Final report for GW20-212
Project Information
Cover crops play important roles in maintaining soil health. This project focuses on identifying a cover crop that have additional benefits besides soil erosion control including disease suppression, and water conservation. In particular, I would like to devote my Ph.D. dissertation into evaluating the multi-facet benefits of sorghum and sorghum-sudangrass hybrids (I hear by refer this as SSgH) for their potential in suppressing plant-parasitic nematodes and soil borne fungi, while contributing to increase soil organic matter that will lead to soil moisture retention. This is the first year of my Ph.D. program. Although I am partially funded by other projects of my adviser, I do not have sufficient funding to carry out multiple components of the soil health assays for SSgH. I am proposing to work with researchers, two edible crop extension agents and three local farmers with different farming operations. I aim to evaluate SSgH as a viable tropical cover crop for their 1) soil building and water conservation properties; 2) plant growth enhancement potential following a no-till SSgH cover cropping practice, and 3) ability to enhance beneficial soil microbiome that are associated with various soil health improvement properties. While PI Wang had been demonstrating the great benefits of another tropical cover crop, sunn hemp, for soil health management, this project come in critical now as sunn hemp is suffering from soilborne fusarium disease throughout Hawaii. By collaborating with extension agents, we also will work with a new farmers training program in Hawaii to reach out to wider audience.
The overall goal of this project is to identify sorghum and sorghum-sudangrass hybrids that are most efficient in tropical climate for suppressing soil-borne pathogens, improving soil water holding properties and enhancing soil microbiome that can contribute to better soil nutrient cycling and ecosystem functioning. Under a no-till cover cropping system, this healthy soil microbiome would not be disturbed and can help to increase soil organic matter faster. These would improve soil aggregates, water infiltration, thus a better water conservation. Specific objectives of this project are:
- Evaluate sorghum/sorghum-sudangrass hybrids for soil building and water conservation properties in a no-till farming eggplant agroecosystem (by Paudel, Wang, Silva)
- Evaluate eggplant growth and soil-borne disease suppression following no-till planting of different sorghum/sorghum-sudangrass hybrids (by Paudel, Ahmad).
- Identify sorghum/sorghum-sudangrass hybrids with distinct microbiome that are associated with good water conservation properties, soil-borne disease suppression and nutrient cycling (by Paudel, Wang, Waisen)
This project will be carried out over a 2-year period at three locations, one at Poamoho Experiment Station, University of Hawaii, the others at Kahumana Farm, an organic farm advocate for permaculture, and Tolentino Farm, a family farm at Waianae. The best outcome of this study will also be demonstrated at a conventional leafy green farm that is always challenged by soil-borne fungal disease problem (Owen Kaneshiro Farm). Detail of measurable outcomes of each objective are listed in Materials and Methods section below. Field days will be organized by the PI and the collaborative extension agents who are also our co-PIs multiple times a year to update findings to local farmers in Hawaii, in particular to new farmers under GoFarm Hawaii New Farmers training program as well as clients of CTAHR Sustainable and Organic Agriculture Program (SOAP). We aim to provide more incentive for farmers to practice no-till cover cropping in Hawaii.
