Final report for GS23-286
Project Information
The delicious peach is a foundational crop in the Southeastern United States, with deep cultural roots and major economic importance. In 2021, over 120,000 tons of peaches across South Carolina and Georgia were harvested, valued at more than $170 million. Despite this success, peach production is facing new challenges. The U.S. Environmental Protection Agency (EPA) recently restricted the use of chlorpyrifos, a broad-spectrum insecticide that growers have long relied on for the management of key insect pests. For many foliar pests, there are still alternative insecticides available. However, for borer pests like the lesser peachtree borer (LPTB), effective chemical options are lacking.
Adult moths seek out peach trees where the females will lay their eggs. The LPTB larvae bore into the wood of peach trees to feed, subsequently damaging vascular tissue, weakening branches and scaffolds, reducing tree vigor, and making trees more susceptible destructive pathogens. Over time, infestations can lead to significant yield losses by reducing fruit-bearing capacity and/or, in severe cases, killing entire trees. Because the larvae remain hidden inside the tree, LPTB is especially problematic because it is difficult to detect early, and once established, infestations are hard to control with conventional insecticides. With growing restrictions on insecticide use, LPTB poses a persistent and costly threat to southeastern peach production, challenging growers to find sustainable and effective management strategies.
One potential alternative management strategy is the use of fungi that naturally infect insects—known as entomopathogenic fungi—which have historically been explored for pest management in other systems. Unfortunately, these fungi can be difficult to use in the field because they are sensitive to environmental conditions, and their effectiveness is limited when pests live hidden inside the tree. However, new research is showing promise by using these fungi in a different way: as endophytes, or beneficial fungi that live inside the plant itself. This approach has already been tested in other crops like pecan, coffee, and cacao. Building on that progress, our study is working to establish the entomopathogenic fungus Beauveria bassiana as an endophyte in peach. If successful, this could provide growers with a safe, sustainable tool to help manage LPTB and protect their orchards for the long term.
This project investigated whether the beneficial entomopathogenic fungus B. bassiana can live inside peach seedlings as an endophyte and produce the insecticidal compound beauvericin (BEA) systemically within plant tissues. The goal was to determine if this endophytic relationship could improve sustainable options for insect pest management in peach fruit production.
We found that B. bassiana successfully colonized peach leaves, stems, and roots through both foliar spray and soil drench application methods, confirming that peach can host this fungus endophytically. However, BEA production within plant tissues was minimal and unlikely to provide direct systemic insect control. By contrast, foliar spray applications resulted in high levels of BEA on plant surfaces, especially leaves at two weeks post treatment with the fungus. Feeding bioassays with mealworms (Tenebrio molitor) confirmed that these surface residues can negatively impact insect survival, supporting the role of B. bassiana as a contact-based insect biocontrol agent in peach.
The ability of B. bassiana to establish inside peach plants still has important implications for integrated pest management (IPM). Endophytic B. bassiana in other crops has been shown to improve plant stress tolerance, growth, and disease resistance. While more research is required to test these possibilities in peaches, this work provides the foundation for exploring B. bassiana and other fungal entomopathogens as a sustainable, multifunctional tool in peach production.
- Determine if B. bassiana can establish inside peach seedlings as an endophyte through foliar spray and soil drench applications with a fungal inoculum.
- Quantify and compare production of the insecticidal compound beauvericin (BEA) on plant surfaces versus inside colonized tissues, using surface sterilization to distinguish external vs. internal presence.
- Assess the functional potential of endophytic and epiphytic B. bassiana as a biocontrol strategy by conducting insect feeding bioassays with the model insect the yellow mealworm, Tenebrio molitor, to test whether fungal and mycotoxin presence impact insect survival.
Cooperators
- (Educator and Researcher)
Research
We asked three linked questions:
- Can B. bassiana (strain GHA) establish inside peach seedlings as an endophyte after we apply the fungal inoculum as either a foliar spray or a soil drench?
- Where does the insecticidal metabolite beauvericin (BEA) show up, on surfaces (epiphytic context) or inside tissues (endophytic context) after B. bassiana inoculation?
