Final report for GNE22-277
Project Information
Maine is a unique state with a wealth of agricultural production amidst the lobster industry. Using lobster processing byproducts as a soil amendment could benefit both industries. Post-processed lobster shells can be finely ground to produce lobster shell meal (LSM), which contains chitin, much like the key components of fungal cells and their overwintering structures. Adding chitin-rich material such as LSM has been shown to increase the populations of some chitin-degrading microorganisms capable of suppressing soilborne pathogens. In greenhouse trials, the efficacy of LSM was determined using potato ‘Shepody’ inoculated with the soilborne pathogens, Verticillium dahliae, Streptomyces scabies, or Rhizoctonia solani. In field trials, LSM was tested with potato ‘Russet Burbank’ by amending the soil in either the fall or spring, inoculating with V. dahliae, and using soil fumigation. Potato yield, disease intensity, and microbial communities were examined. LSM in a greenhouse study significantly increased the aboveground biomass of potato plants, but this did not translate to the field effects. Disease incidences were also not significantly different in greenhouse nor field trials, but the severity of potato early dying was seen to be significantly reduced within fumigated treatments amended with LSM compared to those that were only fumigated and not amended with LSM. Fungal communities were more sensitive to LSM as a soil amendment than bacterial communities. Both fall and spring amendments of LSM reduced fungal diversity, while fungal evenness was only reduced in the fall amended trial. Bacterial communities were not significantly impacted by LSM, but evenness was reduced with the use of fumigation. Core communities of bacteria with LSM amendment were larger in the fall amended trial compared to the spring LSM-amended trial. The use of LSM presents an opportunity to connect the potato and lobster industries in Maine and transform shellfish industry byproducts into a useful resource.
- Determine the efficacy of LSM for promoting beneficial soil microbial communities and reducing soilborne diseases of potato
- Analyze soil microbial community dynamics under LSM treatment
The purpose of this project is to reduce disease-related losses in potatoes by enhancing soil health with the addition of a shellfish industry byproduct. Potatoes are the most valuable crop in Maine and have been part of the economy since the mid-1700’s (USDA 2021; Johnston 1972). In 2019, 51,500 acres of potatoes totaling a yield of 16,738,000 hundredweight were grown in Maine (USDA 2020). Unfortunately, the potato industry has been challenged by the ever-present issue of soilborne diseases.
The first problem to be addressed is decreasing the impact of soilborne diseases and consequently increasing yields of potatoes. Soilborne diseases have been managed in various strategies, including using tolerant or resistant varieties, chemical treatments, and soil fumigation. They also can be managed with the addition of soil amendments which can stimulate disease-suppressive beneficial microbial communities in addition to improving soil quality and nutrients (Larkin 2015).
Depending on the source, soil amendments have different functions and effects on soilborne pathogens and potato (Hao and Ashley 2021). Therefore, it is critical to understand how the amendment works before using it. An amendment such as LSM contains a high concentration of chitin, which provides nutrients for microbes which degrade and utilize chitin for their growth (Andreo-Jimenez et al. 2021). Some of these microorganisms can suppress plant pathogens with one or more modes of actions, such as directly degrading pathogen cell walls or overwintering structures that contain chitin as a major structural component (Ramirez et al. 2010). This increase to beneficial microbial communities through the introduction of organic matter could also be described as an improvement to soil health. Soil health is a critical factor of sustainability and longevity of agricultural lands. In order for farmers to continue practicing agriculture for generations to come, sustainable practices which contribute to soil health and subsequent plant health are of utmost importance (Hao and Ashley 2021; Larkin 2015). Agricultural practices which rely on the existing ecology of microbes in the soil to assist in disease suppression as opposed to solely chemical application methods encourage this longevity and reduce the impact on the environment caused by chemical application.
The second problem which is being addressed in this study is with the innovative utilization of lobster byproducts post-processing to remove them from the waste stream. Disposing of lobster shell waste is highly costly, for example, a facility processing 15,000 pounds of lobster per day can expect to pay upwards of $4000 per month for disposal services. A small portion of lobster shell waste is composted, but the majority is sent to landfills (Fulton et al. 2013). Maine is uniquely positioned as a state with a wealth of agricultural production amidst the highly valuable lobster industry. The use of lobster processing waste as a soil amendment could help build collaboration between these two vital industries in the state of Maine. Overall, this study will address two problems with a solution which advances sustainable agriculture and longevity of agriculture through soil health.
Cooperators
- (Educator)
- (Educator and Researcher)
- (Educator)
Research
The lack of field research and technical application of shellfish byproducts in agriculture indicates that field research is of most use. However, because of the intrinsic challenges and large scale of field research, preliminary studies took place in the greenhouse located at The University of Maine in Orono. Following the greenhouse trial, a field trial took place. Lessons learned in the greenhouse trial aided in determining the best direction for field application, experimental design, and rates.