|
Activities / Milestones |
2020 |
2021 |
2022 |
|||||
|
F |
W |
Sp |
Su |
F |
W |
Sp |
Su
|
|
|
Objective 1 |
|
|
|
|
|
|
|
|
|
Conduct field experiment to evaluate nine different sorghum/sorghum-sudangrass hybrids for soil health and water conservation. |
× |
|
|
|
× |
|
|
|
|
Objective 2 |
|
|
|
|
|
|
|
|
|
Experiment: Conduct field experiment to evaluate the growth and disease suppression of eggplant following the sorghum/sorghum-sudangrass termination. |
|
× |
× |
× |
|
× |
× |
× |
|
Objective 3 |
|
|
|
|
|
|
|
|
|
Experiment: Identify microbiome associated with good water conservation properties using bioinformatics and statistical analysis. |
× |
|
|
|
× |
|
|
|
|
Field day 1: Demonstrate the growth and performance of eggplant under different cover crop treatments. |
|
× |
|
|
|
× |
|
|
|
Field day 2: Demonstrate the effect of the best sorghum/sorghum-sudangrass hybrid on soil properties and water conservation. |
|
|
|
|
× |
|
|
|
|
Farmers’ evaluation during field days and guest lectures. |
|
× |
× |
|
× |
× |
|
× |
|
Data analysis and manuscript writing |
|
|
× |
|
× |
|
× |
×
|
Cooperators
- - Producer
- - Producer
- - Producer
- (Educator)
- (Educator)
Research
Objective 1
Greenhouse trials
Two greenhouse pot experiments were conducted to screen SSgH varieties that are most suppressive against M. incognita. The first greenhouse trial was conducted on March 27, 2020. Eleven SSgH varieties tested included ‘Elite Brown Mid Rib’, ‘Bundle King’, ‘Monster II’, ‘Big Kahuna Plus’, ‘Cow Vittles II’, ‘512×14’, ‘Latte BMR’, ‘535×14’, ‘Latte’, ‘NX 4264’, and ’NX-D-61’ (Table 1). Sunn hemp (Crotalaria juncea) was included as a positive control, and a no amendment was included as a negative control. The second trial conducted on June 8, 2020, was a repeat of the first trial with an additional sudangrass variety, ‘Piper’ and forage sorghum ‘EBMR’. All SSgH were grown in the field at Magoon Teaching Facility, University of Hawaii at Manoa. SSgH shoot biomass was collected at 1, 2, and 3 months after planting and brought back to the laboratory to set up the greenhouse pot trials. Fresh SSgH shoot tissues were chopped into small pieces of 1 cm consistency prior to amending into the soil at 1% (w/w) dry weight equivalent. Each pot consisted of 103 g dry weight of sterile sand: soil mix (1:1 v/v). Sterile soil was an autoclaved Wahiawa soil (Tro-peptic Eutrustox, clayey, kaolinitic, isohyperthermic soil) collected from Poamoho Experiment Station. Shoot tissues and sterile soil were placed in a plastic bag and thoroughly mixed before transferring into each pot. The experiment was arranged in a 12×3 (amendment × plant age) factorial design with 4 replications on a greenhouse bench. A 5-week-old ‘Hirayama’ kai choi seedlings were transplanted into each pot and 220 J2/plant were inoculated on the same day. Root penetration by the nematodes was observed 1 month after soil amendment by staining a 0.3 g subsample of roots using acid fuchsin (Bybd et al., 1983). Stained roots were observed under a dissecting microscope (Leica Microsystems Company, Wetzlar, Germany) to quantify the number of J2, J3-4, and females per sample.
Table 1: Sorghum/Sorghum-sudan grass varieities and their charecteristic features.
|
Variety |
Type |
Properties |
|
Bundle King/Elite BMR |
Forage sorghum |
High yielding, juicy midrib, sterile |
|
Cowvittles |
Forage sorghum |
High yielding, juicy midrib |
|
Big Kahuna Plus |
Forage sorghum |
Less lignin, photo sensitive |
|
Monster ll |
Forage sorghum |
Photo sensitive, 8-9 ft in height |
|
512×14 |
Sorghum-sudangrass hybrid |
6-8 ft in height, early maturing |
|
535×14 |
Sorghum-sudangrass hybrid |
Sterile hybrid, 8-9 ft height |
|
Latte |
Sorghum-sudangrass hybrid |
Drought resistant, 7-8 ft height |
|
Latte BMR |
Sorghum-sudangrass hybrid |
Drought resistant, 7-8 ft height |
|
D61 (NX2) |
Energy sorghum |
Large biomass, photo period neutral |
|
4264 (NX1) |
Energy sorghum |
Large biomass, 15-20 ft in height |
|
Piper |
Sudangrass |
Annual grass, rapid growth, lower prussic acid content than sorghum-sudangrass |
Field trials
