Final report for GW18-106
Project Information
Brown Marmorated Stink Bug (BMSB), native to eastern Asia, was first detected
in the U.S. in Allentown, PA in 1996, and by 2010 populations increased significantly
in the mid-Atlantic region. Multiple high value fruit and vegetable crops experienced
extreme damage, stone fruit losses being particularly severe, many times sustaining
100% crop loss (Hoebeke and Carter 2003, Leskey et al. 2012). Following range
expansion and multiple introductions, BMSB was first found in Utah in Salt Lake City
in 2012 (Gariepy et al. 2014, Haye et al. 2015, Dr. L. Spears, personal
communication). BMSB adults are strong fliers, immature and adult life stages feed
on over 100 plant species often resulting in economic damage, the insect is tolerant
of many insecticides, and it is well adapted to overwintering in human structures
(Wiman et al. 2015, Rice et al. 2014). All of these characteristics make BMSB a
significant threat to U.S. agriculture, especially specialty crops like fruits and
vegetables. Little research has addressed BMSB status and management in the high
elevation, arid environments of the Intermountain West. I propose to conduct
critical research and outreach education on BMSB through host plant use surveys,
development of life stage degree models (for egg, nymph and adult activity),
assessment of native and introduced biological controls through sentinel egg mass
deployment and field surveys, grower and public presentations on study findings,
and publication of results in an extension factsheet, news articles, and pest
advisories. In 2017, BMSB economic crop damage was reported for the first time in
Utah on peach, apple, squash, and corn. My project will provide valuable insight for
better understanding and managing BMSB in the urban-agricultural landscapes of
northern Utah. I will help train the public sector on prevention, monitoring, and
management of BMSB. I will coordinate my outreach education efforts with the USU
Extension Invasive Pest Program.
1. Host Plant Surveys (2018-2019)
Purpose: Host plant surveys will be conducted May through October of 2018 and 2019 to
determine BMSB plant use for spring emergence/acclimation, feeding (adult and nymph
stages), and reproduction. Surveys will be in suburban and rural locations of the Wasatch
Front. Surveys in 2017 were focused in suburban areas near human structures that
support overwintering. These results showed that BMSB is expanding into the suburbanagricultural/rural
interface.
a. Host plant surveys of residential areas will be conducted in Provo, Salt Lake City,
Kaysville, Layton, Roy, and Ogden for 2018 (surveys were conducted in similar
locations in 2017) to compile a list of common plants associated with BMSB.
b. In 2019, host plant survey sites will be positioned next to commercial farms and
community gardens producing fruits and vegetables. The results from these
surveys will expand the Utah host plant list to include agriculturally significant
plants, especially those in close proximity to residential neighborhoods.
c. With the assistance of several undergraduate student research technicians, I will
document which BMSB developmental life stages (egg, nymph, adult) are
present, and an estimate of their abundance.
2. Phenology (2018-2019)
Purpose: A seasonal timeline of BMSB development in Utah is imperative to empower
producers to manage BMSB. I intend to employ traps and host plant surveys described in
Objective 1 to characterize BMSB phenology in northern Utah.
a. Seasonal development of BMSB will be documented from late April to October in
both 2018 and 2019. Host plant surveys and trap deployment at each of the survey
sites will support observation of life stage occurrences and population abundance
patterns during the growing season.
~Narrative~
*Reference figures and tables can be found in addenda document 5
b. Based on observed phenological activity and temperature, a BMSB degree-day
model will be developed for Utah. With this information, growers and the private
sector will be able to better anticipate optimal timings in the BMSB seasonal
cycle to interrupt population growth.
c. It has been speculated that BMSB is univoltine (one generation per season) in
Utah, but one year of data collection in 2017 indicated that a small second adult
generation may occur in the late fall (Fig. 7-8). During 2018 surveys, a subset of
first generation adults will be marked and confined on host plants with mesh
netting; their progeny will be marked to determine if a second adult generation is
produced before the onset of winter.
3. Biological Control (2018-2019)
Purpose: My focus for this objective is to discover natural enemies of BMSB present in
Utah. Parasitoid wasps are of specific interest, along with documentation of generalist
predators and microsporidia (Nosema spp.) in the gut of wild caught BMSB. It is
important that growers in Utah gain a better understanding of the potential for natural
control, and which organisms contribute the most to BMSB population suppression.
a. In the spring/summer of each 2018 and 2019 season, I will survey for parasitoid
wasps that specifically target BMSB eggs. Sentinel egg mass deployments
(BMSB egg masses reared in a lab colony that are deployed on host plants and
collected 2-3 days later) will survey for parasitoid wasps in suburban and
rural/agricultural environments, and determine if native (or introduced) wasps can
successfully kill and parasitize BMSB eggs.
b. Preliminary sentinel egg mass deployments in 2017 found several native egg
parasitoids. Parasitoid surveys in 2018 and 2019 will target native and exotic
wasps, such as the samurai wasp (Trissolcus japonicus), which can provide high
egg parasitism rates in the native range of BMSB and in laboratory studies (and
has now been detected in 9 U.S. states).
c. Generalist insect predators (e.g. praying mantis, assassin bugs, robber flies,
spiders, etc.) will be documented as observed in host plant surveys and sentinel
egg mass assessments.
d. Microsporidia have been identified as a potential population control agent of
BMSB in the eastern U.S. Microsporidia live in the intestinal tract of BMSB.
Wild caught BMSB will be dissected in the lab and evaluated for the presence of
microsporidia (Nosema spp.) spores.
