Final report for GS19-207
Project Information
Current agricultural conventions tend to favor unbroken landscapes of monoculture crops. Though these fields can be efficient for farm equipment, there is evidence that they are inadequate for soil, pollinator, and ecosystem health. With this study, we aim to investigate whether the addition of native plant mixes and a higher diversity of floral resources in an area can improve the nutrient quality of the soil and the healthy development of native bees. We divided 12 plots of land into three treatment groups: a buckwheat monoculture control and two different seed mixes of native and naturalized North American flowers. To investigate soil health, we analyzed the pH and the levels of nitrogen, potassium, and phosphorus in the soil before and after planting, and we will perform an analysis of the soil microbial community using 16S ribosomal RNA sequencing. To assess native bee health, we constructed wood and mesh cages over each plot to house blue orchard bees (Osmia lignaria). After being exposed to either the buckwheat crop or one of the wildflower mixes, the bees were measured on several endpoints to determine fitness, including foraging activity, nesting activity, number of offspring produced, offspring emergence as adults, and offspring longevity after emergence. We then performed a comparative analysis to determine whether the native flower mixes had a positive effect on soil quality and bee development when compared to the monoculture control.
- Determine whether native seed mixes, when compared to monoculture plantings, have a positive impact on native bee development and health.
- Evaluate the soil quality of plots separately planted with native seed mixes and nonnative monocultures.
- Recommend more sustainable agriculture systems that support soil health and robust pollinator populations.
Research
This was a semi-field study on the effects of different wildflower or monoculture plantings on bee and soil health. The field plots were located at the University of Arkansas Milo J. Shult Agricultural Research and Extension Center in Fayetteville, AR.
Flower Planting
| Planting Group | Cage ID | 2021 Wildflower List | 2022 Wildflower List |
| Buckwheat | A | Fagopyrum esculentum | Fagopyrum esculentum |
| D | Fagopyrum esculentum | Fagopyrum esculentum | |
| F | Fagopyrum esculentum | Fagopyrum esculentum | |
| L | Fagopyrum esculentum | Fagopyrum esculentum | |
| Honey Bee Wildflower Mix | C | Asclepius tuberosa, Aster oblongifolius, Chamaecrista fasciculata, Coreopsis lanceolata, Coreopsis verticillata, Desmodium canadense, Echinacea purpurea, Erigeron annuus, Eryngium yuccifolium, Gaillardia aristata, Heliopsis helianthoides, Heterotheca subaxillaris, Liatris spicata, Papaver rhoeas, Rudbeckia fulgida, Rudbeckia hirta, Scrophularia marilandica, Trifolium pratense | Asclepius tuberosa, Daucus carota, Desmodium canadense, Eryngium yuccifolium, Heterotheca subaxillaris |
| E | Aster oblongifolius, Chamaecrista fasciculata, Coreopsis lanceolata, Coreopsis verticillata, Erigeron annuus, Gaillardia aristata, Heliopsis helianthoides, Heterotheca subaxillaris, Liatris spicata, Monarda citriodora, Oenothera lamarckiana, Papaver rhoeas, Rudbeckia hirta, Scrophularia marilandica, Trifolium pratense |
Erigeron annuus, Heliopsis helianthoides, Heterotheca subaxillaris, Lactuca spp. (likely serriola), Monarda citriodora | |
| I | Aster oblongifolius, Chamaecrista fasciculata, Coreopsis lanceolata, Coreopsis verticillata, Desmodium canadense, Erigeron annuus, Eryngium yuccifolium, Gaillardia aristata, Heliopsis helianthoides, Liatris spicata, Monarda citriodora, Rudbeckia fulgida, Rudbeckia hirta, Scrophularia marilandica, Trifolium repens |
Coreopsis lanceolata, Desmodium canadense, Erigeron annuus, Eryngium yuccifolium, Gaillardia aristata, Heterotheca subaxillaris, Monarda citriodora | |
| K | Asclepius tuberosa, Aster oblongifolius, Coreopsis lanceolata, Coreopsis verticillata, Desmodium canadense, Echinacea purpurea, Erigeron annuus, Eryngium yuccifolium, Gaillardia aristata, Heliopsis helianthoides, Liatris spicata, Rudbeckia fulgida, Rudbeckia hirta, Torilis sp., Trifolium pratense, Trifolium repens | Asclepius tuberosa, Desmodium canadense, Erigeron annuus, Rudbeckia hirta | |
| Eastern Wildflower Mix | B | Aster oblongifolius, Buddleia sp. (hybrid), Chamaecrista fasciculata, Coreopsis lanceolata, Echinacea purpurea, Erigeron annuus, Gaillardia aristata, Medicago lupulina, Monarda citriodora, Oenothera lamarckiana, Rudbeckia hirta, Stokesia laevis, Trifolium pratense | Chamaecrista fasciculata, Erigeron annuus, Gaillardia aristata, Heterotheca subaxillaris, Lactuca spp. (likelyserriola), Monarda citriodora |