Two field trials were conducted on May 28, 2020, and May 26, 2021, at Poamoho Experiment Station, Waialua, HI to compare the SSgH varieties for their potential to improve soil health in no-till and strip-till agroecosystems. Soil at the test site was a Wahiawa Soil Series, Oxisol, Tropeptic Eutrustox clayey, kaolinitic, isohyperthermic with 18.6% sand, 37.7% silt, 43.7% clay and pH 6.7. Two fields with high natural population of root-knot and reniform nematodes were used for Trial I and Trial II, respectively. Seven SSgH varieties with an assortment of allelopathic effects against root-knot nematodes penetration and development based on the greenhouse experiment results, SSgH types with high sugar content or high biomass production were selected for the field trial. A bare ground plot was included as a control. Each SSgH variety was seeded at 56 kg seeds/ha in 3.6 × 1.2-m2 plots. Experimental plots were arranged in a randomized complete block design (RCBD) with 4 replications. Cover crops were drip irrigated for 2.5 months. In trial I, cover crops were terminated in a no-till system using a flail mower operated by BCS walk-behind tractor (Model 853, BCS America, LLC, Portland, OR). In trial II, SSgH biomass was incorporated into the soil following a low-till practice where cover crop residues were shallowly tilled (10 cm deep) in narrow strips (20 cm wide) using a two-tine tiller. Prior to the termination of SSgH, the biomass from each plot was estimated using three 0.1-m2 quadrants. Each plot was 0.5 m away from the adjacent rows, with a 1.5-m bare ground area between plots in a row. A total of 32 plots were established. Six-week-old ‘Shikou’ eggplant seedlings were transplanted after 2 weeks of SSgH termination. Each plot had 7 eggplant seedlings planted at 0.5 m spacing between plants in a zigzag pattern in plot. Eggplants were fertilized using Sustane 8-2-4 organic fertilizer (Sustane Natural Fertilizer, Inc., Cannon Falls, MN) at a rate of 73 kg N/ha.
Soil samples were collected systematically from 4 spots per plot in a zigzag pattern from the top 10-cm of soil using a GroundShark shovel (Forestry Suppliers Inc., Jackson, Mississippi). Soil cores from each plot were composited in a plastic bag and transported to the laboratory for nematode extraction and soil nutrient analysis. Soil samples were collected 2 weeks after cover crop termination, and at 3 months after eggplant planting. Soil samples were submitted to Agricultural Diagnostic Services Center (ADSC) of the University of Hawaii, Honolulu, Hawaii to analyze for total C and N content using LECO TruSpec CN (LECO Corporation, Saint Joseph, MI). FieldScout TDR 100 Soil Moisture Meter (Spectrum Technologies, Inc., Aurora, IL) was used to measure volumetric soil moistures twice during the eggplant growing season with 12-cm rods at the rhizosphere from 3 randomly selected spots per plot. Soil from each plot was measured for infiltration rate at 2 months after growing sorghum and 3 months after eggplant planting using a double-ring infiltration method (Fares et al., 2008).
Objective 2
Soil samples were collected as described in Objective 1 at the initiation of the experiment, and at monthly intervals thereafter from cover crop to eggplant growing seasons. All soil samples were sieved through a 0.5 cm2 mesh screen and homogenized before sub-sampling 250 cm3 soil for nematode extraction using elutriation and centrifugal floatation method (Jenkins, 1964; Byrd et al., 1976; Barker, 1985). All nematodes extracted were identified to the genus level wherever possible, counted under an inverted microscope (Leica DMIL, Leica Microsystems Company, Wetzlar, Germany), and assigned to trophic groups (algivores, bacterivores, fungivores, herbivores, omnivores, or predators) based on categorization of Yeates et al. (1993). Nematode richness was calculated as the total number of different taxa recorded per sample. Simpson’s index of dominance was calculated using λ = Σ (pi)2, where pi is the proportion of each of the i genera present, and Simpson’s index of diversity was calculated as 1/λ (Simpson, 1949). The fungivore to fungivore and bacterivore ratio (F/F+B) was calculated to characterize the decomposition and mineralization pathways (Freckman and Ettema, 1993). Maturity index (MI) of free-living nematodes was calculated as Σ (pi ci), where pi is the proportion of the taxon, and ci is the c-p rating of taxon i according to the 1 to 5 c-p scale (Bongers and Bongers, 1998). Enrichment index (EI) and Structure index (SI) was calculated as EI = 100 × [e/(e+b)] and SI = 100 × [s/(s+b)] where e, s, and b are the abundance of nematodes in guilds representing enrichment (e = Ba1 and Fu2 guilds, where Ba1 = guild of bacterivores with c-p value of 1, Fu2 = fungivores with c-p value of 2), structure (s = Ba3-Ba5, Fu3-Fu5, Om3-Om5, Ca2-Ca5 guilds, where Om = omnivores, Ca = carnivores), and basal (b = Ba2 and Fu2 guilds) food web components, respectively. The channel index (CI) was calculated as CI = 100 × [0.8Fu2/ (3.2Ba1+ 0.8Fu2)] (Ferris et al., 2001).