1. Effects of lobster shell meal (LSM) on soilborne pathogens and potato plants, Preliminary greenhouse studies. In the University of Maine greenhouse, the potato variety ‘Shepody’ was planted in a mix of garden soil and potting soil (1:1) in one-gallon pots in March and July of 2022. ‘Shepody’ was selected because it is susceptible to both V. dahliae and S. scabies. The soil was inoculated with either Verticillium dahliae or Streptomyces scabies inoculums, the causal agents of potato early dying (PED) and common scab, respectively, and non-inoculated soil was used for a non-diseased control. Prior to planting, inoculum for S. scabies was made by growing the bacteria in vermiculite mixed with liquid medium (Say’s solution: 40 g sucrose, 2.4 g asparagine, 1.2 g K2HPO4, 20 g yeast, 1 L sterile water). The bacteria and vermiculite combination was added to the soil in pots at a rate of 1:10 inoculum to soil, and mixed thoroughly. The final inoculum concentration was 105 colony forming units (CFU) per gram of soil. For V. dahliae inoculum preparation, microsclerotia, the rigid overwintering structure of V. dahliae, were used. Twenty microsclerotia per gram of soil were added to pots by suspending microsclerotia in sterile water and adding to vermiculite. Lobster shells collected from local processors were dried and ground using a grain mill to produce a fine powder, which was used as the standard LSM. Soil samples were collected and stored in a -80°C freezer until further processing to represent soil before adding potato seed pieces, pathogen inoculum, or LSM. LSM was then blended into the soil at various rates (0%, 2%, and 8% w/w). These ranges were selected based on previous studies and to help narrow in on appropriate rates for subsequent work. There were initially five pots per treatment within each pathogen inoculation category (V. dahliae, S. scabies, or uninoculated) and both planting groups. In the first planting group, the pots were immediately planted with sprouting seed tubers, and the second group remained without tubers for the first three months before planting with sprouting seed tubers. This gave an indication of how microbial communities were changing without the addition of plant tissues, but with the addition of LSM alone, and model a fall or early spring amendment timeline. This also gave time for microbial communities to respond to the addition of LSM. Pots were set up and randomly arranged in the greenhouse bench. Plants were watered as needed throughout their growth and until the plants began to naturally senesce around 3 months after planting.
Plant heights were measured over the growing season until the natural death of the plants occurred, after which, plants were harvested. To harvest the plants, plants were cut at the soil line and all plant tissue were placed into paper bags to dry in a heated room for approximately one week, or until the plant matter was fully desiccated. Desiccated plant matter was weighed and recorded as aboveground biomass. A few days after the removal of aboveground plant tissue, tubers were removed from the soil, the surfaces were cleaned of any residual soil, and they were processed immediately. The total weight of the tubers, tuber numbers, and disease symptoms were recorded. Disease incidence described the presence or absence of symptomology and disease severity was recorded in various ways depending on the disease. To determine PED infection symptom severity, tubers were cut in half longitudinally, the percent of the vascular ring which was discolored was recorded. To determine the severity of symptoms of infection by S. scabies or Rhizoctonia solani, the causal agent black scurf, the percent of the tuber surface which was covered by scabs or sclerotia, respectively, was recorded. Indigenous populations of R. solani produced disease symptoms in tuber without inoculating the soils, and this was recorded as well.
A follow-up greenhouse study was performed in March and July of 2023. Six treatments with five replications were established, building upon challenges associated with the first greenhouse trial. Fertilizer was added as a treatment factor and inoculum was combined in equal parts from R. solani and V. dahliae to ensure proper inoculum levels to cause disease, and is described below. Treatments were once again prepared twice to simulate a spring and fall amendment timeline as described in greenhouse trial 1. To best compare to the field trial, rates of inoculum, fertilizer, and LSM were all as similar as possible to those used in the field trial. The rate of inoculum was 20ml/foot, and as the pots were 8” in diameter, a rate of 14ml/pot was used. Virgin grain was added at the same rate to non-inoculated pots. The rate of fertilizer (14-14-14) was applied at a rate of 1100lbs/acre, typically incorporated at a depth of 12”, so each pot received 2.99grams of fertilizer. The rate of LSM was described by Coast of Maine to be applied at a rate of 5lbs/100ft2. In the field, the LSM was incorporated to a depth of approximately 4”, resulting in a rate of approximately 1.7% w/w. Data was collected in the same fashion as in the first greenhouse study, with the exception of plant height, which was not collected.




2. Field Studies. Following the greenhouse studies, two field trials took place in Presque Isle at the Aroostook Research Farm, Maine. Potato ‘Russet Burbank’ was planted in three rows within 25-foot-long plots with four replicates. Soil treatments included unamended soil, unamended soil inoculated with V. dahliae, LSM amendment alone, LSM amendment with V. dahliae inoculum added, soil fumigation with metam sodium at 50 gal/A amended with LSM and inoculated with V. dahliae, and soil fumigation with metam sodium at 50 gal/A inoculated with V. dahliae. These additional treatments gave a comparison to typical potato agricultural practices. The fumigation was performed in the fall of 2022 under favorable weather conditions. These trials took place in adjacent fields which have not had exposure to LSM previously and had been planted in oats for at least one year prior to these trials. Lobster shell amendments were applied in the fall of 2022 before planting seed tubers in the spring. The rate of LSM as an amendment was informed by industry standards provided by Coast of Maine, a compost and soil amendment company. The rate of LSM for the field trial was consequently 5lbs per 100 ft2 (approximately 0.35-0.7% of the soil % w/w). LSM was also procured from Coastal Chitin, LLC, a local processor, which aided in the industry collaborations and simplified the process of preparing large volumes of LSM. This prepared LSM was received pre-dried, pre-ground, and ready to use. The field was maintained during the growing season using standard operational practices, including fertilizer application and pest control. The fields were irrigated with rainwater only. This field evaluation also served as a demonstration plot, because of its location at the research farm, and was shared with farmers at the annual summer meeting. A second field trial was established in the spring of 2023 to simulate a spring amendment timeline. However, because of the difficulty of fumigation in the spring, this factor was not included in this trial. Consequently, the experimental design was simpler and consisted of four treatments: unamended and not inoculated, unamended and inoculated, amended with LSM only, and amended with LSM and inoculated. Soil was sampled at planting, mid-season, and before harvest.