| G | Asclepius tuberosa, Aster oblongifolius, Buddleia sp. (hybrid), Centaurea cyanus, Chamaecrista fasciculata, Coreopsis lanceolata, Coreopsis tinctoria, Cosmos bipinnatus, Desmodium canadense, Echinacea purpurea, Erigeron annuus, Eryngium yuccifolium, Gaillardia aristata, Heterotheca subaxillaris, Monarda citriodora, Phlox drummondii, Plantago lanceolata, Rudbeckia fulgida, Rudbeckia hirta, Stokesia laevis, Trifolium pratense, Trifolium repens | Desmodium canadense, Echinacea purpurea, Heterotheca subaxillaris, Monarda citriodora | |
| H | Asclepius tuberosa, Aster oblongifolius, Buddleia sp. (hybrid), Centaurea cyanus, Coreopsis lanceolata, Coreopsis tinctoria, Cosmos bipinnatus, Echinacea purpurea, Erigeron annuus, Eryngium yuccifolium, Gaillardia aristata, Heterotheca subaxillaris, Monarda citriodora, Phlox drummondii, Plantago lanceolata, Rudbeckia fulgida, Rudbeckia hirta, Stokesia laevis, Trifolium pratense, Trifolium repens |
Asclepias tuberosa, Coreopsis lanceolata, Desmodium canadense, Echinacea purpurea, Erigeron annuus, Eryngium yuccifolium, Gaillardia aristata, Rudbeckia hirta | |
| J | Asclepius tuberosa, Aster oblongifolius, Buddleia sp. (hybrid), Chamaecrista fasciculata, Coreopsis lanceolata, Coreopsis tinctoria, Cosmos sulphureus, Desmodium canadense, Echinacea purpurea, Erigeron annuus, Eryngium yuccifolium, Gaillardia aristata, Heterotheca subaxillaris, Monarda citriodora, Phlox drummondii, Plantago lanceolata, Rudbeckia fulgida, Rudbeckia hirta, Stokesia laevis, Trifolium pratense, Trifolium repens | Chamaecrista fasciculata, Desmodium canadense, Erigeron annuus, Heterotheca subaxillaris |
In the springs of 2020, 2021, and 2022, the field plots were planted with either a buckwheat monoculture or with a wildflower seed mix. Plots were labelled A-L, and randomly assigned a treatment group: buckwheat (Plots A, D, F, and L), honey bee wildflower mix (Plots C, E, I, and K), and eastern wildflower mix (Plots B, G, H, and J). Buckwheat (Fagopyrum esculentum) was chosen due to its fast development time (~4-6 weeks from planting to bloom period) and its long period of bloom. It is also a fairly heat tolerant crop and can grow well in the rising temperatures of late spring in Arkansas. The other eight field plots were planted with wildflowers, four with a "honey bee flower mix" and four with a "eastern pollinator flower mix," both purchased from the Sustainable Seed Company. The honey bee flower mix notably contained lacy phacelia (Phacelia tanacetifolia), a wildflower that is preferred by Osmia lignaria, as well as several other mason bee species, including Osmia bicornis, though it did not grow well or bloom at the field site. Additionally, several wildflower species had already been established at the research site, including dandelion (Taraxacum officinale), red clover (Trifolium pratense), and butterfly weed (Asclepias tuberosa). These wildflowers were removed from the buckwheat plots, but allowed to grow in the wildflower plots. The list of successfully flowering plants in each cage is listed in Table 1. Wildflower growth and diversity was better in the 2021 season, due to heat waves and drought in the 2022 season.
Soil Sampling and Analysis
Soil samples were collected from each plot before planting and ~3 months following planting each year (2020, 2021, and 2022). 15cm soil cores were taken with a sterile soil probe. 7 cores were taken from each research plot, as well as from 3 plots in an adjacent grassy field. Cores from each plot were homogenized in a sterile bucket and collected. An amount of soil (~56.7 g) was set aside from each plot and stored in a -80°C freezer for later soil microbiome analysis, which is still ongoing. The bacterial 16S rRNA gene from these samples will be amplified by PCR and then sent for sequencing, in order to measure the diversity of the bacterial communities. The rest of the soil samples (~475 mL per plot) were dried for a week and then sent to the Marianna Soil Test Laboratory in Marianna, AR for analysis of the pH and nutrient content (levels of nitrogen, phosphorus, and potassium).
Bee Cage Preparation

Wooden cage frames, measuring 8'x5'x5' were built over each plot in 2020, but due to working restrictions related to the Covid-19 pandemic, the cages were not able to be completed in time to release the blue orchard bees that year.
In the spring of 2021, the bee cage construction was completed, with aluminum mesh screen around the wood frame cages. Inside each cage was placed a mason bee nest box filled with plastic and paper nest tubes of diameter 0.25" and length 7", as well as a water dish and a dish of river sand. Mason bees, like Osmia lignaria, collect mud to use in their nest construction and can be particular in their preferences for soil textures. The soil at the field site was primarily clay and silt loam, so this larger grain sand was provided to allow the bees to mix soil as needed to their preferred texture.