Following the termination of the SSgH, eggplant plant height was monitored at 2-month intervals after transplanting. Eggplant fruits were harvested beginning at 2 months after planting, and weekly thereafter. Numbers of fruits and fruit weight per plot were recorded. Fruits were sorted to marketable and unmarketable categories. Unmarketable fruits were due to thrips damage. At the end of the experiment, three plants from each plot were uprooted, washed, weighted, and rated for root-gall index (RGI) based on a 0-10 scale according to Netscher and Sikora (1990).
Objective 3
Soil samples were collected from the rhizosphere of SSgH or eggplants at 3 plants/plot at 2-week after SSgH termination, and at 2 months after eggplant planting for microbial biomass estimation using phospholipid fatty acid (PLFA) analysis. Rhizosphere soils were collected by screening through a 0.5-cm2 mesh metal sieve. A 10-g subsample from the composited rhizosphere soil was placed in a 14-ml Falcon tube (cat. No. 352059, Becton Dickinson, Lakes, NJ), transported to the laboratory with dry ice, and stored at -80◦C (PHCBI, cat. No. MDF-DU702VHA-PA, PHC corporation, Wood Dale, IL) before shipping to Microbial ID Laboratory (MIDI Inc., Newark, DE) for PLFA analysis. Based on PLFA, total and relative (%) microbial biomass of G+ bacteria, G- bacteria, actinomyces, arbuscular mycorrhizae (AMF), non-AMF, and protozoa were estimated.
Statistical analyses
Data were checked for normality using Proc Univariate in SAS Version 9.4 (SAS Institute Inc., Cary, NC). Wherever necessary, data were normalized using log10(x + 1) or square root transformation prior to analysis of variance (ANOVA) using Proc GLM in the SAS. Nematode data were subjected to repeated-measures ANOVA and checked for interaction between treatment and sampling date. If significant interaction between treatment and date occurred, data were subjected to one-way ANOVA by date. Means were separated using Waller-Duncan k-ratio (k = 100) t-test and only the true means were presented. Canonical correspondence analysis (CCA) was performed for field trial I and II to detect relationships between environmental and species variables using CANOCO 4.5 for Windows (ter Braak and Smilauer, 2002). Species variables included total PLFA biomass, G+ bacteria, G- bacteria, actinomyces, arbuscular mycorrhizae (AMF), non-AMF, and eukaryotes. Environmental variables included nematode community indices [EI, F/(F+B), CI, MI, SI and richness], abundance of nematode trophic groups (bacterivores, fungivores, herbivores, omnivores, and predators) including root-knot and reniform nematodes in the soil, soil carbon, infiltration, volumetric soil moisture, Solvita (https://solvita.com) respiration rate, SSgH biomass, eggplant yield (fruit weight), RGI.
References:
- Bybd, D.W.; Kirkpatrick, T.; Barker, K.R. An Improved Technique for Clearing and Staining Plant Tissues for Detection of Nematodes. Nematol.1983, 15, 142–143.