At harvest, potato yield, disease incidence and severity of PED were evaluated to determine the impacts of these treatments. Additionally, incidences of hollow heart, a physiological condition, were noted if tubers had hollowing in their core. Data was analyzed using similar methods for both greenhouse field studies. Statistical analyses were performed using the R package Agricolae. Effects of treatments on disease incidence, disease severity, and plant biomass were determined using analysis of variance and Fisher’s Least Significant Differences tests.


3. Soil microbial community dynamics analysis. In the trials described in objective 1, field soil samples were collected in the fall-amended trial after fumigation, then in both before planting, in the middle of the growing season, and before harvest. Each soil sample consisted of a minimum of ten cores randomly selected in the plot which were homogenized in a gallon size Ziploc bag to give a representative composite sample of the plot. This helped us catalog changes to the microbial community over time. Soil samples were sieved and stored at -80°C until processed. DNA was extracted using 0.25 grams of soil and a DNEasy PowerSoil Pro Kit according to the provided instruction. DNA was quantified, and purity was ensured before sending for sequencing analysis at The University of Michigan Genomic Sequencing Facility. Samples were processed using NextGen sequencing on an Illumina MiSeq to return sequencing reads which were used to identify fungal and bacterial community members. Amplicons of the samples were generated using the V3-V4 hypervariable regions of 16S rRNA region to determine the presence of various bacteria present in the soil samples. For fungal populations, the ITS2 rDNA region was amplified (van Horn et al. 2021).
Raw data of Illumina sequencing was processed to filter sequences, remove chimeras and other unwanted contaminating sequences, and clustered into amplicon sequence variants (ASVs) at 97% sequence similarity, which was analyzed using the DADA2 pipeline. R packages such as Phyloseq, DeSeq2, and Microbiome were used to quantify and visualize community differences between samples and treatments. Microbial diversity was measured by a series of ASV-based analyses of alpha and beta diversity (Rosenzweig et al. 2012; Rajendhran et al. 2011). Community differences were visualized using ordinations of Bray-Curtis and Jaccard dissimilarity (van Horn et al. 2021; Callahan et al. 2016). Core members which were unique to treatments were also called and described, should they match a previously described organism to genus and species (Callahan et al. 2016). Differential abundance of microbes was performed using DeSeq2 to determine if specific ASVs or phyla were enhanced or reduced following our treatments (Love et. al 2016).
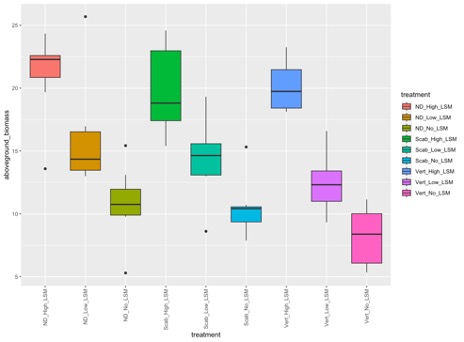
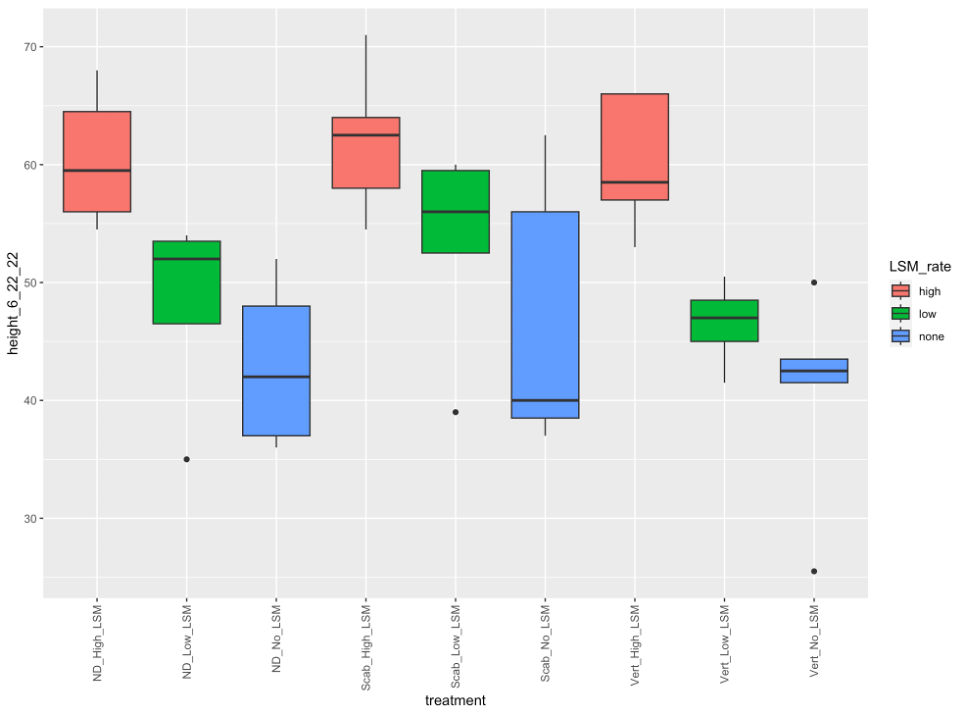