- Do B. bassiana and BEA presence in plant tissue have lethal effects on insects tested with a feeding bioassay?
We ran greenhouse experiments with a randomized block design, sampled multiple tissues (leaf, stem, root) across multiple time points (2, 4, 6, 8, and 12 weeks post-inoculation; WPI), and analyzed (a) endophytic colonization by re-culturing B. bassiana from tissues and species and strain confirmation with DNA bar coding at each of the sample periods, (b) BEA quantification by ultra-high performance liquid chromatography coupled with electrospray ionization and tandem mass spectrometry (UHPLC-ESI-MS) at 2 WPI, and (c) insect impact by a standardized feeding assay using leaf tissues 2 WPI and the model insect the yellow mealworm, Tenebrio molitor. Surface sterilization was the key method used to distinguish epiphytic vs. endophytic fungal states throughout our study.
Plants
Plant Material: Guardian ® rootstock peach puts were surface-sterilized (0.5% NaOCl 2 mins → 70% EtOH 2 mins → 3X sterile water rinse), hydrated in sterile water for 4 days at room temperature, cold-stratified in sterile perlite (~ 3 months at 4 °C), germinated, and transplanted to sterilized propagation mix with slow-release fertilizer. Clean starts reduced background fungi; fungicides normally used in the stratification process could not be utilized so clean seed preparation was essential.
Greenhouse Conditions: ~ 25 °C, natural light, drip irrigation every other day for 1 minute. Water withheld 48 h pre-treatment with fungal inoculum. Brief drought improves uptake/adhesion of inoculum and movement into rhizosphere without overstressing seedlings.
Fungal Inoculum:
Beauveria bassiana strain GHA (BotaniGard 22WP) was utilized due to its commercial availability and broad-spectrum insecticidal effects. To create the fungal inoculum used to treat peach seedlings, the fungus was grown on potato dextrose agar media (PDA) for approximately two weeks at 25 °C in the dark. Conidia were then harvested into sterile water and filtered through sterile cheesecloths. We chose to use the standard rate for entomopathogenic fungal work as determined in previous work in other cropping systems as 1×10⁸ conidia mL⁻¹ in sterile water with 0.05% Silwet L-77 (spreader-sticker), validated using a hemocytometer. Prior to inoculating plants, the viability of the fungal inoculum was confirmed by plating a 100 µL aliquot of the inoculum on PDA and ensuring at least a >90% germination rate at 24 h. Inoculum was stored at 4 °C in the dark until use.
Treatments and Application Methods
Foliar Spray: CO2 -pressurized backpack sprayed (TeeJet 8002 tio), ~ 10mL of B. bassiana 1×10⁸ conidia mL⁻¹ in sterile water with 0.05% Silwet L-77 per plant.
Soil Drench: 150 mL B. bassiana 1×10⁸ conidia mL⁻¹ in sterile water with 0.05% Silwet L-77 per plant applied to media surface at stem base.
Control Treatment: Water + 0.05% Silwet L-77 both foliar spray of 10 mL and soil drench of 150 mL per plant to control for any surfactant or handling effects.
After treatment, plants were placed in greenhouse in a randomized block design to minimize micro-environment effects.
Endophytic Colonization Assessment
Sampling Schedule: 2, 4, 6, 8, 12 weeks post inoculation (WPI); n = 5 plants/treatment/time
Tissue Sampling: Two leaflets from mid canopy; two main stem sections (mid-stem and near soil line); two taproot sections.
Surface Sterilization Treatment: 0.5% NaOCl 30 s → 70% EtOH 30 s → 3× sterile water; plate last rinse (100 µL) on PDA to verify sterilization; replace samples if rinse grows.
Plating: Trim tissues edges, cut into ~ 4X4 mm pieces, plate on DBT media (selective media to grow B. bassiana); incubate for ~ 14 days in the dark at 25 °C.