Research
Host Plant Surveys
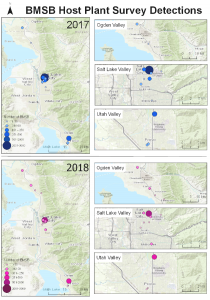
Due to the fact that BMSB was originally discovered in Salt Lake City, UT on the University of Utah campus, host plant survey locations were concentrated within Salt Lake county (eight sites). The remaining seven sites were selected in adjacent counties like Utah (three sites), Davis (three sites), and Weber (one site). Selecting 15 total sites was based on time needed to survey each site every other week from May to September. Our crew of three to four people were usually able to survey an average of four sites a day, twice a week. Each site was selected based on homeowner reports from previous years provided by Dr. Lori Spears. Of the approximately 52 mapped/documented homeowner reports (as of spring 2017) of BMSB, 15 of these sites were selected based on access to an adequate number of plants (>20), along with homeowner permission to conduct surveys on plants that may have been on/over their property lines. The 20 plants randomly selected of those that were available at each site were within our 40x200 m long transect. Each plant was inspected using a beat sheet technique (a padded dowel/stick and canvas beating sheet) and visual observation by at least two people for three minutes, less time if a full plant inspection could be completed in under 3 minutes (e.g. plants with little foliage present). Access to higher branches with the beat sheet technique was enabled by a 1.5 m tall step ladder. Plants that were observed with at least one BMSB life stage (egg, nymph, or adult) present were allotted another seven minutes of inspection. The number of BMSB from each life stage was recorded for every plant. Every plant surveyed was identified to species level. A foliage sample from each species was pressed and mounted in accordance to protocols set forth by the Intermountain Herbarium.
Phenology
In documenting BMSB phenology, host plant surveys, trapping, and controlled voltinism field experiments have been employed.
Trap Data
Three traps were deployed at each of the host plant survey sites from approximately May 8 through October 25, 2018. Trap types included: 1) Dead-Inn 1.2 m tall black corrugated plastic pyramid trap (AgBio Inc., Westminster, CO), 2) Trece STKY dual panel adhesive trap (Trece Inc., Adair, OK) mounted on a wooden stake 1.2 m above ground height, and 3) Trece dual funnel tube trap (Trece Inc., Adair, OK) attached to a tree trunk 2-3 m above ground height. All traps were baited with the Trece BMSB dual lure (murganitol + aggregation pheromone MDT) and replaced every 12 weeks. The sticky panels were replaced at 6-week intervals or when 50% of trap surface was covered by debris or insects. Traps were positioned in the urban landscape sites to avoid conflict with human activity as much as possible. All traps were serviced weekly, and specimens recorded by number of adults (males and female) and nymphs (by 2nd-3rd or 4th-5th instars).
In 2019, early season trapping was initiated at eight sites within Salt Lake City to determine day length and temperature associated with adult BMSB emergence from overwintering diapause. Only the pyramid trap mentioned above was used in 2019, employing the same Trece dual lure as before. These traps were assessed once a week starting on March 29, 2019 to document the presence or absence of BMSB. All sites were in urban landscapes with a variety of managed ornamental plants nearby.
Voltinism
The number of full and partial generations of BMSB each season in Utah has not yet been confirmed. Current knowledge asserts that BMSB is univoltine (one adult generation per season) in the Intermountain West based on latitude and elevation; however, late season occurrence of nymphs in 2017 trapping trials and host plant surveys suggest that there may be a partial second generation occurring. To determine generation times for northern Utah, overwintering adults were collected in May 2018 (n=70) and 2019 (n=60), and confined within mesh fabric sleeve cages (made from 30x80 cm piece of mosquito netting, MosquitoCurtains.com, Alpharetta, GA) on preferential hosts (host plants that offer season long nutrition, shelter, and substrate for oviposition) catalpa and tart cherry in 2018 and tart cherry, catalpa, peach in 2019. Two additional cohorts of BMSB were placed on multiple hosts in 2019, including tart cherry-peach and catalpa-peach combinations. A total of seven mesh bags each containing 10 overwintered adult BMSB were placed on individual tree branches (three on catalpa and four on tart cherry) in the USU Kaysville Farm/Arboretum in 2018. A total of 10 mesh bags were deployed in 2019, with two bags per plant treatment (tart cherry, catalpa, peach, catalpa-peach cross, and tart cherry-peach cross), each containing six BMSB adults. Each mesh bag was marked and monitored once weekly to assess survival status and longevity of captive BMSB. Every bag was sealed with both a plastic zip tie and a metal twisty tie. Leaves with new egg masses produced by adults were removed, placed on a new branch, and enclosed in a new cage on the same host tree for continued monitoring. Nymphs that hatched from these eggs (F1 generation) were counted weekly by nymphal instar (Instars 1-5). Adults that developed from these F1 nymphs were again recorded and monitored for survival and longevity. Overwintering behaviors, such as aggregation, were also monitored for. Any new egg masses produced by these F1 adults were transferred to a new cage as before. Nymphs from these egg masses marked the beginning of the F2 generation, which were then monitored weekly into mid-October in a similar fashion to the F1 generation. Counting the number of individuals still alive each week was done by hand. Our lab group found that counting was easiest when all counted insects were placed into a temporary holding container (e.g. plastic container with a funnel for a lid as to allow for easy bug transfer into the container) and then placed back into the bag after the count was finished. Relative humidity and temperature were logged with a HOBO data logger (UX100, Onset Computer Co., Bourne, MA), on a tree within each of the plant host treatment types.
Biological Control
Parasitoid Wasp Surveys
Survey sites: Surveys for native and exotic parasitoid wasps of BMSB eggs in northern Utah were conducted from June through September in 2017, 2018, and 2019. The surveys included a total of 17 field sites. Sites 1, 4, 5, 8, and 10-17 were located in suburban landscapes containing mixed woody ornamental trees and shrubs. Sites 2, 3, 6, 7, and 9 were in conventionally managed agricultural row crops and orchards (Figure 1).
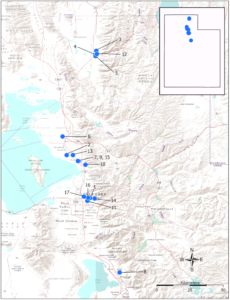
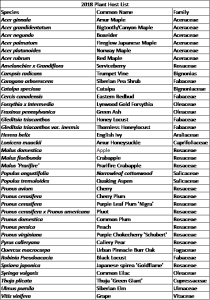
Survey sites were chosen based on areas of established BMSB populations and preferred host plant availability (Tables 1 and 2).