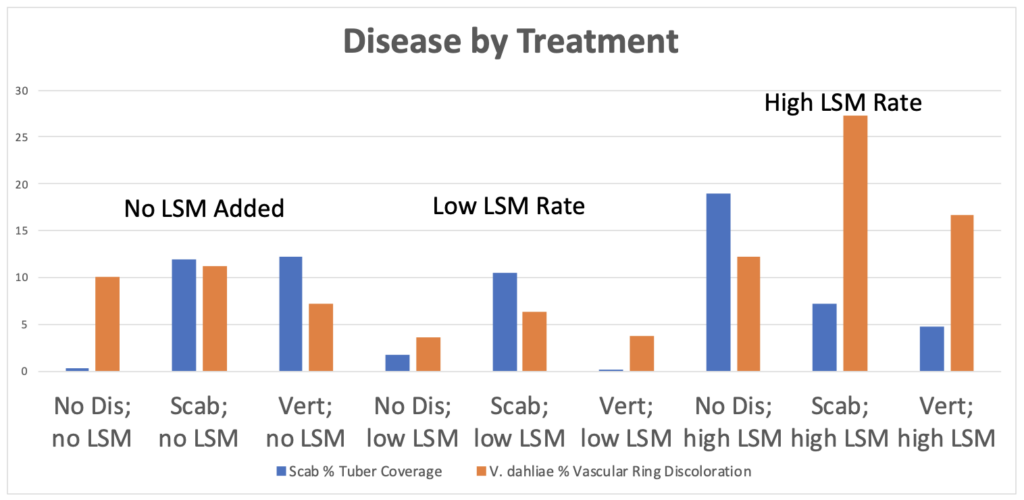
1. Greenhouse Trial Results. In the initial greenhouse trial, the aboveground biomass of potato plants increased significantly with increasing rates of LSM, regardless of if plants were inoculated with a fungal or bacterial pathogen, or not inoculated at all (p=2e-16; Figure 1). However, the highest aboveground biomass was seen in non-inoculated plants with the highest rate of LSM. The number of tubers was not significantly different between treatments, but this could also be an artifact of the small volume of soil in which the plants were being grown (p=0.26; data not shown). Plants that were not inoculated grew taller overtime when amended with the highest rate of LSM, as is seen in Figure 2 (p=4.11e-7). Disease severity was inconclusive from this initial greenhouse trial and no significant differences were seen in pots inoculated with V. dahliae or S. scabies (p=0.152 and 0.251, respectively); however, one trend was noticed. While not significant, disease severity overall was lowest in the treatments which were amended with the lower rate of LSM compared to non-amended or the higher rate of LSM. Severity of PED, as measured by the percent of the tuber vascular ring which was discolored, was also not significantly different (p=0.967). Scab severity was not significantly different, but the lowest rate of LSM had the lowest severity numerically, regardless of treatment (p=0.669; Figure 3).
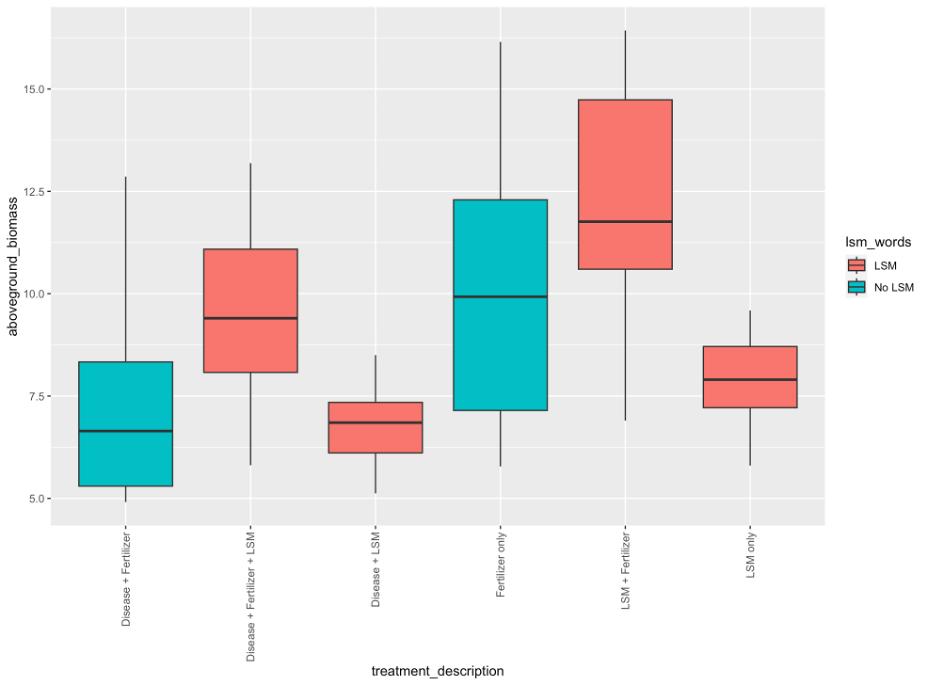
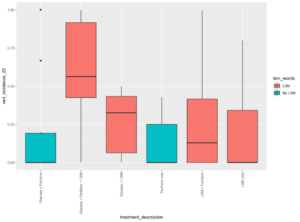
In the second greenhouse trial, aboveground biomass was significantly different between treatments (p=2.05e-5), and the heaviest group was the combined LSM and fertilizer treatment (Figure 4). Total tuber weight and number were once again not significant, likely as a result of the highly restricted environment in which these plants were grown (p=0.693 and 0.348, respectively). Disease incidence was variable within treatments, but consistently significantly different. The incidence of PED was shown to be significantly higher than any other treatment in pots inoculated with disease and amended with both LSM and fertilizer (p=00825; Figure 5). Incidence of black scurf was highest in treatments which were amended with LSM and fertilizer, however interestingly, these pots were not inoculated and indicated that this was R. solani which was naturally occurring in the soil (p=0.0027). A similar trend was seen with respect to the incidence of common scab, in that treatments which had both LSM and fertilizer, and disease and LSM had the highest incidences of disease symptoms (p=5.75e-6). It is worth noting that these two treatments were the only ones to express symptoms of common scab, and this could be related to the irregular microbial content of commercially available potting soil. Pots were prepared in batches and this could have played a role in the irregularity.