Confirmation of B. bassiana strain GHA: Colonies that morphologically appeared to be B. bassiana (Figure 1) were subcultured, DNA extracted (fungal kit); ITSF1-F/ITS4 PCR primers used for DNA bar coding (~464 bp); agarose gel check; Sanger sequencing (Eurofins genomics); BLAST vs. B. bassiana reference genome on GenBank; GHA strain used as positive control and water negative control.

Quantifying Internal vs. External BEA in Peach Tissues
Design: Independent cohort of peach seedlings (n = 10 plants/treatment). At 2 WPI, collect six leaves, stems, and roots per plant as previously described. Half of the samples of each tissue type subjected to surface sterilization. Therefore, half the samples represent surface associated (epiphytic) and internal BEA and surface sterilized samples represent internal (endophytic) BEA only.
Processing of tissues and BEA Extraction:
After harvest, and if necessary, surface sterilization, tissues were flash frozen in liquid nitrogen, freeze dried, ground and weighed into 10 mg per sample. Extraction of BEA was performed using 3:1 MeOH:water with internal standard (¹³C₃-cinnamic acid). Samples were vortexed on ice for 15 minutes, pelleted (4 °C, 15k rpm, 5 min), the pellet re-extracted, supernatants pooled then filtered (0.22 µm PTFE filter) N2 – dried, re-suspended, then stored at -80 °C until LC/MS analysis. The samples were analyzed utilizing UHPLC-ESI-MS with an Agilent 1290 UHPLC + 6546 QTOF, Zorbax Eclipse Plus C18 (2.1×50 mm, 1.8 µm). 40 °C. The limit of detection (LOD) and limit of quantification (LOQ) were used to evaluate method sensitivity, which were determined to be 1.950 ng g⁻¹ and 5.910 ng g⁻¹, respectively. We considered samples with resulting BEA concentrations that fell between LOD and LOQ to contain trace amounts of the mycotoxin and samples containing values below the LOD to contain none of the mycotoxin.
Insect Feeding Bioassay
Insect Model: The yellow mealworm Tenebrio molitor (3rd instar)
Plant Material: Leaves of the same size were collected from plants treated with the fungus via foliar spray or soil drench or the control 2 WPI. Half the leaves were surface sterilized (no surface residues present), and half the leaves were not surface sterilized (surface fungus present).
Feeding Chamber: 100 mm Petri dish, lined with moist filter paper, one larva added per dish (20 dishes per treatment X sterilization), sealed with parafilm, incubated at 25 °C; mortality monitored for 21 days, larvae considered dead if they did not respond to gentle probing. The entire experiment was conducted twice.
Statistical Analysis:
All statistical analyses and data visualization were performed in R version [4.3.2] (R Core Team, 2023) using base functions and relevant packages.
Endophytic Colonization:
Presence and absence of B. bassiana as an endophyte in tissue samples was coded as 1 and 0, respectively. The main effects of tissue type (leaf, stem, or root), time (WPI), and treatment (foliar spray, soil drench, or control) as well as their interactions on the probability of observing B. bassiana as an endophyte were modeled using a GLMM with a binomial link function. Plant ID was included as a random intercept to account for multiple tissue sampling from individual plants. The model was checked to ensure it met proper assumptions. Significance was determined using a Type-III Anova, and Sidak’s post hoc test for comparisons of estimated marginal means (α = 0.05).
BEA Quantification:
Samples containing BEA below the limit of detection (LOD = 1.950 μg/g) were recorded as zero. The treatment of surface sterilization was removed from our analysis because all sterilized samples were below the LOD and contained no detectable BEA. Our data was zero-inflated and right-skewed therefore we utilized a two-part hurdle model. The first part of the model was a logistic part to determine if there were significant effects of treatment tissue and their interaction on our ability to detect BEA >LOD. Model significance was assessed with a Type III Anova, and post hoc comparisons of estimated marginal means were conducted using Sidak’s test (α = 0.05). The second part of the model was a gamma regression with a log-link function to determine the effect of treatment, tissue type, and their interaction on samples containing positive concentrations of BEA. Model significance was assessed with a Type III Anova, and post hoc comparisons of estimated marginal means were conducted using Sidak’s test (α = 0.05). To report the overall expected concentration (including zeros) we multiplied the probability of detection by the conditional mean.