- Fares, A.; Abbas, F.; Ahmad, A.; Deenik, J.L.; Safeeq, M. Response of Selected Soil Physical and Hydrologic Properties to Manure Amendment Rates, Levels, Andtypes. Soil Sci.2008, 173, 522–533.
- Byrd, D.W.; Barker, K.R.; Ferris, H.; Nusbaum, C.J.; Griffin, W.E.; Small, R.H.; Stone, C.A. Two semi-automatic elutriators for extracting nematodes and certain fungi from soil. Nematol.
- Jenkins, W.R. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Report.1964, 48, 692.
- Barker, K. R. (1985). Nematode extraction and bioassays. An advanced treatise on Meloidogyne, 2, 19-35.
- Yeates, G.W.; Bongers, T.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera-an outline for soil ecologists. Nematol.1993, 25, 315–331.
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688.
- Freckman, D. W., & Ettema, C. H. (1993). Assessing nematode communities in agroecosystems of varying human intervention. Agriculture, Ecosystems & Environment, 45(3-4), 239-261.
- Bongers, T., & Bongers, M. (1998). Functional diversity of nematodes. Applied soil ecology, 10(3), 239-251.
- Ferris, H.; Bongers, T.; De Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Soil Ecol.2001, 18, 13–29.
- Netscher, C.; Sikora, R.A. Others Nematode parasites of vegetables. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; Luc, M., Sikora, R., Bridge, J., Eds.; CAB International: Wallingford, UK, 2005; pp. 319–392. ISBN 9781845931445.
- Ter Braak, C.; Smilauer, P. Canoco Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5)—[email protected] online: https://research.wur.nl/en/publications/canoco-reference-manual-and-canodraw-for-windows-users-guide-soft (accessed on 15 July 2021).
Objective 1
-
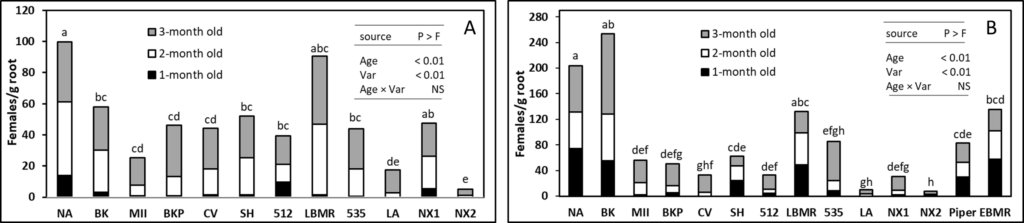
In greenhouse trial I, energy sorghum ‘NX2’ was most suppressive (P ≤ 0.05) to M. incognita female development for all three ages of sorghum biomass amended compared to the no amendment control (Figure 1A). Sorghum-sudangrass hybrid ‘LA’ was suppressive to M. incognita when using 1- and 2-month-old tissue, however, it could not suppress the number of females when using 3-month-old biomass. In greenhouse trial II, ‘NX2’ and ‘LA’ were again found to be most suppressive to M. incognita infection and lead to lower number of females compared to the control (Figure 1B). Interestingly, ‘NX2’ and ‘LA’ suppressed the number of females more effectively than sunn hemp amendment. Results from both trials showed a clear trend of decrease in the allelopathic effect of SSgH against M. incognita as the age of the biomass increase.
Figure 1: Effect of 1-, 2-, and 3-month-old SSgH biomass on the number of root-knot nematode females on mustard green roots in Trial I (A) and Trial II (B). NA (no amendment), BK (Bundle King), MII (Monster II), BKP (Big Kahuna Plus), CV (Cow Vittles), SH (sunn hemp), 512 (512 × 14), LBMR (Latte BMR), 535 (535 × 14), LA (Latte), NX1 (NX 4264), and NX2 (NX-D-61). Means (n = 12) followed by the same letter(s) are not different according to Waller–Duncan k-ratio (k = 100) t-test.