BMSB Colony: BMSB egg masses were reared in the Department of Biology at Utah State University, Logan, UT. The colony was initiated and continuously supplemented from wild BMSB collections in northern Utah beginning in 2016, and further supplemented in 2019 by egg masses from the New Jersey Department of Agriculture colony. The lab colony was maintained at 25-28°C, 40-60% RH, and 16:8 hr photoperiod. Fresh lab-reared egg masses were deployed within 24-48 hr post-oviposition. Egg masses were oviposited onto paper towels, and attached to wax-covered cardstock (4x4 cm) using double-sided sticky tape with sand to cover excess adhesive.
Survey methods: Cardstock cutouts were attached to the underside of plant leaves (Table 1) 2-3 m above the ground using metal safety pins, and collected approximately 48 hr after
deployment. The number of lab-reared egg masses deployed each season was dependent on lab colony fecundity: 114, 93, and 28 in 2017, 2018, and 2019, respectively. Wild BMSB egg masses were identified through 30-min bouts of physical inspection of preferred host plants (Table 2). Each branch was inspected up to a height of 3 m using a step ladder. The number of wild egg masses identified in the survey was 5, 8, and 106 in 2017, 2018, and 2019, respectively. Wild egg masses were collected at the time of detection.
Upon collection, all egg masses were inspected for the presence of guarding parasitoid wasps. If present, wasps were collected with an aspirator (Carolina Scientific Supply Co. Burlington, NC) and placed into a 47 mm plastic petri dish (Fisher Scientific Co. L.L.C. Pittsburgh, PA) with the associated egg mass to allow for further oviposition during transport to the lab in a cooler at ambient temperature, 15.5-24°C.
In the lab, egg masses were stored under the same conditions as the BMSB colony described above. Guarding female wasps were removed upon arrival to the lab, preserved in ethanol, and later pinned for identification. Incubating egg masses were observed once for the presence of hatched, parasitized (emerged parasitoid wasp), missing, or predated eggs (e.g., chewing or sucking damage) approximately one week after collection, following similar procedures established by Ogburn et al. (2016). Egg masses were observed again six weeks after collection to identify late-emerging wasps or those with partially developed wasps within eggs (Stahl et al. 2019). Wasp species were identified using the keys to Nearctic Trissolcus (Talamas et al. 2015b), Nearctic Telenomus (Johnson 1984), and Nearctic Anastatus (Burks 1967).
Statistical methods: Parasitism (percent eggs producing adult wasps) was compared among years and between lab-reared and wild egg masses with a generalized linear model and a quasi-binomial distribution to account for over-dispersion, (R version 3.6.1; R Core Team, 2019), using packages car (Fox and Weisberg 2019) and emmeans (Lenth 2019). The alpha level was set to 0.05.
Natural Enemy Surveys and the Search for Microsporidia
Host plant surveys previously described were also used to document the occurrence of natural enemies considered capable of feeding on BMSB eggs and other life stages as defined by Morrison et al. (2016) and observations by our lab in Utah. Each plant was given a total number of each insect family or order in cases in which family was too difficult to discern in the field.
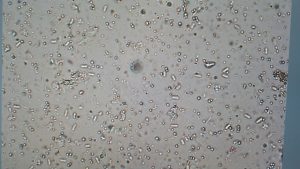
Another portion of our biological control research was focused on documenting the percentage of BMSB infected with the microsporidia species Nosema maddoxi. This microsporidian has only recently been discovered to infect BMSB by Dr. Ann Hajek, her research gives hope to future possibilities of biological control of BMSB (Hajek et al. 2017). All urban trap specimens of BMSB were taken from traps once a week, put into bags and labeled with date and location information, and finally stored in the freezer at -15°C. Stink bug specimen were then prepared for dissection according to procedures set for by Dr. Ann Hajek’s lab. Each stink was crushed with dissection probes/pestles inside of a 2 mL micro centrifuge tube. Crushed stink bugs were then mixed with 500, 200, 140, 60, 50, and 50 µL of deionized water for each life stage (adult, 5th, 4th, 3rd, 2nd, and 1st instar nymph) respectively. A 12 µL sample of the crushed stink bug solution was then placed on a microscope slide and covered with 22x22 mm cover slip. The slide was then viewed under a phase contrast microscope at 400x magnification. Visual confirmation of microsporidia infection was confirmed by the presence of spores (figure 2). After viewing each sample under the microscope, the original micro centrifuge samples were then frozen at -80°C.
Host Plants
Urban host plant surveys conducted in 2018 documented 2552 BMSB, this total being made of up egg masses (37), nymphs (2170), and adults (345). The occurrences of each life stage over time is shown in figure 3. There were 37 plant species with BMSB present in 2018 (Table 3), 13 of which were novel BMSB host plants to the state of Utah (Table 4). These 2018 BMSB plant data also contributed to the total list of 63 known Utah BMSB host plants (Table 5), which is currently published on the Utah Pests website through Utah State University Extension.
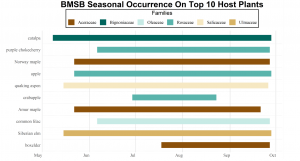
Host plant data from 2018 surveys were compared to identical surveys conducted by our lab in 2017 and the findings shed some light on the most commonly encountered host plants (Figure 4) and numbers of BMSB found at each survey site in both years (Figure 5).