2. Effects of lobster shell meal amended field trials. In the spring-amended field trials, there were no significant differences among treatments in yield, PED incidence or severity, or incidence of hollow heart (p = 0.59, 0.969, 0.894, and 0.799, respectively; Table 1). While PED incidence was not significant, the nominally highest incidence was observed in the unamended and inoculated treatment, and similarly, this treatment also had the most severe PED symptoms.
In the fall-amended field trial, there were no significant differences among treatments in yield (p = 0.059; Table 1), but the non-inoculated LSM treatment showed somewhat lower yield than the inoculated LSM treatments (at p = 0.06). However, there was a significant blocking effect (p < 0.001) which was driven by the replication layout in this trial. Both PED incidence, and hollow heart incidences were not significantly different among treatments (p = 0.221 and 0.999, respectively). However, the severity of PED was significantly different among treatments, with the most severe symptoms occurring in the fumigated and inoculated treatment, and the least severe symptoms occurring in the treatment which was not inoculated and amended with LSM, and the treatment which was inoculated, fumigated, and amended with LSM (p = 0.048; Table 1).
|
Amendment Timing |
Tmt. |
Description |
Total Yield (Mg ha-1) |
Yield (z-Score) |
PED Incidence |
PED Severity |
Hollow Heart Incidence |
|
Spring |
1 |
Unamended; Non-inoculated |
33.59 |
0.07 |
0.27 |
0.70 |
0.29 |
|
2 |
Unamended; V. dahliae Inoculated |
35.74 |
0.43 |
0.31 |
0.81 |
0.36 |
|
|
3 |
LSM; Non-inoculated |
36.34 |
0.53 |
0.30 |
0.78 |
0.35 |
|
|
4 |
LSM; V. dahliae Inoculated |
31.63 |
-0.25 |
0.30 |
0.65 |
0.37 |
|
|
p = |
0.59 |
0.59 |
0.969 |
0.894 |
0.799 |
||
|
Fall |
5 |
Unamended; Non-inoculated |
33.24 |
-0.30 ab |
0.39 |
1.08 ab |
0.21 |
|
6 |
Unamended; V. dahliae Inoculated |
38.02 |
0.01 ab |
0.31 |
0.71 bc |
0.24 |
|
|
7 |
LSM; Non-inoculated |
24.98 |
-0.85 b |
0.26 |
0.59 c |
0.22 |
|
|
8 |
LSM; V. dahliae Inoculated |
43.23 |
0.36 ab |
0.36 |
0.98 abc |
0.22 |
|
|
9 |
Fumigated; V. dahliae Inoculated |
47.61 |
0.65 a |
0.35 |
1.165 a |
0.21 |
|
|
10 |
LSM & Fumigated; V. dahliae Inoculated |
39.87 |
0.14 ab |
0.25 |
0.615 c |
0.20 |
|
|
p = |
0.059 |
0.059 |
0.221 |
0.048 |
0.999 |
||
Table 1 Effects of soil treatments, including amendment of lobster shell meal (LSM), Verticillium dahliae inoculation, and fumigation on potato early dying (PED) and yield of potatoes in the fall- and spring-amended field trials. Means were separated by protected Fisher’s least significant difference tests.
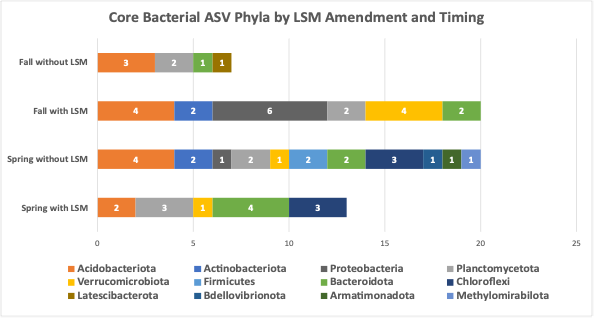
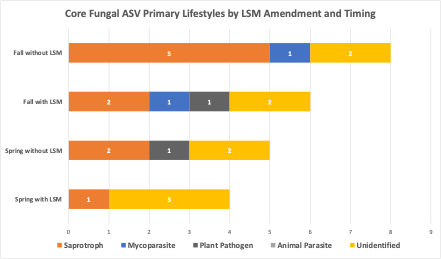
3. Lobster shell meal effects to soil microbial communities in the field. Over both fall and spring amended trial samples, 9,180,019 bacterial sequences were delivered which resulted in 6,049 bacterial ASVs across 155 samples. Within the fungal sequences, 3,028,243 reads were delivered, resulting in 14,246 ASVs across 155 samples. In spring-LSM-amended soils, microbial community diversity metrics, including observed ASVs, Shannon diversity index, evenness, and richness indicators ACE and Chao1, were not significantly different for soil bacterial communities among treatments. Fungal Shannon diversity was significantly different among soil treatments and was the lowest in soils amended with LSM and highest in the unamended, uninoculated treatment (Table 2). Within bacterial communities in soils amended with LSM in the spring, 127 ASVs were core with LSM, and 135 ASVs were core without LSM amendments (Table 3). By excluding ASVs which were common core members, and likely represented simply common soil microorganisms, 13 were conditionally core to LSM amended soils only and 20 were conditionally core to soils without LSM amendment. Of the top three bacterial phyla of ASVs which were conditionally core with LSM amendment, 30.7% were in the phylum Bacteriodota, followed by 23.1% Chloroflexi, and 23.1% Planctomycetota (Figure 6). The top two bacterial phyla which were conditionally core without LSM amendment, were Acidobacteriota and Chloroflexi (20% and 15%, respectively; Figure 7). For fungal core communities, 79 were found to be core with LSM amendment, and 78 were core without LSM amendment. After common core fungal ASVs were removed, 4 remained as conditionally core to LSM amended treatments, and 6 remained conditionally core to that without LSM amendment (Table 3). Of those fungal ASVs which were conditionally core with LSM amendments, three were unidentified, but one was identified by FungalTraits as a litter saprotroph. Of the fungal ASVs which were conditionally core without LSM, two were unidentified, two were saprotrophs, one was a plant pathogen, and one was an animal parasite. In spring amended treatments, only one bacterial ASV was more abundant with LSM amendment and was identified in the order Solirubrobacterales and family 67-14 and one fungal ASV was more abundant with LSM and was identified to the family Nectriaceae.