-
In no-till field trial/field trial I, soil carbon was significantly higher (P ≤ 0.05) in ‘NX2’ sorghum compared to the bare ground control at 2.5 months after planting SSgH cover crop, however this effect dissipated at 3 months after eggplant planting, soil carbon was not different (P > 0.05) among SSgH varieties. Energy sorghum ‘NX2’ and forage sorghum ‘BKP’ had higher (P ≤ 0.05) soil respiration rates compared to the bare ground control. However, water infiltration rate though highly variable, was not different among treatments (Table 2). In the low-till field trial/field trail II, soil carbon was not different among SSgH varieties and bare ground control (P > 0.05) except for ‘5355’ which had lowest soil carbon. Soil respiration was higher in ‘NX2’ and ‘BK’ sorghum. In the low-till trial, as opposed to the no-till trial, infiltration was enhanced by SSgH cultivars "NX2" and "512” (P ≤ 0.05) (Table 3).
Table 2. Effect of SSgH on soil carbon, soil respiration, and water infiltration rate in no-till field trial/Field trial I.
|
|
Treatments |
|||||||
|
Parameters |
BG |
512 |
LA |
BK |
BKP |
CV |
NX1 |
NX2 |
|
Soil carbon (%) |
1.48 b y |
1.39 b |
1.41 b |
1.41 b |
1.43 b |
1.45 b |
1.62 ab |
1.83 a |
|
Soil respiration (lbs Co2/acre) |
3.97 c |
7.55 bc |
7.08 bc |
5.8 bc |
8.65 ab |
6.78 bc |
7.53 bc |
11.89 a |
|
Water infiltration (mm/hr) |
943.09 a |
441.62 ab |
539.17 ab |
163.33 b |
478.93 ab |
252.74 b |
292.73 b |
422.01 ab |
Means (n = 8) are from repeated measures over 2 sampling dates for soil carbon and water infiltration. Means (n = 16) for soil respiration. Values followed by the same letter(s) in a row are not different based on Waller–Duncan k-ratio (k = 100) t-test.
Table 3. Effect of SSgH on soil carbon, soil respiration, and water infiltration rate in low-till field trial/Field trial II.
|
|
Treatments |
|||||||
|
Parameters |
BG |
512 |
5355 |
542 |
LA |
BK |
NX2 |
Piper |
|
Soil carbon (%) |
1.53 c |
1.58 bc |
1.61 abc |
1.71 abc |
1.57 bc |
1.68 abc |
1.79 a |
1.75 ab |
|
Soil respiration (lbs Co2/acre) |
4.84 d |
19.00 ab |
9.44 c |
18.81 ab |
12.87 ab |
23.10 bc |
13.41a |
11.89 bc |
|
Water infiltration (mm/hr) |
52.62 b |
277.93 ab |
164.87 ab |
174.49 ab |
264.96 a |
154.42 ab |
269.12 a |
253.58 a |
Means (n = 8) are from repeated measures over 2 sampling dates for soil carbon and water infiltration. Means (n = 16) for soil respiration. Values followed by the same letter(s) in a row are not different based on Waller–Duncan k-ratio (k = 100) t-test.
Objective 2
Field trial I (No-till system)
When terminating SSgH cover crops in a no-till system, SSgH treatment did not affect the abundance of reniform nematodes, but population density of root-knot nematodes was numerically lowest in the bare ground (BG) control but statistically lower in ‘CV’ than BG. However, increase of root-knot nematode numbers was only occurring towards the end of the eggplant crops. In terms of beneficial nematodes involved in soil nutrient cycling, abundance of bacterivorous nematodes was increased by ‘512’ (P ≤ 0.05), whereas omnivorous nematodes were increased by ‘NX2’, ‘BKP’, ‘BK’ and ‘512’ (P ≤ 0.05) compared to BG (Table 4). In general, all SSgH treatments increased abundance of omnivorous nematodes compared to BG numerically. When using nematode community indices to evaluate soil health, all SSgH treatments increased nematode richness compared to BG (P ≤ 0.05) except for ‘CV’ and ‘BKP’. There was also a trend that all SSgH increased CI compared to BG but this was most significant in ‘BK’, ‘BKP’ and ‘NX2’ (P ≤ 0.05). Though not significant, all SSgH also increased SI compared to the BG.