Phenology
Trap Data
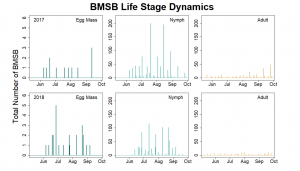
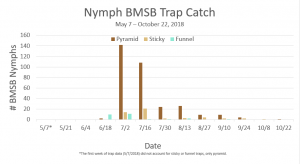
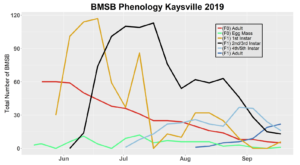
Trap data collected in 2018 trapping trials brought in a total of 818 BMSB (sum of all three trap types). The pyramid trap performed the best in overall trap catch (584), followed by the dual panel adhesive trap (168), and the dual funnel tube trap with the least (66). Trap data was recorded for each of the three trap types at all 15 host plant survey locations every week. Peak numbers of BMSB adults occurred in late May and early June, along with mid-July through early August, and once again in late September (Figure 6). Nymph numbers peaked most notably in early July (Figure 7).
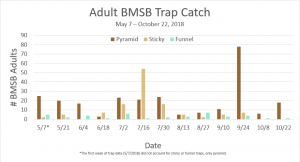
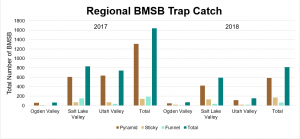
The four sites located within Weber and Davis counties (Ogden Valley) recorded 72 BMSB by the end of the season, the eight sites in Salt Lake county (Salt Lake Valley) accumulated 591 BMSB, and the three sites in Utah county (Utah Valley) had 155 BMSB. The overall trap catch of BMSB decreased dramatically in 2018 when compared to the overall total catch in 2017 (Figure 8).
In 2019, early season trapping was initiated March 29 and revealed that initial spring adult BMSB detection began on April 22. This data helps us to identify important abiotic factors like day length and temperature related to adult emergence from winter diapause. We can establish a biofix, or initiation point with this information, enabling predictive degree day modeling of BMSB develpment. Nielsen et al. (2008) have established data on normal developmental rates for BMSB and we intend to use our trapping data (along with voltinism data discussed below) to compare our observations of BMSB in the field to these predictive models. We will replicate this early season trapping in March 2020 to account for any yearly discrepancies that may exist between the two years, hopefully revealing a consistent date in which adult spring emergence occurs each year based on day length and temperature.
Voltinism
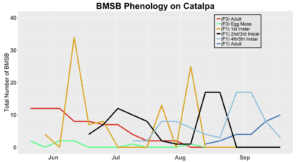
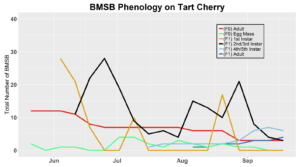
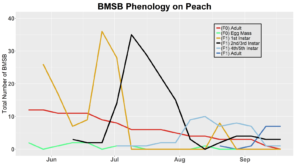
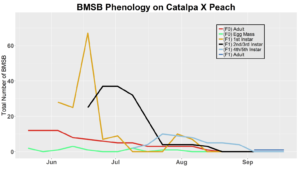
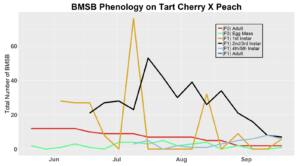
After season long observations of BMSB on both catalpa and tart cherry in 2018, tart cherry was found to be the only plant successful in harboring a partial F2 generation(Figure 12). Catalpa supported only the F1 generation and these adults produced no eggs (Figure 13). These results are not entirely surprising, as it was previously understood that northern Utah would most likely only support a single adult generation given historical weather and other environmental factors. In light of these results, our lab is still not convinced that this experiment has fully exposed the truth behind BMSB voltinism in Utah. A major problem with our design was the initiation date of the experiment. Originally the start date was set for mid-May, but the tart cherry plot wasn’t started until June 4, 2018 and catalpa not until June 18, 2018 (with only 30 total F0 adults instead of 40 as in tart cherry). This delayed and disjointed start was a product of low numbers of collected wild adults in May. We started the experiment as fast as we could with the population supply that was available us, but it was too little, too late. Even with a delayed start on tart cherry, BMSB did experience a partial second generation. It is thought that an earlier start date may enable better observation of a full second adult generation in September.
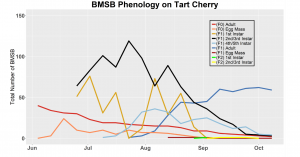
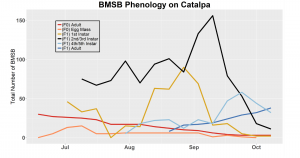
In 2019 voltinism trials were initiated May 21, all plant host treatment types initiated at the same time, accounting for an earlier start date over 2018 trials. Results from all five different plant treatments, including those plant treatments in which BMSB was transfered to multiple host species, there were no F2 nymphs or adults observed (Figures 11-15). Combining all five plant types, a plot of overall BMSB population size can be viewed in figure 16.
Ultimately this observation data on BMSB voltinism and seasonal development will be combined with trapping data results above to confirm our predictive degree day models. These models are still being constructed and the analysis is ongoing. Having a firm understanding of when certain developmental stages of BMSB in Utah occur is imperative in building effective pest management programs. Once constructed, our models will ideally predict future phenological trends before they occur, providing farmers with foresight in planning their management strategies each season.
