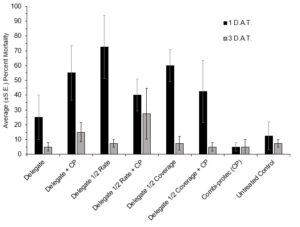
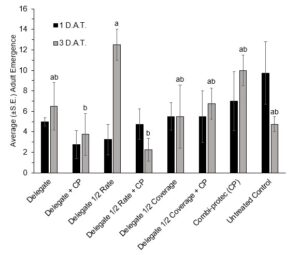
The 2022 semi-field bioassays saw no significant difference in adult mortality between the treatments at the 1 D.A.T. (H(7)=13.648, p=0.058) or 3 D.A.T. (H(7)=3.318, p=0.854) time points (Figure 11). There was no significant difference in emergence at the 1 D.A.T. (H(7)=6.3818, P=0.4959). However, at the 3 D.A.T. the ANOVA showed significant differences between treatments (F(7,24)=3.2416, P=0.0145) and the Tukey revealed that the Delegate half rate treatment had significantly higher emergence than both the Delegate full rate + Combi-protec (P=0.0473) and the Delegate half rate + Combi-protec (P=0.0130). The fact that Delegate half rate saw a significantly higher emergence than Delegate half rate + Combi-protec indicates that the addition of Combi-protec to a half rate spray may provide greater protection than the half rate insecticide alone (Figure 12).
Objective 1: Optimization of Trap Captures and Threshold Modeling
Monitoring is a key component of integrated pest management. We encourage all of our growers to monitor their fields for the presence of SWD adults, and to take regular fruit samples to look for larval infestation. While our trials did not result in one trap style or bait type that consistently outperformed the others, each trap and bait will still alert growers to the presence of SWD. We recommend that growers use a trap they are comfortable with, and that they set the traps early and monitor them throughout the growing season. The existing threshold model for probability of larval infestation based on adult male SWD captures should still be used, but we warn growers that in years with warmer than normal summer temperatures, additional caution should be made and tolerance levels should be lowered.
Objective 2: Exploration of Reduced Risk Management Strategies
Perimeter sprays have been shown to be valid management strategies for the control of blueberry maggot fly (Rhagoletis mendax) in Maine's wild blueberry, as well as for SWD in other states and crops. More research needs to be done before concluding its effectiveness for SWD in Maine. Phagostimulants show promise as a potential aid in managing SWD. Based on these limited results we do not recommend spraying any insecticide at a half rate (with or without a phagostimulant), as this may lead to quicker development of insecticide resistance.
Education & Outreach Activities and Participation Summary
Participation Summary:
During the 2021 and 2022 field seasons I gave interactive talks to approximately 210 (combined) growers in a wild blueberry field at the University of Maine Blueberry Hill Farm Field day. These talks detailed two of the field trials I was running in that field, with hands on options for growers to interact with the different trap styles I was testing.
In February 2022 I gave a 20 minute talk (with 10 minutes of questions) over zoom to 76 growers during the virtual 2022 Wild Blueberry Conference. I presented all of the trials run in 2021 and the results, as well as detailed the expansion of those projects that I would be running during the 2022 field season.
After both the 2021 and 2022 field seasons all experiments were written up into short reports which were included in the Wild Blueberry Research and Extension Reports from the University of Maine Cooperative Extension. These reports were given to growers during the Wild Blueberry Conferences as well as made available online.
I also presented data from this work at the 2022 Entomological Society of America joint annual meeting to approximately 50 other Drosophila suzukii researchers.
All experiments funded by this grant will end up in my Ph.D. dissertation. Finally, experiments 1.1 and 1.2 are currently being written up as a journal article with the intent to submit to a peer reviewed journal by May 2023.
Project Outcomes
This project added information on the efficacy of several reduced risk products (decoy discs, ACTTRA, and Combi-Protec) to a growing body of work. Results from our trials have been shared with collaborators who are working with the companies producing these products. If a phagostimulant adjuvant does end up registered for use in blueberries and is able to reduce the amount of insecticides required for control, it will save growers money on insecticide costs as well as have beneficial impacts on the environment. We are still collaborating with Maine blueberry growers to get a comparison of control based on full field versus perimeter sprays, as well as comparing efficiency and costs of monitoring with traps for adult presence, fruit samples for larval presence, or a combination of both.
When this grant was being written I was in my first year of a Master's program. I have since decided to switch into a Doctoral program, and I still have two and a half years left. Two of my thesis chapters include the work completed here, and I have added in two additional chapters focused on integrated pest management and D. suzukii in Maine's wild blueberries. One chapter is focused on classical biocontrol for SWD. The other chapter is based off the abnormal weather seen during the summer 2022 growing season. This chapter is focused on modeling and predicting SWD populations based off of climatic variables such as winter low and high temperatures, rate of spring warming, amount of snowfall, first fall freeze. I am also in the process of creating a second threshold model for SWD infestation based off of male captures from red panel traps. Having an accurate working threshold model for two different trap types will give growers more options in how they choose to monitor their fields.
One of the biggest downfalls to the design of this project was not including a check on the Delegate half rate + Combi-Protec treatment in the 2021 trials. Having this would have helped clear up some of the results seen that year. It was unfortunate for our research that the 2022 growing season was plagued by warm and dry weather as it kept populations of SWD well below normal, however that same weather was fortunate for growers as it meant less management was needed for SWD control.
Further study is needed on how weather impacts trapping ability. Looking at the rate of decay of volatiles of all the baits and lures used in our trial would be fascinating. Another potential project could focus on trapping results across different states and crops and trap styles, but from the lens of climate: does warmer weather impact how well some traps work - will a trap that is effective in the cooler north be less effective in the warmer southern summers? There is also more work to be done with the threshold model, specifically focused on years where it does not accurately predict SWD larval infestation based off male trap captures and what can be done to change that.