Table 4. Effect of SSgH cover crop on the abundance of nematode trophic groups and nematode community indices throughout the SSgH–eggplant cropping cycle in no-till system.
|
Parameters |
Treatments |
|||||||
|
BG |
512 |
LA |
BK |
BKP |
CV |
NX1 |
NX2 |
|
|
Abundance |
-----------250 cm3 soil----------- |
|||||||
|
Root-knot |
447 ab y |
966 bc |
1556 a |
957 a |
966 ab |
923 c |
812 a |
725 ab |
|
Reniform |
800 a |
666 a |
595 a |
376 a |
568 a |
709 a |
466 a |
489 a |
|
Bacterivores |
164 b |
293 a |
206 ab |
179 b |
224 b |
325 ab |
220 ab |
310 ab |
|
Fungivores |
69 a |
134 a |
82 a |
99 a |
90 a |
105 a |
117 a |
148 a |
|
Herbivores |
1249 a |
1636 a |
2161 a |
1335 a |
1534 a |
1642 a |
466 a |
489 a |
|
Omnivores |
4 b |
16 a |
15 ab |
16 a |
18 a |
8 ab |
11 ab |
19 a |
|
Indices |
||||||||
|
Richness |
8 c |
10 a |
9 ab |
10 ab |
9 bc |
9 bc |
10 ab |
10 a |
|
Diversity |
2.57 a |
2.84 a |
2.48 a |
2.77 a |
2.78 a |
2.41 a |
3.03 a |
2.99 a |
|
EI (%)z |
1.96 a |
2.02 a |
2 a |
2.02 a |
2 a |
1.97 a |
1.96 a |
1.99 a |
|
SI (%) |
45.58 a |
47.19 a |
48.52 a |
50.75 a |
50.51 a |
53.02 a |
54.81 a |
52.18 a |
|
MI (%) |
0.36 a |
0.33 a |
0.37 a |
0.38 a |
0.39 a |
0.4 a |
0.39 a |
0.37 a |
|
CI (%) |
11.39 b |
23.97 ab |
20.52 ab |
24.34 a |
24.49 a |
22.33 ab |
20.31 ab |
25.42 a |
Means ± standard error (n = 28) are averaged from repeated measures over seven sampling dates. Values followed by the same letter(s) in a row are not different based on Waller–Duncan k-ratio (k = 100) t-test. z EI = Enrichment index; SI = Structure index; MI = Maturity index; CI = Channel index.
Eggplant height (5 weeks after transplanting) was not different (P > 0.05) among treatments. Eggplant fruit weight was numerically higher in all SSgH treatments compared to BG control, though it was not significantly different (P > 0.05) (Table 5). A higher (P ≤ 0.05) fruit number was observed in ‘CV’ plots. Root weight was increased (P ≤ 0.05) by ‘BK’ sorghum and all SSgH treatments had a higher root weight than BG control but root-gall index was not different (P > 0.05) among treatments.
Table 5. Effect of SSgH cover cropping on eggplant growth and yield in no-till field trial.
|
|
Treatments |
|||||||
|
Parameters |
BG |
512 |
LA |
BK |
BKP |
CV |
NX1 |
NX2 |
|
Height (cm) |
29.7 a y |
40.64 a |
35.61 a |
35.31 a |
34.82 a |
36.42 a |
36.83 a |
36.7 a |
|
Yield (kg) |
2.47 a |
3.64 a |
2.15 a |
3.4 a |
2.45 a |
3.63 a |
2.6 a |
3.63 a |
|
Fruit no. |
27 a |
31 a |
25 a |
28 a |
30 a |
38 a |
27 a |
29 a |
|
Root wt (kg) |
0.07 b |
0.1 ab |
0.09 ab |
0.11 a |
0.08 ab |
0.10 ab |
0.11 ab |
0.1 ab |
|
RGI (0–10) z |
5.43 a |
5.50 a |
6.13 a |
6.73 a |
5.33 a |
5.50 a |
5.98 a |
6.10 a |
y Means (n = 40) are from repeated measures over 10 sampling dates for total fruit number/plot and total fruit weight/plot. Means (n = 4) for root-gall index, root weight, and plant height per plant/plot. Values followed by the same letter(s) in a row are not different based on Waller–Duncan k-ratio (k = 100) t-test. z RGI = Root-gall index on a 0–10 scale.