Biological Control
Parasitoid Wasps
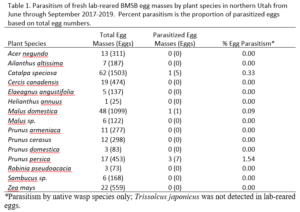
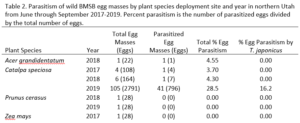
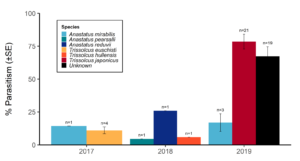
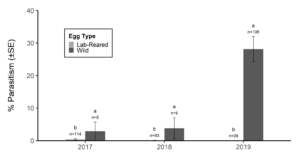
Over our three-year survey period, a total of 39 individuals from five native parasitoid wasp species were found emerging from six wild and five lab-reared BMSB egg masses. The species Anastatus mirabilis (Walsh & Riley), A. pearsalli Ashmead, A. reduvii Ashmead, Trissolcus eushisti (Ashmead), and Tr. hullensis (Harrington) were documented in both guarding and successful emergence from BMSB egg masses (Table 1 and Figure 2). Trissolcus utahensis (Ashmead) and Telenomus podisi Ashmead were observed guarding BMSB eggs, but were never documented in successful adult emergence. In June 2019, the Asian parasitoid Trissolcus japonicus (Ashmead) was first discovered in Utah emerging from two wild BMSB egg masses on Catalpa speciosa (Warder) in Salt Lake City (Table 2; Figures 1, 17, and 18). Trissolcus japonicus was then detected consistently from June through September, 2019, with 452 wasps emerging from 21 wild egg masses (Table 2). Mean parasitoid emergence from lab-reared egg masses was 0.39 and 0.06% in 2017 and 2018, with no wasps emerging in 2019. Mean parasitoid emergence rates from wild collected egg masses in 2017, 2018, and 2019 were 2.85, 3.81, and 28.1%, respectively (Figure 19). Significantly more wasps emerged from wild than lab-reared egg masses (p<0.05; F1,348= 9.4984; df= 1). There were no significant differences in mean wasp emergence among years (p>0.05; F2,348= 0.2267; df= 2) (Figure 19).
Catalpa speciosa, Malus domestica Borkh., and Prunus persica (L.) Batsch were the only plant species to attract parasitism of lab-reared egg masses (Table 1). Parasitized wild egg masses were collected on two tree species, C. speciosa and Acer grandidentatum Nutt, with attack by T. japonicus only on C. speciosa and accounting for 16% of all parasitism in 2019 (Table 2).
When native wasp species successfully emerged from BMSB eggs, the mean number of parasitized eggs per affected egg mass was low. In 2019, mean egg parasitism rates per mass for T. japonicus was 78.5%. Also in 2019, across all wild BMSB egg masses, mean wasp emergence rates were 67.3%; however, in these cases the parasitoids had already emerged and their identification was unknown (Figure 17).
Surveys of wild and lab-reared BMSB eggs in northern Utah demonstrated relatively high diversity of native parasitoid wasps (five species), but low parasitism rates of less than 4% in a given year. These findings are in line with other North American surveys of BMSB egg parasitoids (Dieckhoff et al. 2017; Abram et al. 2019). Low native parasitism rates could be caused by deterrence from natural chemical defenses on BMSB eggs, or a lack of effective venom at the time of female oviposition needed for successful wasp development in the exotic host egg (Haye et al. 2015; Tognon et al. 2016). Other research suggests that using parasitism (i.e., the complete emergence of adult wasps) as a metric of native parasitoid effectiveness may underestimate native wasp effects on BMSB eggs, as unmerged or partially developed wasps often kill the stink bug host (Cornelius et al. 2016; Z. Schumm unpublished data). Though our egg dissections revealed many unhatched eggs with undifferentiated contents, the ultimate cause of egg death could not be ascertained; no partially developed wasps were found in our BMSB egg mass inspections post field collection.
Our work and those of Jones et al. (2014) also suggests that wild egg masses more accurately detect the presence and ability of parasitoid wasps to emerge from BMSB eggs when compared to lab-reared egg masses deployed in the field. This may be due to a variety factors, such as time of egg mass exposure to field conditions or deployment height of egg mass in host tree. Hedstrom et al. (2017) note the importance of semiochemical cues associated with the success of T. japonicus in finding and stinging BMSB egg masses. Therefore, lower parasitism rates of lab-reared egg masses could be due to chemical contamination during transport or effects from lab rearing conditions; however, this question cannot be adequately addressed within the bounds of this study.
Our results indicate that the exotic T. japonicus has the potential to provide meaningful biological control of BMSB in northern Utah. Though current parasitism rates provided by T. japonicus in northern Utah are modest relative to those in its native range; since its first detection in 2019, T. japonicus has already contributed to higher BMSB egg parasitism rates than in the years prior to its presence (Yang et al. 2009; Zhang et al. 2017). Trissolcus japonicus may have killed more BMSB eggs than we were able to document based on identification of the causal wasp. Indeed, many egg masses were attacked by “unknown” parasitoids in 2019, with higher mean parasitism rates than observed for native wasp species, suggesting that at least some of the “unknowns” were likely T. japonicus.
The northern Utah region differs in its climate and topography from most locations in which T. japonicus has been documented or predicted to become established in North America (Avila and Charles 2018). Given the arid, high elevation conditions of northern Utah that include cold winters and hot summers, the detection of an adventive T. japonicus population implies novel range expansion into the greater Intermountain West region. These results support the possibility of an eventual intersection of eastern and western T. japonicus populations in North America (Jarrett et al. 2019; Milnes et al. 2016; Talamas et al. 2015a). Further research should be focused on the ability of T. japonicus to persist in the Intermountain West, specifically data on overwintering behavior where heavy snow fall accumulation and consistent sub-zero temperatures occur (Lowenstein et al. 2019; Nystrom Santacruz et al. 2017). Laboratory rearing and wild releases, in conjunction with conservation efforts, are critical next steps in supporting the future establishment of T. japonicus populations in Utah.

Natural Enemies and the Search for Microsporidia
Currently the raw data for all natural enemies included in the orders/families (Anthocoridae, Asilidae, Cantharidae, Carabidae, Chrysopidae, Coccinellidae, Dermaptera, Dolichopodidae, Formicidae, Gryllidae, Mantodea, Nabidae, Pentatomidae, Reduviidae, Salticidae, Tettigonidae, Thomisidae, Eutichuridae, Araneidae, and Hemerobiidae) have been recorded alongside host plant survey data and are waiting to be analyzed for possible correlation of occurrence with BMSB.