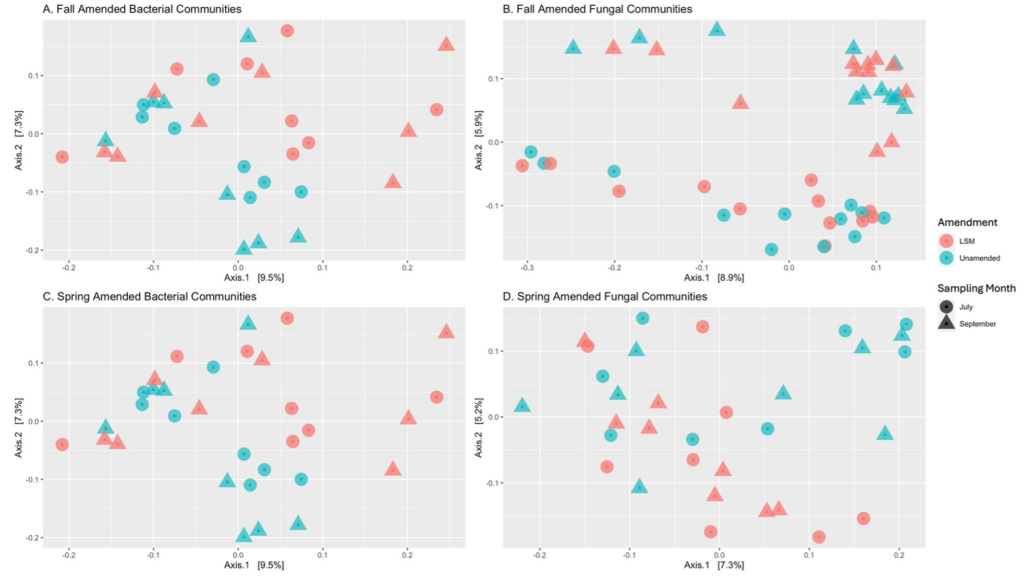
In fall-amended microbial communities, treatment alone was responsible for significant differences in bacterial evenness and fungal Shannon diversity and evenness (Table 2). When different factors within the treatments were analyzed, fumigation was found to significantly reduce bacterial evenness, particularly in the treatment which was fumigated and amended with LSM (Table 2). When core communities were identified, 123 bacterial ASVs were core to LSM-amended soils, and 109 bacterial ASVs were core to treatments without LSM amendment. When common core members were removed, 20 ASVs were found to be conditionally core with LSM amendment, and 7 were found to be conditionally core without LSM amendment (Table 3). The top three bacterial phyla of conditionally core ASVs with LSM amendment, were Proteobacteria, Verrucomicrobiota, and Acidobacteriota (30%, 20%, and 20%, respectively; Figure 6). The top two bacterial phyla of conditionally core ASVs without LSM amendment were Acidobacteriota and Planctomycetota (42.9% and 28.6%, respectively; Figure 6). For fall amended fungal core communities, 61 ASVs were identified as core with LSM amendment and 69 were identified as core without LSM amendment (Table 3). Once common core members were removed, 5 fungal ASVs were conditionally core with LSM amendment and 8 were conditionally core without LSM amendment. FungalTraits identified those conditionally core ASVs with LSM amendment as having the primary lifestyles of two saprotrophs, one mycoparasite, one plant pathogen, and one was unidentified. Of those conditionally core ASVs without LSM amendments, FungalTraits identified five to be saprotrophs, one to be a mycoparasite, and two were unidentified. There were not any differentially abundant bacterial ASVs in the fall amended microbial communities, however one fungal ASV was significantly less abundant with LSM amendment and was identified as Linnemannia hyalina. PCoA ordinations of bacterial and fungal communities in both fall and spring amended trials did not indicate differentiation by LSM amendment, except by sampling timepoint (Figure 7).
