Progress report for GS23-286
Project Information
Peach (Prunus persica) is a valued specialty crop in the Southeastern United States both culturally and economically. In 2021, South Carolina and Georgia produced over 120,000 tons of fruit valued at over $170 million. Recently the sustainability and high-quality production of this nutritional fruit has been challenged due to the restrictions on the use of the broad-spectrum insecticide chlorpyrifos set by the U.S. Environmental Protection Agency. While there are alternative insecticide options for many foliar pests of peaches, there are currently no effective chemical solutions for borer pests such as the Lesser Peachtree borer (LPTB). Fungal entomopathogens have historically been utilized for insect pest management, however commercial adaptation is limited by fungal sensitivity to environmental factors and the cryptic nature of target pests. Researchers have recently begun to overcome these limitations by establishing fungal entomopathogens as endophytes (fungi living inside of plant tissue) in crop plants including the woody perennials pecan, coffee, and cacao. This study aims to develop the use of the entomopathogenic fungi (EPF) Beauveria bassiana as an endophyte in peach as an alternative, safe and sustainable integrated pest management strategy for the key insect pest, the LPTB.
Objective 1: Establish B. bassiana as an endophyte in peach (P. persica)
Inoculation of host plants with EPF can vary based on specific plant propagation requirements. Different inoculation methods result in varying successes of endophyte establishment in distinct plant tissues. In most studies seed, root, and soil inoculation methods are overall less successful compared to foliar and stem injection methods. However, foliar application results in better colonization of leaf tissues while soil drenching and seed soaking favor root colonization (Bamisile et al., 2018). The purpose of this objective is to confirm commercially available B. bassiana strain GHA can become endophytic in a Guardian® rootstock of peach and determine the best method of inoculation.
Objective 2: Evaluate and compare the presence of B. bassiana in peach plant tissues (leaves, stem, roots) over time post inoculation with soil drench and foliar spray methods.
Previous studies have reported a decrease in recovery of EPF from inoculated plant tissues over time, indicating the EPF is only present and protecting plant tissues for a limited time. To properly integrate the use of endophytic EPF into a sustainable IPM program for peach it is important to determine how long endophytic B. bassiana lasts within each tissue type (leaves, roots, and stems) and compare this retention based on each inoculation method employed (soil drench and foliar spray).
Objective 3A: Determine virulence of peach tissue containing endophytic B. bassiana against an insect
B. bassiana has been established as an endophyte in over thirty plant species and plant tissues containing endophytic B. bassiana have been shown to be pathogenic to eight different orders of insects. The goal of this objective is to confirm all peach tissue types (leaves, stem, and roots) containing endophytic B. bassiana are effective at killing insects post consumption. Mealworms, larvae of the beetle Tenebrio molitor, are voracious consumers often added to compost systems because they are known to eat a variety of materials including all types of plant tissues. The objective of this study is to develop a feeding assay using mealworms and peach plant tissues than can be utilized in any plant system to confirm that endophytic B. bassiana is effective at killing an insect. This will be an essential assay to continue the work in broadly developing EPF as tools in sustainable agricultural systems.
Objective 3B: Determine the virulence of peach tissue containing endophytic B. bassiana against LPTB larvae
The purpose of this objective is to confirm peach tissues containing endophytic B. bassiana are pathogenic to larvae of the prominent peach pest, the Lesser Peachtree borer, confirming this tactic can be utilized as a management tool for this insect.
Cooperators
- (Educator and Researcher)
Research
1. Source of EPF, Beauveria bassiana, inoculum
Beauveria bassiana strain GHA (BotaniGard 22WP) was purchased from Arbico Organics (Oro Valley, AZ) and subcultured on potato dextrose agar (PDA). The fungus was incubated for about two weeks, when conidia were harvested by scraping the agar surface with a sterile spatula. Conidial concentrations were determined using an improved Bright-Line™ Hemacytometer (Hausser Scientific, Horsham, PA) and the suspensions were adjusted to 1 × 108 conidia mL− 1 in sterile distilled water containing 0.05% Silwet L-77 (Fisher Scientific, Waltham, MA) according to Parsa et al. (2013). For all experiments, conidial viability was evaluated by taking a 100 ml sample of each inoculum, plating it on PDA, incubating at 25 °C for 24 h in the dark and assessing germination under light microscopy by counting germinated spores from a total of 100 randomly selected conidia. Conidia were deemed to have germinated if the germ tube was at least twice the length of the conidia. Only inoculum with a germination of ≥ 90% were used for experiments.
2. Seed sterilization and aseptic stratification
Following methods similar to Ramakuwela et al. (2020), peach pits were surface sterilized by immersion for two minutes in 0.5% sodium hypochlorite and two minutes in 70% ethanol. Pits were rinsed three times in sterile distilled water. Success of sterilization was evaluated by plating 100µL of last rinsing water on PDA media, incubating the plate for 10 days at 25° C and checking for contaminant growth. If growth was seen these seeds were removed from the experiment. Seeds aseptically removed from pits were hydrated in sterile water for five days, changing water daily. Seeds were then placed in stratifying bags containing moist sterile perlite and left for about three months in the dark at 4° C to germinate.