Acquiring and Releasing Osmia lignaria
The blue orchard bees (Osmia lignaria) were ordered from a well-established commercial supplier and arrived as adults still in cocoons. They were stored at 4°C until their emergence and release. Blue orchard bees were chosen for several reasons. They are a native species, found widespread across the United States. They are efficient pollinators of several fruit, vegetable, and nut crops, and because of this are one of the few solitary bee species to be commercially managed. Finally, they are a generalist species and able to feed on a variety of flower species. Generalist feeders can be more flexible to changes in their diet, but in many cases can benefit from having a variety of floral resources available. Blue orchard bees naturally emerge from their cocoons in mid-Spring, though the exact time can vary depending on the region and weather conditions. The bees were taken from the 4°C refrigerator, allowed to emerge at room temperature, and then released into the cages in June 2021 and then again in July 2022, coinciding with the peak bloom of the wildflowers and buckwheat. In 2021, 50 female and ~25 males were released, and in 2022, 40 females and 8 males were released into each cage. The ratio of males to females was decreased in 2022, as the males do not participate in nest construction or provisioning, and are only needed for mating. Excessive males seem to interfere with female foraging activity, as well, as they can knock females off of flowers during mating attempts.
Following release, the adult bees mated. After mating, females began foraging and nest building within the cages. Blue orchard bee females build their nests in hollow tubes. In the wild, they often use hollow reeds and stems, or old holes made by other arthropods, and lay their eggs in a series of sequential cells down the tube. Females gather pollen and nectar to make a provision for each offspring. Once the provision is made, they lay an egg and then build a partition of mud before starting again on the next egg cell. When a tube is filled, they add a thick plug of mud at the opening to deter predators or parasites from reaching the offspring inside the tube. Offspring develop in the tubes, pupate in the autumn, and overwinter as adults still inside their pupal cocoons. They emerge the following spring.
Foraging, Nesting, and Fecundity Observations
During this time, we observed and recorded the bee foraging activity and behavior (number of bees foraging in each cage, number of floral visits within a 2 minute period at 3 times of day - morning, noon, and late afternoon, and floral preferences). We also observed their nesting activity (number of trips to the nest within a 2 minute period, number of cells with mud or pollen residues, and number of capped tubes).
In 2021, the adult female blue orchard bees lived about a month following their emergence, which was their expected natural lifespan. After the adults died off for the year, the nest boxes were left undisturbed, so the offspring could complete their development into adults to overwinter. In the summer of 2022, however, an extended heat wave and drought in Arkansas caused the bees to die prematurely, before nesting could be completed.
In the spring of 2022, nest boxes from the 2021 foraging period were collected and measured on the following endpoints: number of capped nest tubes (paper or plastic), number of nest tubes with pollen or mud residues, number of tubes with offspring cells, total number of offspring cells, number of empty cells, number of cells with pollen and nectar provision, number of cells with larvae, and number of cells with cocoons. Any nest parasites, parasitoids, or scavengers were also noted. Cocoons were collected, weighed, and then placed in clean plastic lab cages at room temperature to allow emergence. Emerged adult bees were given 50:50 w/v organic honey in DI water solution daily and their longevity was measured.
Soil Nutrient Analysis
Soil samples were collected in the Spring (March/April) and Fall (September/October) of 2020, 2021, and 2022 in order to analyze the nutrient content and microbial communities in the soil. Soil samples were sent to the Marianna Soil Test Laboratory in Marianna, AR for nutrient analysis. The analysis included the concentrations of phosphorus, potassium, zinc, sulfate, nitrate, magnesium, and iron in the soil, as well as the pH and the estimated cation exchange capacity (CEC). The results of the pre-planting and post-planting soil nutrients were compared each year, as nutrients can fluctuate by season. Results were analyzed using a pooled t-test. The results from 2020 and 2021 are shown here, but not 2022, as the 2022 post-planting nutrient analysis reports have not yet been received from the test laboratory.
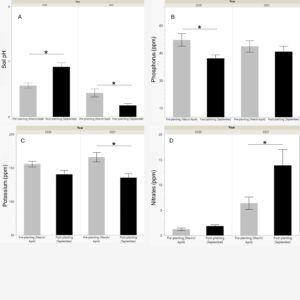
The pH was significantly different by season for both 2020 (p=0.0015) and 2021 (p=0.0102). However, pH increased, becoming closer to neutral, during the post-planting soil collection in 2020, whereas it decreased, becoming more acidic in the post-planting of 2021 (Fig. 2). pH fluctuated between pH 6 and 7.5 for all soil collection results. Phosphorus concentration in the soil decreased from pre-planting to post-planting collections, but this decrease was only significant in 2020 (p=0.0122). Potassium concentration also decreased in the post-planting collections, though this was only significant in 2021 (0.0024). Nitrate (NO3) concentration in the post-planting soil increased slightly in 2020 and significantly in 2021 (p=0.0369). Nitrate levels were also significantly higher for both pre-planting and post-planting soil in 2021 compared to 2020 (Fig. 2). Before the buckwheat and wildflower plots were established in 2020, the area had been a frequently-mowed, but unplanted, grassy field. In 2021, the field plots had all been planted for a growing season with buckwheat or wildflowers. This could account for the overall increase in nitrate concentrations in 2021; however, soil samples taken from a grassy field adjacent to the field plots also showed higher nitrate concentrations in 2021 than in 2020. This would suggest that the planting activity was not responsible for causing the increase in nitrates from 2020 to 2021, and that instead weather conditions, natural variation, or equipment failures could account for this increase.