Insect Feeding Bioassay:
Yellow mealworm mortality was recorded as 1 for alive and 0 for deceased larvae. To test the effect of treatment (foliar spray, soil drench, or control) and surface sterilization status (yes or no) on the probability of yellow mealworm mortality we used a generalized linear mixed model with a binomial distribution and logit link function with experimental trial designated as a random effect. Model significance was assessed with a Type III Anova, and post hoc comparisons of estimated marginal means were conducted using Sidak’s test (α = 0.05).
Endophytic establishment of B. bassiana in peach
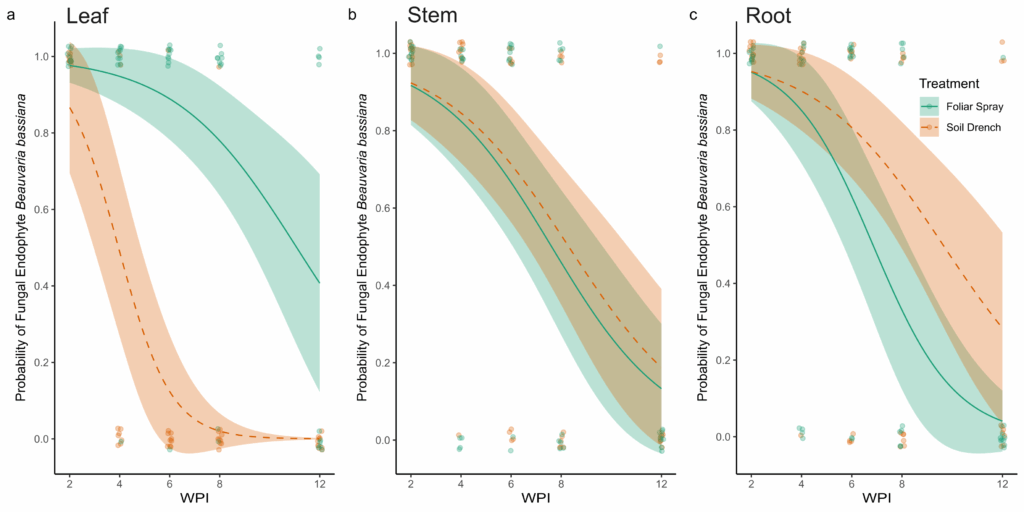
Results from fungal re-isolation via tissue culturing and molecular validation indicate that the entomopathogenic fungus B. bassiana can be successfully established as an endophyte in peach seedlings using both foliar spray and soil drench inoculation methods (Figure 2). The PCR amplification was consistent in generating an amplicon of the appropriate size (~464 bp), specific to B. bassiana. Sequencing confirmed the identity of amplified fragments as B. bassiana and pairwise alignment against previously characterized sequences of B. bassiana on GenBank (Accession number: PP318546) was recorded for all positive samples. The main effects of Treatment (χ² = 23.72, df = 1, p < 0.001), Tissue type (χ² = 6.17, df = 2, p = 0.046), and Time (WPI; χ² = 33.17, df = 1, p < 0.001) significantly affected the probability of recovery of B. bassiana as an endophyte from peach seedlings. In addition, a significant Treatment × Tissue interaction was detected (χ² = 27.59, df = 2, p < 0.001), indicating that the effect of inoculation treatment varied among plant tissues.