Whole body BMSB dissections looking for microsporidial infections began in October 2018. Undergraduate student research assistant James Withers has been trained in visual inspection and microscope techniques in searching for microsporidia, specifically Nosema maddoxi (Figure 2). Due to a slow learning curve by our lab group (myself included) in determining absence or presence of spores visually, dissections of trap caught BMSB are still ongoing. Of the 516 BMSB assessed so far, less than 1% of those individuals have been found to be positively infected with Nosema maddoxi spores. Though we still have a few hundred more BMSB to assess from 2019, our preliminary results indicate that either our methods for detecting the microsporidial spores are insufficient or that microsporidia are not commonly found in wild populations of BMSB in Utah. Our dissections searching for Nosema maddoxi on 2019 BMSB specimens will be continued, but as of this time parasitoid wasps remain the most viable biological control option in Utah thus far.
References:
Abram, P. K., Talamas, E. J., Acheampong, S., Mason, P. G., and Gariepy, T. D. (2019). First detection of the samurai wasp, Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae), in Canada. Journal of Hymenoptera Research, 68, 29–36. https://doi.org/10.3897/jhr.68.32203
Avila, G. A., and Charles, J. G. (2018). Modelling the potential geographic distribution of Trissolcus japonicus: A biological control agent of the brown marmorated stink bug, Halyomorpha halys. BioControl, 63(4), 505–518. https://doi.org/10.1007/s10526-018-9866-8
Burks, B.D. (1967). The North American species of Anastatus Motschulsky (Hymenoptera, Eupelmidae). Transactions of the American Entomological Society. 93, 423–431.
Cornelius, M. L., Dieckhoff, C., Vinyard, B. T., and Hoelmer, K. A. (2016). Parasitism and predation on sentinel egg masses of the brown marmorated stink bug (Hemiptera: Pentatomidae) in three vegetable crops: Importance of dissections for evaluating the impact of native parasitoids on an exotic pest. Environmental Entomology, 45(6), 1536–1542. https://doi.org/10.1093/ee/nvw134
Dieckhoff, C., Tatman, K. M., and Hoelmer, K. A. (2017). Natural biological control of Halyomorpha halys by native egg parasitoids: A multi-year survey in northern Delaware. Journal of Pest Science, 16. https://doi.org/10.1007/s10340-017-0868-6
Fox, J., and Weisberg, S. (2019). An {R} companion to applied regression, third edition. Thousand Oaks CA: Sage. https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Hajek, A. E., Solter, L. F., Maddox, J. V., Huang, W. F., Estep, A. S., Krawczyk, G., … Becnel, J. J. (2017). Nosema maddoxi sp. nov. (Microsporidia, Nosematidae), a Widespread Pathogen of the Green Stink Bug Chinavia hilaris (Say) and the Brown Marmorated Stink Bug Halyomorpha halys (Stål). Journal of Eukaryotic Microbiology. https://doi.org/10.1111/jeu.12475
Haye, T., Fischer, S., Zhang, J., and Gariepy, T. (2015). Can native egg parasitoids adopt the invasive brown marmorated stink bug, Halyomorpha halys (Heteroptera: Pentatomidae), in Europe? Journal of Pest Science, 88(4), 693–705. https://doi.org/10.1007/s10340-015-0671-1
Hedstrom, C., Lowenstein, D., Andrews, H., Bai, B., and Wiman, N. (2017). Pentatomid host suitability and the discovery of introduced populations of Trissolcus japonicus in Oregon. Journal of Pest Science, 90(4), 1169–1179. https://doi.org/10.1007/s10340-017-0892-6
Jarrett, B. J. M., Pote, J., Talamas, E., Gut, L., and Szucs, M. (2019). The discovery of Trissolcus japonicus (Hymenoptera: Scelionidae) in Michigan. The Great Lakes Entomologist, 52, 8.
Johnson, N. F. (1984). Systematics of Nearctic Telenomus: classification and revisions of the Podisi and Phymatae species groups (Hymenoptera: Scelionidae). Columbus: College of Biological Sciences, Ohio State University.
Jones, A. L., Jennings, D. E., Hooks, C. R. R., and Shrewsbury, P. M. (2014). Sentinel eggs underestimate rates of parasitism of the exotic brown marmorated stink bug, Halyomorpha halys. Biological Control, 78, 61–66. https://doi.org/10.1016/j.biocontrol.2014.07.011
Lenth, R. (2019). emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.4.1. https://CRAN.R-project.org/package=emmeans
Lowenstein, D.M., Andrews, H., Hilton, R.J., Kaiser, C., Wiman, N.G. (2019). Establishment in an introduced range: dispersal capacity and winter survival of Trissolcus japonicus, an adventive egg parasitoid. Insects. 10, 443.
Morrison, W. R., Mathews, C. R., & Leskey, T. C. (2016). Frequency, efficiency, and physical characteristics of predation by generalist predators of brown marmorated stink bug (Hemiptera: Pentatomidae) eggs. Biological Control, 97, 120–130. https://doi.org/10.1016/j.biocontrol.2016.03.008
Milnes, J. M., Wiman, N. G., Talamas, E. J., Brunner, J. F., Hoelmer, K. A., Buffington, M. L., and Beers, E. H. (2016). Discovery of an Exotic Egg Parasitoid of the Brown Marmorated Stink Bug, Halyomorpha halys (Stål) in the Pacific Northwest. Proceedings of the Entomological Society of Washington, 118(3), 466–470. https://doi.org/10.4289/0013-8797.118.3.466
Nielsen, A. L., Hamilton, G. C., & Matadha, D. (2008). Developmental rate estimation and life table analysis for Halyomorpha halys (Hemiptera: Pentatomidae). Environmental Entomology, 37(2), 348-355.