3. Inoculation of peach seedlings with B. bassiana
Sixty germinated seeds were planted in 15.2 cm X 15.2 cm plastic pots containing steam sterilized propagation media and Osmocote 18-6-12 slow-release fertilizer. Plants were then placed in the greenhouse at 25° C under natural light where they were watered via drip irrigation for two minutes twice a day until they reached their first true leaf stage. A graduated cylinder was used to apply 150 mL suspension of B. bassiana strain GHA (1 x 108 conidia mL-1 containing 0.05% Silwet L-77) to the surface of the soil at the base of fifteen plants. An additional fifteen plants were treated with a control consisting of 150 mL of sterile distilled water containing 0.05% Silwet L-77. For foliar inoculation, a 2-L pressurized backpack sprayer was used to apply approximately 10 mL of B. bassiana inoculum (1 x 108 conidia mL-1 containing 0.05% Silwet L-77) or a control treatment (10 mL of 0.05% Silwet L-77) to the surfaces of leaves of the remaining thirty seedlings. After inoculation plants were arranged in a randomized block design and allowed to continue to grow in the greenhouse until subsequent tissue harvesting to confirm endophytic B. bassiana presence.
4. Assessment of endophytic B. bassiana colonization in plants by re-isolation and culturing
Two weeks, six weeks, and twelve weeks post inoculation five peach seedlings treated with B. bassiana through soil drench and foliar spray methods along with their corresponding controls were harvested to confirm endophytic colonization of the fungus. Plants were carefully uprooted and rinsed thoroughly under running tap water. Two leaflets, two pieces of stem, and two pieces of tap root were sampled from each plant. The leaves were randomly selected from the middle of the seedling; no leaves were selected from the apical or basal area of the plant. Two parts of the stem were sampled, one towards the middle of the plant and the second closer to the soil surface. The tap root was sampled by dividing it into two parts. Under the laminar flow hood all tissue samples were surface sterilized separately by immersion in 0.5% sodium hypochlorite for one minute, followed by immersion in 70% ethanol for one minute, and rinsing in sterile distilled water three times. Samples were surface-dried on sterile cheesecloth. The outer edges of each tissue sample were trimmed and samples were further cut into six ~CA. 4 mm pieces. Tissues pieces were then plated on Doberski and Tribe medium (Doberski and Tribe, 1980). Cultures were incubated at room temperature in the dark for 14 days. Tissue cultures exhibiting typical mycelial growth of B. bassiana were re-isolated onto fresh DBT and allowed to grow for 21 days at room temperature in the dark for subsequent molecular identification.
5. Molecular identification of bassiana
5a. DNA Extraction
Re-isolated fungal cultures grown on DBT were subjected to molecular diagnosis to confirm the identity of B. bassiana. The commercial B.bassiana strain GHA grown on PDA was used as a positive control. DNA was extracted from samples using a ZR Fungal/ Bacterial DNA MiniPrep Kit (Zymo Research, Irvine, CA) following the manufacturer’s protocol. Sterile distilled water was used as a negative control to check for contamination and DNA samples were stored at –20° C until they were processed for PCR.
5b. PCR amplification and DNA sequencing
Following methods similar to Posada et al., 2006, the ITS region of the nu-rDNA repeat was sequenced for each re-isolate utilizing primers ITS1-F (fungal-specific) (Gardes & Bruns 1993) and ITS4 (White et al. 1990) for both amplification and sequencing. PCRs were performed in 25 ul reaction volumes with 12.5 ul GoTaq Green Master Mix (Promega, Madison, WI), 1 ul each of 10 mM primers, and 1 ul of diluted (10- to 100-fold) DNA template. Amplification was achieved with an initial denaturation step of 5 min at 94° C; 35 cycles of 30 s at 94° C, 45 s at 50° C, and 45 s at 72° C; and a final extension of 7 min at 72° C. PCR products were run on an agarose gel, stained with SYBR Safe, and viewed under UV light. An All-Purpose Hi-Lo DNA Marker (Bio-nexus, Oakland CA) was used to visually estimate amplicon size. Samples with amplicons of the correct size (~464 bp) were sent to Eurofins Genomics for Sanger sequencing. Sequencing reactions were edited as needed and contiguous sequences for each isolate were assembled in Geneious Prime version 2024.0.3 (www.geneious.com). Sequences were aligned using the BLAST tool on GenBank (National Center for Biotechnology Institute, National Institute for Health, Bethesda, MD) to confirm identity.