At two WPI, all sampled tissues (leaves, stems, and roots) from both foliar-sprayed and soil-drenched plants tested positive for endophytic colonization by B. bassiana (Figure 2). Over time, the probability of recovering B. bassiana from leaf tissues was higher in foliar-sprayed plants compared to soil-drenched plants (Figure 2A). In soil-drenched plants, recovery from leaves declined sharply around six WPI and ceased entirely by twelve weeks (Figure 2A). In contrast, recovery from stem tissues was similar between foliar spray and soil drench treatments throughout the study period (Figure 2B). By eight WPI, the likelihood of recovery from stems was approximately 50%, regardless of treatment method (Figure 2B). For root tissues, the probability of endophytic recovery was slightly higher in soil-drenched plants compared to those treated via foliar spray (Figure 2C). Overall, the probability of recovering B. bassiana from all tissue types declined over time. However, in foliar-sprayed plants, the fungus was still detectable in representative samples of all tissue types at twelve WPI (Figure 2). There was no recovery of B. bassiana in any of the plants treated with the control.
Quantification of BEA
Surface sterilized tissues consistently contained BEA concentrations below the limit of detection (LOD = 1.95 µg/g), indicating that in this study measurable BEA production was only present epiphytically. Therefore, analyses were restricted to non-sterilized tissues to evaluate treatment effects on epiphytic BEA production.
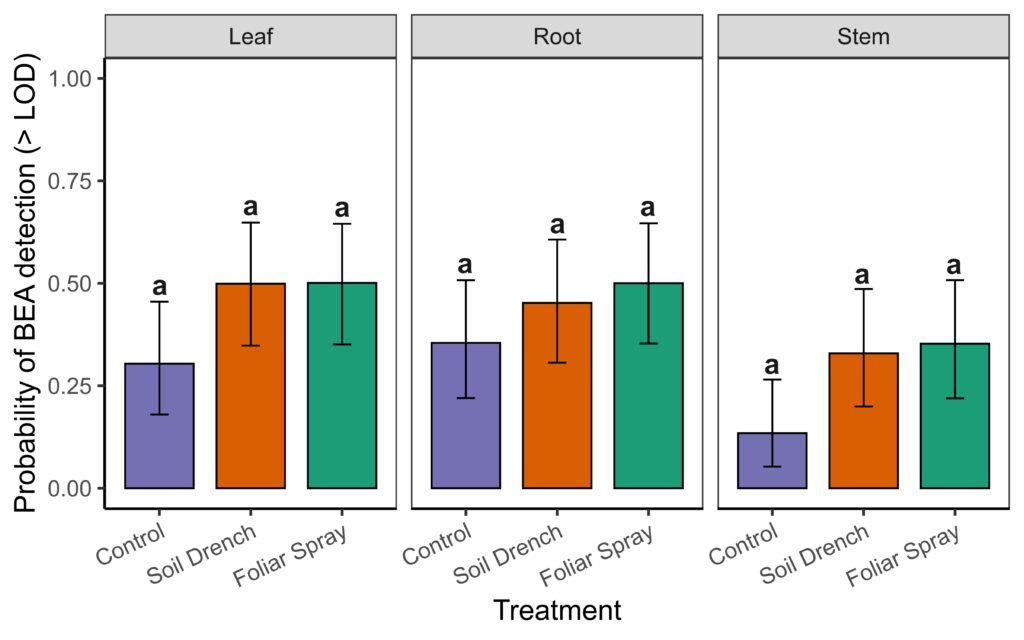
To do so we used a two-part hurdle modeling framework. The first component modeled the probability of detecting BEA above the limit of detection (LOD = 1.95 µg/g) in non-surface sterilized tissues. Detection probability did not differ significantly among Treatments (foliar spray, soil drench, control) , Tissue (leaf, root, stem), or their interaction (Anova, χ² = 4.25, df = 2, p = 0.12 for Treatment; χ² = 5.41, df = 2, p = 0.07 for Tissue; χ² = 1.39, df = 4, p = 0.85 for Treatment × Tissue; Supplemental Fig. 1). Because detectability did not differ among treatments or tissues, subsequent differences reflect true variation in concentrations rather than artifacts of threshold sensitivity.