The post-planting results of the buckwheat, honey bee flower mix, and eastern flower mix groups were then compared to samples taken from nearby grassy fields at the research site. Post-planting samples were analyzed using a one-way ANOVA, by treatment group. In the case of the soil pH and nitrate levels in 2020, a nonparametric Kruskal-Wallis H test was performed instead. There was no significant effect of flower planting type on post-planting soil pH for either 2020 or 2021. In all plots, pH was neutral or mildly acidic, ranging from 6.1-7.4. Post-planting nitrate levels were also not significantly affected by flower planting type. In 2020, the eastern wildflower planting group and the honey bee flower planting groups had higher nitrate concentration averages than both the buckwheat and grassy field groups, though this difference was not significant. In 2021, buckwheat had a lower nitrate concentration means than the honey bee wildflowers, eastern wildflowers, and grassy field, but it was not significant.

In 2020, there was a significant effect of flower planting on post-planting potassium levels, though this was only seen in pairwise comparison between the grassy field and buckwheat groups (p=0.026, Fig. 3). The grassy field has significantly higher potassium levels than the buckwheat plot. Both the eastern flower mix and the honey bee flower mix had average potassium levels higher than the buckwheat and lower than the grassy fields, but these differences were not significant. Both of the wildflower planting groups, the eastern mix and the honey bee mix, had significantly higher post-planting phosphorus concentrations than the grassy field samples (Fig. 3). In 2021, there was no significant difference among treatment groups for either potassium or phosphorus concentrations. For both years, there was a general trend across all treatment groups of phosphorus levels decreasing from the March pre-planting samples to the September post-planting ones. The wildflower groups retained higher phosphorus levels later in the year than the buckwheat and grassy field groups, though these differences were only significant in 2020.
Bee Foraging Activity
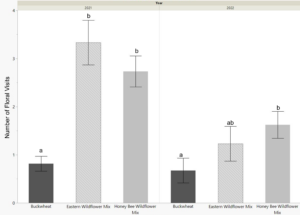
The released bees were active and foraging for about 6 weeks in 2021 and 2 weeks in 2022. During this period they were observed 4-6 times per week during the morning (9am), midday (12pm), or late afternoon (6pm) for 2 minute intervals. The cages planted with eastern wildflower mix and honey bee wildflower mix had a significantly higher average of floral visits in 2021 (p<0.0001, Fig. 4) and higher totals of floral visitation over the whole activity period (Table 2) when compared to the buckwheat cages. In 2022, both wildflower mixes had higher averages than buckwheat, though it was only significantly higher for the honey bee wildflower mix plantings (p=0.0277, Fig. 4). Additionally, the activity period and longevity of the adult bees was longer in the wildflower groups than the buckwheat group for both years; bee foraging activity and signs of live bees ended in the buckwheat cages 1-2 weeks before that in the wildflower planted cages.
Osmia lignaria also showed strong feeding preferences for certain wildflowers when given a variety of wildflower options to feed on (Table 2). Lemon bee balm (Monarda citriodora), which was present in the eastern wildflower mix, but also growing wild at the field site and so also present in two of the honey bee mix cages, was most often visited by the bees. Several other wildflowers were commonly visited, including Rudbeckia hirta, Asclepias tuberosa, Heliopsis helianthoides, Coreopsis lanceolata, and Gaillardia aristata.
Foraging activity was also highest in the morning and midday observations, and low in the late afternoon.