Nystrom Santacruz, E., Venette, R., Dieckhoff, C., Hoelmer, K., and Koch, R. L. (2017). Cold tolerance of Trissolcus japonicus and T. cultratus, potential biological control agents of Halyomorpha halys, the brown marmorated stink bug. Biological Control, 107, 11–20. https://doi.org/10.1016/j.biocontrol.2017.01.004
Ogburn, E. C., Bessin, R., Dieckhoff, C., Dobson, R., Grieshop, M., Hoelmer, K. A., Mathews, C., Moore, J., Nielsen, A. L., Poley, K., Pote, J. M., Rogers, M., Welty, C., and Walgenbach, J. F. (2016). Natural enemy impact on eggs of the invasive brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), in organic agroecosystems: A regional assessment. Biological Control, 101, 39–51. https://doi.org/10.1016/j.biocontrol.2016.06.002
Talamas, E. J., Herlihy, M. V., Dieckhoff, C., Hoelmer, K. A., Buffington, M., Bon, M.-C., and Weber, D. C. (2015a). Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae) emerges in North America. Journal of Hymenoptera Research, 43, 119–128. https://doi.org/10.3897/JHR.43.4661
Talamas, E. J., Johnson, N. F., and Buffington, M. (2015b). Key to Nearctic species of Trissolcus Ashmead (Hymenoptera, Scelionidae), natural enemies of native and invasive stink bugs (Hemiptera, Pentatomidae). Journal of Hymenoptera Research, 43, 45–110. https://doi.org/10.3897/JHR.43.8560
Tognon, R., Sant’Ana, J., Zhang, Q.-H., Millar, J. G., Aldrich, J. R., and Zalom, F. G. (2016). Volatiles mediating parasitism of Euschistus conspersus and Halyomorpha halys eggs by Telenomus podisi and Trissolcus erugatus. Journal of Chemical Ecology, 42(10), 1016–1027. https://doi.org/10.1007/s10886-016-0754-3
Yang, Z.-Q., Yao, Y.-X., Qiu, L.-F., and Li, Z.-X. (2009). A new species of Trissolcus (Hymenoptera: Scelionidae) parasitizing eggs of Halyomorpha halys (Heteroptera: Pentatomidae) in China with comments on its biology. Annals of the Entomological Society of America, 102(1), 39–47. https://doi.org/10.1603/008.102.0104
Zhang, J., Zhang, F., Gariepy, T., Mason, P., Gillespie, D., Talamas, E., and Haye, T. (2017). Seasonal parasitism and host specificity of Trissolcus japonicus in northern China. Journal of Pest Science, 90(4), 1127–1141. https://doi.org/10.1007/s10340-017-0863-y
Research Outcomes
Education and Outreach
Participation Summary:
I have split the total number of farmers and agricultural professionals into two groups evenly above. This is because my participants did not all fit into these two categories evenly, there were many other categories represented. There were county extension specialists, growers, farmers, master gardeners, students, agricultural professionals, landowners, etc. I have given an exact count of participants listed under each of the verbal presentation events below. In the two boxes above, I did not include students in the total numbers given, those students are only represented in the event specific participant delineation below.
(Curricula, factsheets or educational tools)
- I created a poster that was displayed at the Entomological Society of America (ESA) Annual Meeting in Vancouver, Canada, displaying current brown marmorated stink bug host plants, voltinism, and trapping data for urban-agricultural landscapes in Utah. My poster received a cash prize for first place in its category and is now displayed in the Utah State University biology department. (November 12, 2018) (Poster PDF file is available on other report page)
- In my presentation for the ESA Annual Meeting in St. Louis Missouri I spoke on voltinism of BMSB in Utah and the species of parasitoid wasps that we had discovered between 2017, 2018, and 2019 field seasons. The audience was primarily graduate students, professors, and several private industry workers. (Nobember 18, 2019) (Presentation PDF file available in report)
- The fact sheet Common Stink Bugs of Utah, was used to inform the public of commonly encountered native and exotic stink bug species in Utah. A general description of each species listed was provided, these included information on beneficial predatory stink bugs. Photos of each species in the adult and juvenile life stage were provided, including eggs. This document was shared via the Utah Pests Website and Utah State University Extension digital commons.
- The fact sheet on Parasitoid Wasps of the Invasive Brown Marmorated Stink Bug in Utah, focused on all native parasitoid wasp species discovered in Utah between 2017 and 2018. The exotic Asian samurai wasp, Trissolcus japonicus had not yet been found during the creation of this document. This fact sheet was aimed at educating the public on biological control of stink bugs (especially BMSB), by way of parasitoid wasps from the families Eupelmidae and Scelionidae. This fact sheet included pictures and locations in which each wasp species has been found.
- In response to the discovery of the exotic Asian parasitoid Trissolcus japonicus, in June 2019, the fact sheet The Samurai Wasp Brings New Hope in the Fight Against Brown Marmorated Stink Bug in Utah was created. This fact sheet detailed the life cycle of the exotic wasp species and informed the reader of how to identify a healthy stink bug egg mass from one that was parasitized. This fact sheet was seminal in providing home owners and growers with hope of effective biological control by parasitoid wasp in Utah, especially in light of the fact that many native species had not shown promise. Readers were encouraged to aid in the preservation of the samurai wasp and any parasitized egg masses that may be found.
(Published press articles, newsletters)
- & 2. My colleagues and I published two different newsletter articles on BMSB in the Utah Pests Spring newsletter publication in 2019 (https://utahpests.usu.edu/files/up-newsletter/2019/UtahPests-Newsletter-spring19.pdf). One article was titled Seasonal
Development and Occurrence of Brown Marmorated Stink Bug in Utah and the other Biological Control of Brown Marmorated Stink Bug. The first deals with monitoring seasonal development of BMSB, the second detailing parasitoid wasp species that may provide biological control of BMSB. These articles targeted a diverse audience of growers, master gardeners, and other people from the public that rely on the Utah State Extension.
(Tours)
- Utah State University Entomology Club Insect Tours: September 26, 2018 Cedar Ridge Elementary (15 participants)
- Utah State University Entomology Club Insect Tours: October 16, 2018 Edith Bowen Laboratory School (20 participants)
Insect tours at USU are a great platform for sharing basic insect facts and information with young people from the Cache county community. When I am in charge of giving a presentation in front of these young people, I include information on the brown marmorated stink bug (BMSB) and why it is significant to all of us here in Utah. I show students live BMSB specimens, help them understand its impact on agriculture and food production, and explain tactics used by my lab to better understand and manage BMSB as a pest.