| Bacterial Diversity Statistics | Fungal Diversity Statistics | |||||||||||
| Amendment Timing | Tmt. | Description | Average Shannon Diversity | Average Observed ASV Diversity | Average Evenness | Average ACE | Average Chao1 | Average Shannon Diversity | Average Observed Diversity | Average Evenness | Average ACE | Average Chao1 |
| Spring | 1 | Unamended; Non-inoculated |
5.8 | 493.5 | 0.94 | 494.84 | 494.94 | 4.94 ab | 435.38 | 0.82 | 466.76 | 462.45 |
| 2 | Unamended; Inoculated |
5.75 | 453.25 | 0.94 | 455.01 | 456.28 | 4.95 a | 419.13 | 0.82 | 449.75 | 448.42 | |
| 3 | LSM; Non-inoculated |
5.76 | 445.38 | 0.94 | 446.23 | 446.92 | 4.82 bc | 383 | 0.81 | 403.78 | 399.76 | |
| 4 | LSM; Inoculated |
5.73 | 451.63 | 0.94 | 452.7 | 453.66 | 4.79 c | 401 | 0.8 | 428.56 | 422.92 | |
| p = | 0.59 | 0.57 | 0.11 | 0.58 | 0.6 | 0.02 | 0.49 | 0.07 | 0.6 | 0.58 | ||
| Fall | 5 | Unamended; Non-inoculated |
5.68 | 422 | 0.94 a | 423.01 | 423.96 | 4.84 ab | 431.88 | 0.80 a | 467.89 | 461.44 |
| 6 | Unamended; Inoculated |
5.64 | 409.75 | 0.94 a | 410.5 | 410.6 | 4.73 abc | 439.63 | 0.78 abc | 493.21 | 489.67 | |
| 7 | LSM; Non-inoculated |
5.67 | 412.38 | 0.94 a | 413.08 | 413.57 | 4.62 bc | 430.75 | 0.76 bc | 482.93 | 476.16 | |
| 8 | LSM; Inoculated |
5.61 | 388.63 | 0.94 a | 389.21 | 389.45 | 4.51 c | 419.13 | 0.75 c | 466.42 | 463.18 | |
| 9 | Fumigated; Inoculated |
5.55 | 378.13 | 0.94 a | 378.59 | 378.44 | 4.88 a | 478.25 | 0.79 ab | 532.77 | 526.96 | |
| 10 | LSM & Fumigation; Inoculated |
5.62 | 455.63 | 0.92 b | 457.7 | 457.62 | 4.64 bc | 444.25 | 0.76 bc | 502.81 | 498.21 | |
| p = | 0.79 | 0.5 | 0.01 | 0.49 | 0.49 | 0.02 | 0.3 | 0.02 | 0.36 | 0.37 | ||
Table 2 Alpha and beta diversity indicators of soil bacterial and fungal microbial communities, including Shannon diversity index, observed ASVs, evenness, and richness indicators ACE and Chao, by treatment for the spring and fall LSM amended field trials. Values followed by the same letter within each parameter are not significantly different based on Fisher’s protected LSD test at p < 0.05. Fall and spring amendment time points were analyzed separately.
|
Factor |
16S |
ITS |
|||||
|
Initial Total |
Number w/o shared |
Uniquely Core |
Initial Total |
Number w/o shared |
Uniquely Core |
||
|
Fall Amended |
With LSM |
123 |
28 |
20 |
61 |
6 |
5 |
|
Without LSM |
109 |
14 |
7 |
69 |
14 |
8 |
|
|
Spring Amended |
With LSM |
127 |
24 |
13 |
79 |
13 |
4 |
|
Without LSM |
135 |
32 |
20 |
78 |
12 |
6 |
|
Table 3 Core community members of bacteria and fungi in soils treated with lobster shell meal (LSM) amendment by spring and fall. Initial total included those which were common core members overall. Conditionally core members were core to that management factor level. Uniquely core members were not core to any other management factor or level, but only core to that specific management factor level.
Figure 6 Phyla of amplicon sequence variants (ASVs), of conditionally core bacteria (A) and primary lifestyle assigned by FungalTraits of conditionally core fungal ASVs (B) in soils either amended with lobster shell meal (LSM) or not and amended in the fall or spring. Conditionally core members were core to that management factor level.
Figure 7 Principal Coordinate Analysis (PCoA) of Bray-Curtis dissimilarity of bacteria and fungi in trials amended with lobster shell meal (LSM) in the fall (A, p = 0.501; B, p = 0.371) and spring (C, p = 0.646; D, p = 0.948). Clusters are not significantly different by LSM amendment.
In greenhouse studies, LSM treatments increased aboveground plant growth proportionately with increasing rates, although this trend did not translate to enhanced potato yields in either the greenhouse or field trials. Additionally, in the initial greenhouse trial PED incidence was found to be significantly reduced when planting was time delayed when amended with the higher rate of LSM, however this was not seen in the second greenhouse trial and could point to the stress of these plants not being fertilized. Disease incidences within tubers were not significantly different in either greenhouse or field trials by the treatments, although PED severity was significantly reduced by LSM amendment within fumigated soils. In general, fungi were more sensitive to the addition of LSM. LSM was found to alter fungal communities by reducing evenness in both the fall and spring amended trials, and by reducing Shannon diversity in the fall amended trial only. Bacterial diversity metrics were not significantly affected by LSM amendment, except one bacterial ASV in the phylum Actinobacteriota was found to be significantly more abundant with LSM amendment. Although trial limitations and environmental anomalies may not have resulted in a clear or consistent demonstration of the potential benefits of LSM treatments in this study, LSM as a soil amendment may be a useful tool in restructuring soil microbial communities following fumigation, reducing PED severity, and enhancing plant growth, but requires much additional research. In the future, better field maintenance, increased rates of LSM, and enhanced investigations of the role of LSM in PED disease severity suppression following fumigation could improve its efficacy and use as a soil amendment.