| Planting Mix | Plot ID | Wildflower | 2021 Total Number of Floral Visits | 2022 Total Number of Floral Visits |
| Buckwheat | A | Fagopyrum esculentum | 7 | 6 |
| D | Fagopyrum esculentum | 14 | 5 | |
| F | Fagopyrum esculentum | 18 | 4 | |
| L | Fagopyrum esculentum | 10 | 10 | |
| Honey Bee Wildflower Mix | C | Asclepias tuberosa | 12 | 0 |
| Coreopsis lanceolata | 1 | 0 | ||
| Coreopsis verticillata | 1 | 0 | ||
| Desmodium canadense | 0 | 2 | ||
| Echinacea purpurea | 1 | 0 | ||
| Eryngium yuccifolium | 2 | 0 | ||
| Gaillardia aristata | 2 | 0 | ||
| Heliopsis helianthoides | 1 | 0 | ||
| Heterotheca subaxillaris | 2 | 12 | ||
| Papaver rhoeas | 1 | 0 | ||
| Rudbeckia hirta | 1 | 0 | ||
| Cage Total | 26 | 14 | ||
| E | Coreopsis lanceolata | 1 | 0 | |
| Erigeron annuus | 1 | 0 | ||
| Gaillardia aristata | 1 | 0 | ||
| Heliopsis helianthoides | 1 | 4 | ||
| Heterotheca subaxillaris | 2 | 2 | ||
| Monarda citriodora | 31 | 7 | ||
| Rudbeckia hirta | 1 | 0 | ||
| Cage Total | 38 | 13 | ||
| I | Coreopsis lanceolata | 9 | 1 | |
| Desmodium canadense | 2 | 0 | ||
| Eryngium yuccifolium | 0 | 2 | ||
| Gaillardia aristata | 2 | 0 | ||
| Heliopsis helianthoides | 10 | 0 | ||
| Monarda citriodora | 43 | 8 | ||
| Rudbeckia hirta | 2 | 0 | ||
| Scrophularia marilandica | 2 | 0 | ||
| Cage Total | 70 | 11 | ||
| K | Asclepias tuberosa | 10 | 0 | |
| Coreopsis lanceolata | 2 | 0 | ||
| Coreopsis verticillata | 1 | 0 | ||
| Desmodium canadense | 2 | 0 | ||
| Erigeron annuus | 1 | 0 | ||
| Gaillardia aristata | 1 | 0 | ||
| Heliopsis helianthoides | 4 | 0 | ||
| Rudbeckia hirta | 8 | 2 | ||
| Torilis sp. | 1 | 0 | ||
| Cage Total | 30 | 2 | ||
| Eastern Wildflower Mix | B | Coreopsis lanceolata | 17 | 0 |
| Erigeron annuus | 2 | 0 | ||
| Gaillardia aristata | 16 | 0 | ||
| Medicago lupulina | 1 | 0 | ||
| Monarda citriodora | 40 | 4 | ||
| Rudbeckia hirta | 3 | 0 | ||
| Trifolium pratense | 5 | 0 | ||
| Cage Total | 84 | 4 | ||
| G | Asclepias tuberosa | 12 | 0 | |
| Coreopsis lanceolata | 7 | 0 | ||
| Cosmos bipinnatus | 2 | 0 | ||
| Desmodium canadense | 2 | 1 | ||
| Echinacea purpurea | 3 | 3 | ||
| Gaillardia aristata | 1 | 0 | ||
| Heterotheca subaxillaris | 0 | 3 | ||
| Monarda citriodora | 6 | 1 | ||
| Rudbeckia hirta | 1 | 0 | ||
| Cage Total | 34 | 8 | ||
| H | Asclepias tuberosa | 10 | 16 | |
| Coreopsis lanceolata | 0 | 3 | ||
| Coreopsis tinctoria | 1 | 0 | ||
| Cosmos bipinnatus | 1 | 0 | ||
| Echinacea purpurea | 1 | 0 | ||
| Rudbeckia hirta | 8 | 3 | ||
| Stokesia laevis | 1 | 0 | ||
| Trifolium repens | 1 | 0 | ||
| Cage Total | 23 | 22 | ||
| J | Asclepias tuberosa | 14 | 0 | |
| Coreopsis lanceolata | 7 | 0 | ||
| Coreopsis tinctoria | 1 | 0 | ||
| Desmodium canadense | 0 | 1 | ||
| Echinacea purpurea | 1 | 0 | ||
| Erigeron annuus | 1 | 0 | ||
| Gaillardia aristata | 3 | 0 | ||
| Monarda citriodora | 15 | 0 | ||
| Rudbeckia hirta | 15 | 0 | ||
| Stokesia laevis | 2 | 0 | ||
| Cage Total | 59 | 1 |
Bee Nesting Activity
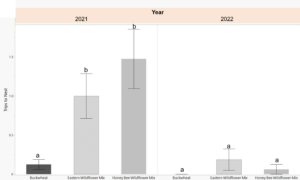
Female bees began visiting the nest boxes and collecting pollen provisions 7 days following their release in the cages. The first capped tubes were observed 14 days after release. Their trips to the nest box, while carrying mud and pollen, were recorded at 2 minute intervals 3-4 times per week. In 2021, bees in the cages with eastern and honey bee wildflower mix had significantly more visits to the nest boxes than those in the buckwheat planted cages (p=0.003). In 2022, overall nesting activity was low. There were no observed nest box visits for the buckwheat planted cages and only few in the wildflower planted cages (Fig. 5).
The bees were also given the option of building their nests in plastic or paper nesting tubes. The plastic tubes were put out to see if bees would nest in them. The development of the offspring through the larval instars and pupation could then be observed through the transparent tubes. The bees had a strong preference for the paper tubes, however, and would not nest in the plastic when paper was available. The plastic tubes were more slippery and retained more humidity, which might have made them less ideal for mason bee nesting.