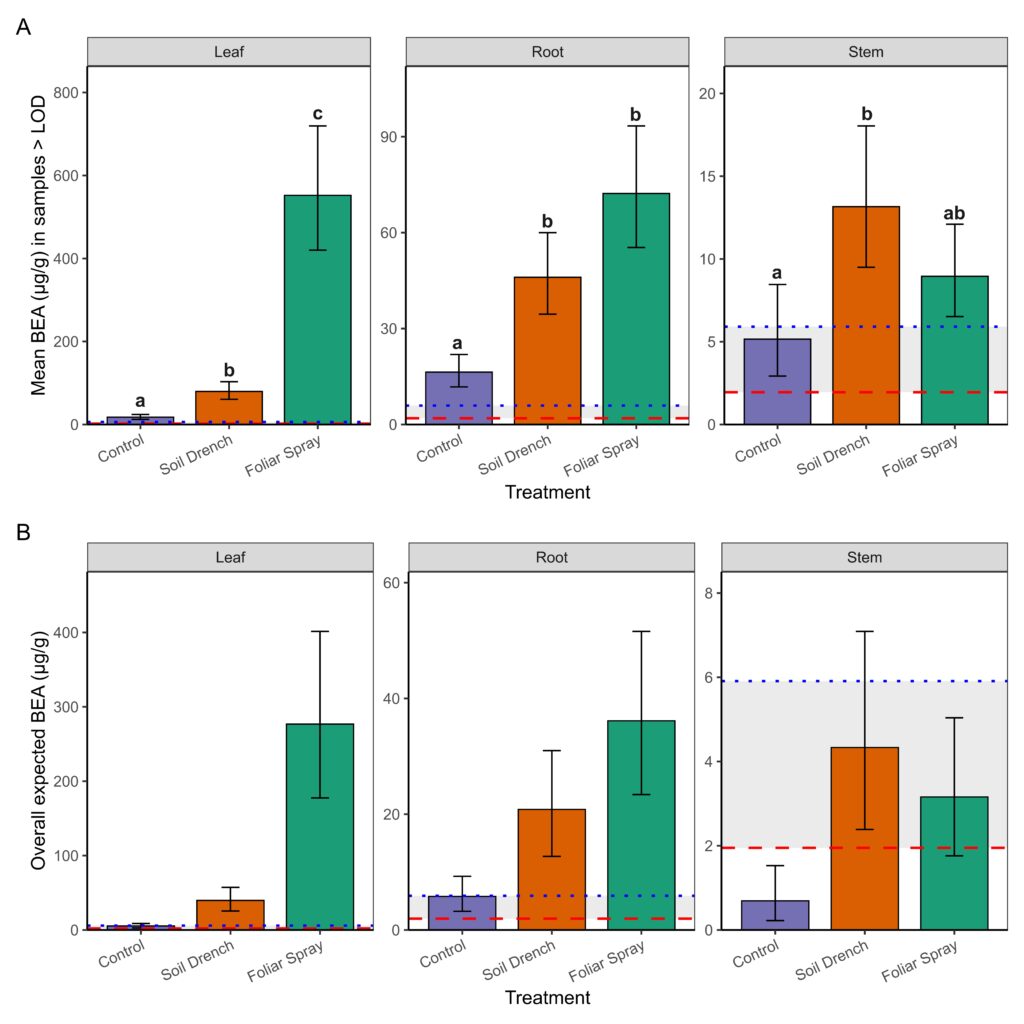
The second component of the analysis estimated mean concentrations conditionally among samples with detectable BEA (BEA >LOD; Figure 3A). Treatment and tissue type as well as the interaction between treatment and tissue type significantly influenced BEA concentrations in non-surface sterilized peach tissue samples. Foliar spray inoculation consistently yielded the highest concentrations, especially in leaves (mean: 549 µg/g; 95% CI: 422–714 µg/g, range: 127 – 1517 µg/g), which were significantly greater than both soil drench and control treatments (p < 0.0001). Roots also exhibited elevated concentrations under foliar spray (71 µg/g; 95% CI: 55–93 µg/g, range: 27.7-119 µg/g) compared to controls (p < 0.0001). Stems contained lower concentrations overall (≤18 µg/g), with only the soil drench treatment producing levels significantly higher than the control (p = 0.0068).