6. Assessment of the pathogenicity of peach tissues containing endophytic B. bassiana post consumption on an insect
To determine if peach tissues containing endophytic B. bassiana fed to an insect are pathogenic, the model susceptible host the yellow mealworm, Tenebrio molitor L. (Coleoptera: Tenebrionidae) was used. Prior to tests, three plants inoculated with B. bassiana by soil drench and three plants inoculated via foliar spray were confirmed to have the fungi endophytically present in all tissue types (leaves, stem, root) based on the procedure described above (Sections 4 and 5). Three plants treated with a control (0.05% Silwet L-77) via soil drench and foliar spray methods were also used and confirmed not to have B. bassiana present within their tissues. Leaves, stems, and roots were harvested from each plant. Tissues were then separately surface sterilized and dried as described in Section 4. Sterilized samples of each tissue type from each plant were then placed in separate deli cups lined with filter paper moistened with 1 mL sterile distilled water to create a feeding chamber. Ten T. molitor larvae were added to each chamber and a lid poked with small holes was added onto the container. Feeding chambers were placed in an incubator at 26° C and mortality was assessed at one week. Deceased larvae were moved to a filter lined petri dish moistened with 1 mL of sterile distilled water and incubated at 26° C in the dark for 48 hours. Each larva was then plated on DBT media and cultures were incubated at room temperature in the dark for days. Cultures exhibiting typical white dense mycelial growth indicative of B. bassiana were re-isolated onto fresh DBT and allowed to grow for 14 days at room temperature in the dark. Microscopic characterization of isolates was completed and a positive identification of B. bassiana was recorded if typical long branched hyphae forming conidiophores and oval shape single cell conidia was observed. Three randomly selected re-isolates from each feeding assay morphologically identified as B. bassiana were subjected to molecular verification as previously described in Section 5.
7. Assessment of the pathogenicity of peach tissues containing endophytic B. bassiana post consumption to larvae of the Lesser Peachtree borer
To determine if consumption of peach tissues containing endophytic B. bassiana is pathogenic to our target pest, the Lesser Peachtree borer (LPTB), feeding chambers will be constructed as previously described (Section 6). Five LPTB larvae will be added to each feeding chamber. Feeding chambers will then be placed in an incubator at 26° C and mortality will be assessed at one week. Deceased larvae will be moved to a filter lined petri dish moistened with 1 mL of sterile distilled water and incubated at 26° C in the dark for 48 hours. Each larva will then be plated on DBT media for re-isolation and morphological and molecular identification of B. bassiana will be conducted as previously described.
Assessment of colonization of B. bassiana in peach tissues
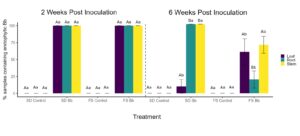

Two weeks post inoculation all tissue samples (2 leaves, 2 pieces of stem, and 2 pieces of tap root) from all plants inoculated with B. bassiana via soil drench (5 plants) or foliar spray methods (5 plants) were molecularly verified to contain B. bassiana as an endophyte while the controls were all negative for EPF (Figure 1 and Figure 2). There was no difference in percent recovery of the EPF from all tissue types (leaves, stem, roots) of plants inoculated with either method (foliar spray or soil drench). This indicates both inoculation methods are proficient at establishing B. bassiana as an endophyte in peach tissues within two weeks of inoculation. Six weeks after inoculation B. bassiana was recovered from significantly less leaf samples compared to stem and root samples of plants soil drenched with the EPF. After six weeks, the percent recovery of B. bassiana from foliar sprayed plants was significantly lower in roots compared to leaves and stems. These results are consistent with reports that B. bassiana colonization is transient in plants and will decrease over time. It is interesting that all plants that were soil drenched with B. bassiana retained the EPF in their roots and stems, but recovery of the EPF was present in only one of two leaf samples in one of the five soil drenched plants. Foliar sprayed plants retained more B.bassiana in leaf samples compared to soil drenched plants but recovery was lower in the roots and stems of foliar sprayed plants six weeks after inoculation. Although the inoculation method did not significantly affect the initial establishment of B. bassiana as an endophyte in peach, there was an effect of inoculation method on recovery of this EPF overtime in the plants. Importantly, these preliminary results indicate plants inoculated by soil drenching retain the EPF longer in stems compared to foliar sprayed plants. Our target pest, the Lesser Peachtree borer predominantly feeds on stem tissues of peaches therefore soil drenching plants may be the best method to utilize endophytic B. bassiana to protect stem tissues from LPTB larvae.
Educational & Outreach Activities
Participation Summary:
After gathering more results and data from these experiments, we plan to create an extension circular to introduce southeastern peach growers to the use of entomopathogenic fungus as endophytes in plants for insect pest management. The results of this study will also be presented at the annual Georgia peach growers extension meeting in January 2025.
Project Outcomes
This study is the first to establish the insect pathogen, the entomopathogenic fungus Beauveria bassiana as an endophyte in peach. This is the first step in developing this sustainable and organically compliant pest management tool for peach production. Due to continued limitations to the use of common synthetic insecticides such as the organophosphate, chlorpyrifos, it is imperative to proactively work to develop new management strategies for prominent pests such as the LPTB that are cryptic and challenging to target beneath the bark even with current management strategies. Completion of this project will aid in understanding the potential and possible limitations of using B. bassiana in peach as endophyte to target insect pests in general.