Bee Fecundity
Bee fecundity was measured as the following endpoints: number of capped tubes, total number of nest cells, number of pollen provisions, number of larvae, and number of cocoons. Each of the nest cells could either be empty, contain a pollen provision and unhatched egg, contain a dead larvae that never pupated, or contain a cocoon. These were recorded in early 2022 for the adult bees that had been active in summer 2021. The buckwheat cages had a little nesting activity, but no successful nest cell construction, provisioning, or egg laying. Three of the honey bee wildflower mix cages and one of the eastern wildflower mix cages managed to produce live offspring. The number of capped tubes, cells, provisions, larvae, cocoons, and emerged adult offspring were all highest in the honey bee wildflower mix cages, lower in the eastern mix cages, and non-existent in the buckwheat cages, though these differences were not significant (Fig. 6), likely due to the low replication.

There were some nest parasites observed in the nest tubes, as well. The first were Dermestid beetle larvae (possibly Trogoderma sp.), which were found in 6 nest cells of the Cage E (honey bee wildflower mix) nest tubes. The beetle larvae had chewed through 3 cocoons and eaten the pupa inside. Dermestid beetles tend to be scavengers, however, rather than predators, so it is likely that the bee pupae had died prior to the beetle feeding. There were also barklice (Liposceles sp.) found in 3 cells of Cage G (eastern wildflower mix) and 6 cells of Cage I (honey bee wildflower mix). They were found in cells with mold and fungal growth, which was probably their food source. They are more likely opportunistic nest scavengers and fungal feeders, rather than posing any direct harm to the bee offspring. There were also a few jumping spiders in the tubes, but only in unoccupied tubes. There were notably no parasitoid wasps or wasp larvae found in the bee nests or parasitizing the bees. Some wasps (likely Chalcidae) were observed in Fall 2021 on the outside of the bee cages, but they were unable to pass through the screen. The aluminum screen seemed to deter parasitoid wasps of O. lignaria from reaching the nests.
The whole cocoons (those not eaten by beetle larvae) were weighed, transferred to clean cages, and allowed to emerge at room temperature in the spring of 2022. Of the 24 cocoons, 16 emerged as adults, 2 females and 14 males. 7 of these managed to live beyond a week and 3 of them beyond 20 days.
|
Planting Group |
Cage | Cocoon Weight (mg) | Sex |
Bee Longevity After Emergence (days) |
| Eastern Wildflower Mix | E | 61 | F | 1 |
| 25 | - | - | ||
| 62 | M | 3 | ||
| 52 | M | 4 | ||
| 42 | M | 4 | ||
| 25 | - | - | ||
| 37 | M | 3 | ||
| 38 | M | 3 | ||
| 35 | M | 3 | ||
| 25 | - | - | ||
| 29 | M | 3 | ||
| I | 50 | M | 14 | |
| 42 | M | 9 | ||
| 8 | - | - | ||
| K | 11 | - | - | |
| 38 | M | 27 | ||
| Honey Bee Wildflower Mix | G | 42 | M | 12 |
| 26 | - | - | ||
| 41 | M | 24 | ||
| 44 | F | 23 | ||
| 27 | M | 6 | ||
| 28 | M | 8 | ||
| 11 | - | - | ||
| 1 | - | - |
Discussion
With modern agriculture, there has been a rise in more intensive growing systems, including monoculture plantings, increased use of pesticides and fertilizers, and frequent tilling. There has also been an increase in the amount of land used either as rangeland for livestock or fields for crop plantings. These changes have brought with them a great deal of investigation into how these intensive agricultural practices may effect ecosystem health and plant growth. Monoculture plantings without crop rotation, for example, have been shown to cause decreasing yields over time. This is likely due to the depletion of soil nutrients taken up by the crop plants, which over time can interfere with plant growth. Crop producers often deal with these problems either through the implementation of a crop rotation system or through the heavy use of fertilizers, though the use of fertilizers can cause further issues, such as algal blooms in local water supplies. As such, it is important to investigate how different agricultural methods can affect soil health and ways to ameliorate the negative effects of intensive agriculture in order to develop more sustainable agricultural practices.
Soil pH can vary based on time of year, region, and habitat type, and can impact nutrient absorption by plants and species richness of soil microbial communities. The highest diversity of microbial communities are generally found in soils near neutral pH, from ~6.5-7.5. Though some plants prefer highly acidic soils, below pH 5.5, these conditions can reduce microbe diversity and interfere with calcium and magnesium absorption. In this study, pH was consistent across planting groups and close to neutral in all. Soil was slightly more acidic in March and became closer to neutral in September in 2020, though this trend was not observed in 2021.
Potassium is an essential nutrient for plant growth, involved in enzyme regulation, gas exchange, and ATP production. Plants with sufficient potassium tend to be hardier in drought condition and more resistant to certain diseases. Potassium levels were highest in the grassy field group. The fields were mowed regularly, but otherwise left undisturbed, without any tilling or planting. After one year of planting, buckwheat had the lowest potassium levels and levels that are considered below the ideal of 131-175 ppm for this region.