Finally, by combining detection probability with conditional means, we derived overall expected BEA concentrations (i.e., the average amount expected across all samples, including non-detects; Figure 3B). This metric revealed the strongest treatment effects in leaves, where foliar spray application produced mean expected concentrations nearly an order of magnitude greater than soil drench and two orders of magnitude greater than controls. Roots showed intermediate effects, while stems exhibited only trace to low expected concentrations. Notably, concentrations between the LOD (1.95 µg/g) and LOQ (5.91 µg/g) were considered trace, and such levels were observed primarily in control and stem tissues. Together, these results demonstrate that foliar spray inoculation is the most effective method for achieving high BEA concentrations in peach seedlings, with the strongest accumulation on leaves. Soil drench application yielded intermediate concentrations, whereas stems rarely accumulated levels above trace amounts.
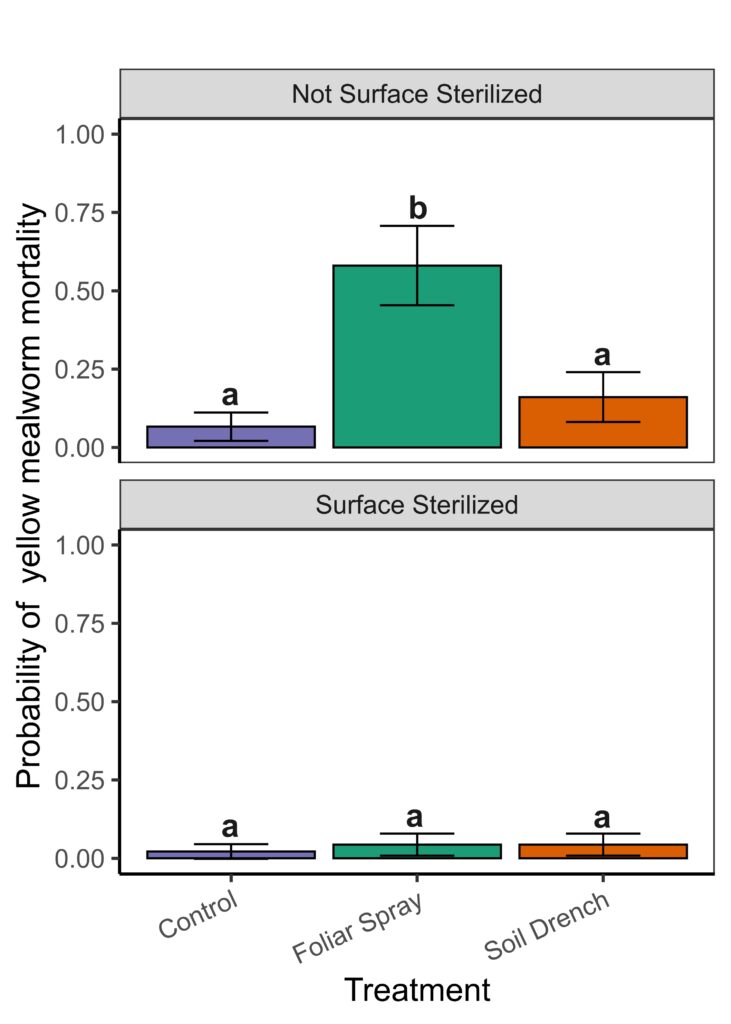
Assessment of Yellow Mealworm Feeding Assays
Discussion
Our study demonstrated that B. bassiana (strain GHA) can successfully colonize peach seedlings as an endophyte through both foliar spray and soil drench applications. However, the insecticidal compound beauvericin (BEA) was detected at low levels inside plant tissues and is therefore unlikely to provide systemic insecticidal properties. In contrast, foliar sprays produced high concentrations of BEA on leaf surfaces, and these residues remained detectable for at least two weeks after application.
Insect feeding assays confirmed that surface residues negatively affected insect survival, showing that B. bassiana functions primarily as a contact-based biocontrol agent when applied to peach foliage. Soil drench applications were less effective at producing surface residues or insect suppression, though they still resulted in endophytic colonization.