(Talks)
- Weber State University October 4, 2018 (25 participants; 45 minutes)
I was invited to share a lecture with students at Weber State University in Odgen, Utah by Dr. John Mull. The presentation explained basic BMSB identification, ecology, and why it is currently considered a major pest in many parts of the world, including Utah. I was able to share findings on some of the most common host plants observed in my surveys, which trap type of the three I compared was most successful in the urban landscape, how many generations of BMSB were observed in controlled outdoor trials, and parasitoid wasps discovered stinging BMSB egg masses. Students were able to ask me questions after the presentation as well.
2. Utah State Horticultural Association: Urban Habitats as Sources of BMSB on Agricultural Lands January 25, 2019 (37 participants)
This presentation was aimed at producers/growers in Utah, informing them of current data on which plants harbor BMSB, effective trapping techniques, and other developments of the BMSB invasion within the urban landscapes of Utah. This presentation emphasized that data on urban settings is extremely applicable to agricultural production because of the close proximity of urban and agricultural lands in northern Utah.
3. First Detector Training September 27, 2019 (45 participants)
I presented on several commonly encountered stink bug species in Utah and provided information on monitoring and management strategies. The audience was made up primarily of master gardeners.
4. Entomological Society of America Annual Meeting Oral Presentation November 18, 2019 (30 participants)
My talk was title Voltinism and parasitoids of the brown marmorated stink bug in Utah. I discussed how many generations of BMSB adults are commonly found each year and what species of parasitoid wasps are present, including the discovery of Trissolcus japonicus. This forum is especially conducive to sharing research methods and results with other entomologists around the nation.
5. Utah Pests In-Service Training February 25, 2020 (15 participants)
I presented on several commonly encountered stink bug species in Utah and provided information on monitoring and management strategies. The audience was made up primarily of county extension agents and a few growers.
6. Utah Urban and Small Farms Conference March 5, 2020 (47 participants)
I gave a 20-minute presentation on brown marmorated stink bug feeding damage and biological control agents specific to cane berry crops. Audience was made up of local growers and those who managed assorted berry crops.
(Workshops)
- Kaysville Fruit and Vegetable Field Day June 20, 2018 (24 participants)
Utah farmers and growers were able to listen to a variety of presentations given at the USU research farm in Kaysville. Dr. Lori Spears, Zach Schumm, and myself shared general information on BMSB. This included traps, fruit feeding damage, and what research was being conducted onsite. I was able to show growers where I would be conducting my voltinism experimentation and display BMSB egg masses deployed in trees in an effort to document local parasitoid wasps. I also had handouts for participants containing data on urban trapping data from 2017 and 2018 summer trials.
2. Kaysville First Detector Workshop September 21, 2018 (47 participants)
Dr. Lori Spears invited me to give a presentation on the current pest status of BMSB at the USU Kaysville Education Center. I presented data on host plants, trapping trials, and biological control agents documented in the summers of 2017 and 2018. The audience was made up of master gardeners and local extension agents. I facilitated a verbal quiz at the end of my talk in which the audience was given two stink bug photos on individual PowerPoint slides and made to select which one was the native look alike species, and which one was BMSB. The audience was receptive to this exercise and I received verbal appreciation after the talk for photos of insects that might commonly be mistaken for BMSB in Utah.
3. Invasive Insect Identification Workshop March 5, 2019 (50 participants)
Workshop emphasized current pest status of several invasive insects in Utah. My role at the workshop was to teach participants how to identify brown marmorated stink bugs in the field using morphological characters and by recognition of feeding damage on common commercial fruit. I also instructed participants on how to purchase and use several different trap types currently on the market.
____________________
(Other Educational Activities)
1. Farmers market attendance and presentations: Wheeler Historic Farm in Murray, UT on July 29 (38 participants)
2. Farmers market attendance and presentations: Wheeler Historic Farm in Murray, UT on August 19, 2018 (42 participants)
3. Farmers market attendance and presentations: Wheeler Historic Farm in Murray, UT on June 9, 2019 (37 participants)
4. Farmers market attendance and presentations: Wheeler Historic Farm in Murray, UT on August 18, 2019 (64 participants)
Utah State University sponsors a tent for master gardeners at many Utah farmer's markets, I happened to volunteer two different Sundays at their Wheeler Historic Farm tent. I was alongside three master gardeners and each of us stood on the ready for any questions the market goers might have about horticultural practices or insect pest problem. When I introduced myself to someone asking about pests I always started by saying that I studied the invasive brown marmorated stink bug. Most of the time I was asked what BMSB looked like, and it was a great way for me to share basic identification information and why BMSB is of such importance in Utah. Many times these people would say that they had seen this insect in their home or yard and I took the time to explain good exclusion practices for their home and how to avoid future problems with BMSB by preventative measures.
(Documents in Progress)
- I am currently working on a manuscript that details our discovery of Trissolcus japonicus in Utah, along with all other other native wasp species detected. This paper highlights our findings in the biological control report section, subheading parasitoid wasps. Our intended journal for publication is the Biodiversity Data Journal.
- Another manuscript that I am currently involved with deals with host plant species of BMSB and the known range the pest inhabits. This paper will include maps of its known detection and predictive maps where the stink bug could potentially spread in the future, based on vegetation cover and a variety of other optimal abiotic factors.
- Trap and voltinism data will be applied to a future manuscript on monitoring and management of BMSB based on degree day modeling.
Information Products
- Tracking Brown Marmorated Stink Bug in Utah's Urban-agricultural Landscapes (Conference/Presentation Material)
- Voltinism and Parasitoids of Brown Marmorated Stink Bug in Utah (Conference/Presentation Material)
- The Samurai Wasp Brings New Hope in the Fight Against Brown Marmorated Stink Bug in Utah (Fact Sheet)
- Common Stink Bugs of Utah (Fact Sheet)
- Parasitoid Wasps of the Invasive Brown Marmorated Stink Bug in Utah (Fact Sheet)