Phosphorus is vital for plants for their energy storage, in the form of ATP. Insufficient phosphorus can slow or even stunt plant development. Uptake of phosphorus by plants depends on both the soil pH, with a pH between 6.5 and 7.0 being the optimum, and organic matter content of the soil, which can be affected by the variety of plants grown in the area. Phosphorus was higher in the pre-planting samples than the post-planting ones, and decreased later in the year, though both time of year averages were within the range for adequate phosphorus levels. In the post-planting analysis, the phosphorus levels in the grassy field samples were significantly lower than those in either of the wildflower planting groups. Buckwheat levels were on average lower than the wildflowers and higher than the grassy field, though not significantly. Wildflower plantings could have the potential to maintain higher phosphorus levels when compared to grassy fields and help prevent any phosphorus deficiencies in nearby crop plants, though the range of these effects are still unknown.
Nitrates are a form of usable nitrogen that can be taken up by plant roots and used for plant growth and leaf development. They are also an essential nutrient and insufficient nitrate levels can interfere with growth. Plants can differ in their nitrate requirements. Some vegetables, such as tomatoes, squash, and sweet corn take up higher levels of usable nitrogen, and over time can deplete the levels in the soil. Others, particularly legumes (Family: Fabaceae), can increase levels of available nitrogen through their association with nitrifying Rhizobium bacteria. Levels of nitrates at the research site were low across all treatment groups. They were on average higher in the two wildflower treatments, though these differences were not significant. Both wildflower mixes included members of the Fabaceae family, which could increase the nitrate content of the soil. There were, however, over 20 species of wildflower in each mix, and of those only 1 species in the honey bee mix and 3 in the eastern mix were legumes, so the nitrifying effect could be minor.
Osmia lignaria is a generalist feeder, but still showed strong floral preferences, particularly to Monarda citriodora, Rudbeckia hirta, and Asclepias tuberosa. Many bee species feed on pollen and nectar as larvae, but only on nectar as adults. Female mason bees (Osmia spp.), however, require pollen in the days following their emergence from cocoons, in order to complete the development of their ovaries and reproduce. Typically, they mate, feed on pollen and nectar for 7-10 days, and then begin provisioning and constructing nest cells. This behavior and morphology could help explain the differences in fecundity for the wildflower treatments and the buckwheat monoculture treatment. Female O. lignaria in the buckwheat cages showed minor interest in the nest tubes early in the observation period, but failed to construct any nest cells, collect any provisions, and lay any eggs. Buckwheat yields are improved by insect pollinator visitation, including that by honey bees (Apis mellifera), bumble bees (Bombus spp.), and mason bees (Osmia spp.), and in return buckwheat can provide a quality source of nectar. The pollen quality and protein content, however, may be inadequate for O. lignaria females to complete their ovary development and produce offspring.
In the wildflower planted cages, foraging activity, nesting activity, and fecundity were all higher than in the buckwheat cages. The successful production of offspring suggests that the female O. lignaria were able to find an adequate pollen source among the mixed wildflowers complete ovary development, and lay eggs. Though O. lignaria, and several other generalist feeding bees, are able to forage and survive on certain monoculture plantings, their health, longevity, and fecundity seem to improve with greater floral diversity and the presence of their more preferred flowers. Additionally, though the activity period of emerged adult mason bees is relatively short, often around 4-6 weeks, it is vital for the females to have access to floral blooms throughout the entirety of this period. Wildflower plantings, especially with staggered bloom times, can help ensure the bees will have a food source throughout their foraging and nesting period, which can help improve the number of offspring they can produce.
In the summer of 2022, the field site in Arkansas, as well as many other areas around the region, experienced an extended drought and heat wave. Wildflower diversity was poor 2022, compared to 2021, and bee health and longevity were poor overall, in all treatment groups.
Though their was a clear benefit of the wildflower plantings on bee health, activity, and fecundity when compared to the buckwheat, improvements to the husbandry of the bees could help improve the bee fecundity overall and thus better show the differences in health and reproduction among the treatment groups. A larger cage size, such as 10'x8'x5' could increase floral availability for each bee in the cage, which could increase the amount of floral provisions and offspring. In 2022, fewer males were released, which seemed to improve female bee activity in the early days following release. Though males are vital for mating and producing female offspring, they can interfere with female bee foraging, as the males can be aggressive in their mating attempts and can knock female bees off of flowers. Mating before release, in a lab setting, and then only releasing female bees into the observation cages could also help improve female foraging and successful reproduction.
Water retention and mold growth was an issue in several of the bee nest boxes, and could have affected bee larval development. Nest cells with mold, sometimes had a pollen provision present, but no larva or developed cocoon. Similarly, nest cells with cocoons did not have any visible mold growth. Removing the nest boxes earlier in the year and storing them in a cool, dry location, such as a 4°C refrigerator, could help reduce moisture damage and mold and improve the bee offspring development into adult bees.