Taken together, these results suggest that foliar application of B. bassiana is the most effective method for achieving meaningful insecticidal activity on peach seedlings. These results suggest that endophytic B. bassiana is unlikely to be an effective management strategy for lesser peachtree borer, particularly once larvae are inside the tree. However, the ability of this fungus to colonize internally provides a foundation for exploring additional benefits, such as improved plant health or stress tolerance, which require further study.
Note: For more in depth methodology, results, and discussion, please read the final research article uploaded to the “Information Products” once it has been published.
Educational & Outreach Activities
Participation summary:
This work has been presented in part at the Entomological Society National Meeting in 2024, the Entomological Society Southeastern Branch Meeting in 2025, and the Georgia Entomological Society Meeting in 2025. Results from the completed project will be presented at the 2025 Entomological Society National Meeting in Portland, Oregon. A manuscript from this work will be submitted to the journal Frontier's in Fungal Biology and results presented to growers at this year's Southeast Regional Fruit and Vegetable Convention as well as the Georgia and South Carolina peach meetings.
Project Outcomes
This project demonstrated that Beauveria bassiana can establish inside peach seedlings as an endophyte, but internal production of the insecticidal metabolite beauvericin (BEA) was minimal. Instead, foliar applications produced high and persistent levels of beauvericin on plant surfaces, particularly on leaves, where residues remained detectable for at least two weeks. Insect feeding bioassays confirmed that these surface residues can negatively impact insect survival, indicating that B. bassiana applied as a foliar spray has value as a contact-based biocontrol tool rather than as a systemic one.
From a grower perspective, this distinction is important. B. bassiana will not replace systemic insecticides in peach production, but it could provide short-term pest suppression when applied as a foliar spray. Like other biological products, repeated applications are likely needed to maintain efficacy. The persistence of beauvericin on leaf surfaces suggests potential utility against pests with exposed feeding stages, such as stink bugs or lesser peachtree borer adults and larvae prior to burrowing.
The establishment of B. bassiana as an endophyte in peach seedlings also has broader sustainability implications. In other crops, endophytic fungi have been linked to improved drought tolerance, growth promotion, and suppression of fungal pathogens. While these benefits were not directly tested here, our findings provide the foundation for exploring similar effects in peach. If confirmed, such multifunctional benefits could reduce chemical inputs, improve orchard resilience, and support more diversified integrated pest management (IPM) programs.
At the same time, adding biologically active metabolites like beauvericin into agricultural systems requires careful consideration of safety and stewardship. While not currently regulated, beauvericin’s activity as a mycotoxin highlights the need for monitoring protocols as fungal-based biocontrol tools gain traction in sustainable and organic agriculture.
Overall, this work shows both the promise and limitations of B. bassiana in peach production. Growers may benefit from incorporating foliar applications as a contact-based biological control option, while researchers now have a foundation for testing additional benefits of fungal endophytes in woody perennial systems under orchard conditions.
This project expanded our understanding of how entomopathogenic fungi like Beauveria bassiana fit into sustainable agriculture. At the start of the project, we expected that endophytic colonization might provide systemic insect control through internal metabolite production. Instead, we learned that while colonization is possible, the insecticidal effects are primarily contact-based through surface residues. This shifted our perspective on how fungal biocontrol products should be applied and managed in perennial fruit systems to manage insect pests.
For me as a graduate student, the project strengthened my skills in designing and conducting greenhouse assays, metabolite analysis, and insect bioassays, as well as communicating results in both scientific and grower-friendly terms. It also deepened my appreciation for the complexity of biological control, since efficacy depends not only on whether a fungus can colonize a plant but also on where and how it produces active compounds.
For my advisor and our research group, the work reinforced the need to integrate both ecological outcomes such as colonization patterns and practical outcomes such as insect mortality and residue persistence when evaluating sustainability tools. It also broadened our awareness of potential multifunctional benefits of fungal endophytes including plant health, stress tolerance, and disease suppression, which opens new avenues for future research relevant to sustainable agriculture.