Education & outreach activities and participation summary
Participation summary:
Presentations and meetings took place to engage a broader audience. One presentation in Bangor, Maine with the Bioscience Association of Maine was awarded third place. This presentation generated interest and established new contacts using a QR code linked to a Google Form which collected contact information, questions, and set up meetings. Meetings took place with individuals associated with the lobster industry, farmers, or other researchers. Additionally, to fully ensure the viability of this project, contact with soil amendment companies and processors was essential to ensure that not only the results of this study, but the actual soil amendments are available to farmers, should they prove useful in the future. Visits and tours of processing plants and soil amendment facilities were conducted early on in the development of this project, as these collaborations are essential to developing an effective distribution pipeline of information and soil amendments. Additionally, a public seminar was given at The University of Maine in December 2022 detailing the specifics of the project. This research project was also selected to be included in the Hall of Flags, where I attended to present this research project informally to legislators in the Maine State Capitol Building in Augusta on March 23, 2023.
We have been featured in three written news articles, one newsletter geared towards the lobster industry, and one video news segment, links to which are included below. The University of Maine News also published a story on this research and included it in the
- News Center Maine Video: https://www.newscentermaine.com/article/tech/science/environment/umaine-researchers-test-lobster-shells-to-combat-soil-borne-pathogens-animals/97-22306c41-3bc7-4731-9606-6175e6b0e9b7
- Bangor Daily News Article: https://www.bangordailynews.com/2022/12/22/news/bangor/lobster-shells-potato-protection-umaine-research-xoasq1i29i/
- MaineBiz Article: https://www.mainebiz.biz/article/from-shells-to-spuds-umaine-using-lobster-by-product-to-fight-potato-pathogens
- University of Maine News Article: https://umaine.edu/news/blog/2022/12/20/umaine-researchers-testing-lobster-shells-to-thwart-potato-soil-pathogens/
- University of Maine Research Report (page 23): https://umaine.edu/research/media/pdf/2/0/2/2022-Research-Report-FINAL-012723-web.pdf
- The Lobster Newsletter: https://www.fish.wa.gov.au/Documents/rock_lobster/the_lobster_newsletter/lobster_newsletter_v35_no1.pdf
- BioScience Association of Maine Presentation: https://biomaine.org/biome-host-fifth-annual-student-showcase/
This work is also in preparation to be submitted for publication in a peer-reviewed journal in the coming months.
Project Outcomes
There was certainly a lot of interest from the public concerning this project. The estimated number reached is a low estimate and takes into account the individuals whom we communicated and formed relationships with following the news reports. This number does not include the large volume of individuals whom were reached who did not follow up concerning the project. Using Google Forms provided after presentations or meetings, we've also built a network of individuals who are interested in learning more and have collected their information to deliver results and potentially build more future collaborations.
With respect to the results, there is still much to be uncovered concerning the potential use of lobster shell as a soil amendment. This study clearly points to the need to increase research utilizing LSM, a product that otherwise is often destined for landfills. We've found that this study generated a lot of interest from the public, and we propose that there are potential benefits with respect to restructuring the soil microbial communities following disturbance or stress, and that LSM can be a useful tool in plant growth promotion. This project only indicated one small piece of the puzzle, and could eventually become a valuable soil amendment for growers in the potato industry, and potentially within other crops. There is also potential to investigate other pathogens and their interactions with LSM.
Ultimately, LSM as a soil amendment provides an opportunity for farmers to reduce their reliance on synthetic fertilizers and pesticides. These products can be incredibly costly, require certifications to handle and apply, cause damage to the environment, and impact soil health. Alternatively, LSM is a safe (baring shellfish allergies) tool which is biobased. There is still much knowledge to be gained on this novel soil amendment, but these initial results are promising. Finally, in the state of Maine, which has a small population, collaborations such as these which we've explored and built in this project are critical for unifying industries and improving economic outcomes overall.
So far in this project, we have learned the delicate nature of collaboration and have worked hard to maintain healthy working relationships. We encountered challenges with respect to producing the volume of LSM needed for the field trial and our collaborators within the lobster industry were instrumental in successful preparation for the 2023 field trial. We have also been featured in a number of news reports and have initiated contact with many other potential collaborators. This is a critical aspect of sustainable agriculture because collaboration and communication are essential for successful adoption of techniques and communication of results.
As a team, we earned an additional $1,500 undergraduate research grant from the University of Maine to support an undergraduate student who is conducting a related project utilizing LSM and compost.
Currently post-graduation, I am working as a postdoctoral researcher funded by a Fulbright Grant in Spain. This experience with grant proposal preparation and report writing has been incredibly useful as I've prepared additional grant proposals and reports. Additionally, this experience has given me more experience in developing and managing an independent research project. Most importantly, this project has given me experience in developing new relationships and collaborating with individuals outside of my discipline. In my future career, I hope to continue similar research utilizing organic soil amendments in disease suppression either in academia as a Cooperative Extension Specialist, or within government as a USDA Plant Pathologist.
The amount of data that would not normally be collected in a plant pathology study contributed to insights into the growth and productivity of potato plants in the greenhouse, especially as they were likely hindered by growing in small, one-gallon pots compared to field conditions. In the future, our greenhouse design will need to be altered, but in the short term, the results provided valuable insight concerning the experimental design of the field trial. Also, our field trial was met with many challenges, often inherent to field trials, but with further replications these issues could be reduced. As previously stated, this topic requires further research but provides a lot of potential as a future tool and soil amendment.