There was also a much higher ratio of male offspring to females. This could be due to unsuccessful mating attempts by the male bees. As with all bees, O. lignaria females are produced from a fertilized egg, while males are produced from an unfertilized egg. If the females were not mated properly by the males, then they would be able to produce male offspring, but not females. It is also possible that moisture retention affected the female nest cells more than the males, causing fewer female offspring to develop. Mason bees lay their female eggs farther back in the tubes and their males closer to the entrance, as the males emerge first from their cocoons. The mold growth was more common farther back in the tubes, and so could have disproportionately affected the female offspring over the males.
Educational & Outreach Activities
Participation Summary:
Published News Articles
-
“Entomology student investigates impact of adding pollinator-friendly plants to pastures, fields” University of Arkansas News. <https://news.uark.edu/articles/57116/entomology-student-investigates-impact-of-adding-pollinator-friendly-plants-to-pastures-fields>
- “Researchers aim to strengthen bee pollinator populations.” Written by George Jared for Talk Business & Politics. <https://talkbusiness.net/2021/08/researchers-aim-to-strengthen-bee-pollinator-populations/>
Webinars, Talks, and Presentations
- Presentation to the Ozark Society, a hiking and conservation club with chapters throughout the southeastern United States. The presentation discussed the project, the importance and diversity of native bees, and ways to improve bee health, diversity, and activity in gardens and local farms. December 2020. (Remote due to Covid-19 Quarantine).
- Talk at the OMNI Center, a local center involved in ecology and conservation, discussing native bees and how to improve pollinator diversity with wildflower plantings. December 2021. The OMNI Center, Fayetteville, AR 72701.
- Talk at Audubon Camp, a summer camp for young and aspiring biologist students, from grades 7-9. The talk covered pollinator diversity and used the research project to highlight the importance of floral diversity in improving bee health. June 2022. Ozark Natural Science Center, Huntsville, AR.
- Presentation at the Botanical Garden of the Ozarks, discussing the relationship between floral diversity and pollinator diversity and health, using the research project as an example. BGO, Fayetteville, AR.
Other
- Radio interview for local radio station, KUAF. “U of A Ph.D. Student Researching Ways to Help Wild Bees in Decline.” Ozarks at Large on KUAF Radio Station. <https://www.kuaf.com/post/u-phd-student-researching-ways-help-wild-bees-decline>
Project Outcomes
During the span of this project, we were able to give several talks, presentations, and interview around Northwest Arkansas and were able to speak with several local farmers, as well as many hobbyist gardeners. Though modern farming systems tend to favor large-scale monoculture plantings, adding wildflower diversity can greatly improve bee activity and fecundity. Our main recommendation throughout this project was the addition of hedgerows or other wildflower strips to attract and support pollinator populations. As we saw in the study, the buckwheat monoculture plantings alone resulted in low foraging activity and no successful reproduction. A buckwheat monoculture field, with an added border of wildflowers, would therefore likely attract more pollinators and support the health of native bees, such as mason bees, better than a buckwheat monoculture unbroken by any other flower plantings.
Four local growers (3 with canola fields, 1 with a pear and persimmon orchard) reached out to us that they had begun to plant wildflower patches alongside their fields following the talks given at the Ozark Society, OMNI Center, and Botanical Gardens.
Many crops are dependent on or benefited by the activity of insect pollinators. Bees, in particularly, can improve the yields of many crops, including buckwheat, almonds, and blueberries (among many others), and therefore the profits for growers. Declines in pollinator populations, range sizes, and activity have caused great concern, both for conservationists and growers of bee-pollinated crops. In some instances, such as the almond blooms in California, honey bee hives have to be rented at great cost to the grower and brought in from out of state in order to ensure adequate pollination of the crop. Finding ways to improve local pollinator populations, of both honey bees and native bees, can help improve crop yields, while saving on the costs of importing honey bee hives. Local wildflowers are often hardy to local conditions and, once established, can grow with minimal maintenance and extra cost. These wildflowers can help support robust local pollinator communities and improve the health and fecundity of solitary bees, like Osmia lignaria. Actively foraging bees in wildflower patches can then spillover into crop fields and improve yields for growers. This method of improving pollinator populations through wildflower plantings may offer a more sustainable and cost-effective method of improving yields of insect-pollinated crops, when compared to importing the non-native honey bees from out of state.
This project highlighted the negative impact that an unbroken monoculture can have on pollinators. Many pollinators have specific preferences and needs for their diet. If these needs are not met by the monoculture, then they will not be able to reproduce or forage well in the area. We observed this in our buckwheat cages, where bee foraging activity was low and the bees failed to reproduce.
In the future, a survey of pollinator abundance, species richness, and species evenness in different farm planting systems (unbroken monocultures, monocultures with wildflower strips, and polycultures) in Northwest Arkansas could help support the results from this semi-field study and help show the impact of floral availability and diversity on pollinator population structure. Some improvements to the husbandry of the blue orchard bees in the foraging cages in future years could also help strengthen the results from the study.