Field Trial II (Low-till system)
Only ‘Piper’ sorghum reduced the reniform nematode population in the field compared to bare ground control (P < 0.05) (Table 6). Root-knot abundance was not different between treatments (P > 0.05). Abundance of fungal feeding nematodes was increased by ‘BK’, ‘NX2’, and ‘Piper’ sorghum, whereas omnivores were increased by ‘BK’ and ‘NX2’ sorghum. Enrichment index was higher in ‘5355’ sorghum. Although not statistically different, all sorghum increased bacterial feeding nematode population. Only ‘542’, ‘BK’, and ‘NX2’ sorghum had numerically higher channel and structure index compared to BG control.
Table 6. Effect of SSgH cover crop on the abundance of nematode trophic groups and nematode community indices throughout the SSgH–eggplant cropping cycle in low-till system.
|
Parameters |
Treatments |
|||||||
|
BG |
512 |
5355 |
542 |
LA |
BK |
NX2 |
Piper |
|
|
Abundance |
-----------250 cm3 soil----------- |
|||||||
|
Root-knot |
35 a |
63 a |
314 a |
49 a |
40 a |
79 a |
105 a |
56 a |
|
Reniform |
426 a |
293 abc |
267 bc |
343 ab |
422 ab |
275 abc |
337 ab |
200 c |
|
Bacterivores |
172 a |
306 a |
259 a |
293 a |
243 a |
252 a |
264 a |
328 a |
|
Fungivores |
111 b |
171 ab |
194 ab |
230 ab |
198 ab |
239 a |
243 a |
251 a |
|
Herbivores |
463 a |
360 a |
587 ab |
399 a |
463 a |
362 a |
446 a |
262 b |
|
Omnivores |
6 b |
13 ab |
25 ab |
19 ab |
19 ab |
27 a |
27 a |
17 ab |
|
Indices |
||||||||
|
Richness |
9 a |
10 |
9 a |
9 a |
10 a |
11 a |
10 a |
10 a |
|
Diversity |
3.54 a |
3.79 a |
3.72 a |
3.65 a |
3.58 a |
3.97 a |
4.25 a |
4.27 a |
|
EI (%)z |
53.65 ab |
58.05 ab |
60.50 a |
49.15 b |
58.00 ab |
55.00 ab |
53.75 ab |
59.20 ab |
|
SI (%) |
24.55 a |
23.80 a |
29.55 a |
26.85 a |
30.15 a |
28.55 a |
32.40 a |
23.55 a |
|
MI (%) |
1.95 a |
1.86 a |
1.90 a |
2.03 a |
1.99 a |
1.99 a |
2.04 a |
1.89 a |
|
CI (%) |
55.00 a |
49.65 a |
51.35 a |
63.25 a |
51.90 a |
58.45 a |
58.05 a |
49.30 a |
Means ± standard error (n = 20) are averaged from repeated measures over five sampling dates. Values followed by the same letter(s) in a row are not different based on Waller–Duncan k-ratio (k = 100) t-test. z EI = Enrichment index; SI = Structure index; MI = Maturity index; CI = Channel index.
Eggplant height recorded at 5 weeks after transplanting was not significantly different (P > 0.05) among treatments (Table 7). Eggplant fruit weight was numerically higher in all SSgH treatments except ‘5355’ and ‘Piper’ compared to BG control, though it was not significantly different (P > 0.05). There were no significant differences (P > 0.05) among treatments for root-gall formation.
Table 7. Effect of SSgH cover cropping on eggplant growth and total yield in low-till field trial.
|
|
Treatments |
|||||||
|
Parameters |
BG |
512 |
5335 |
542 |
LA |
BK |
NX2 |
Piper |
|
Height (cm) |
27.34 a |
33.06 a |
33.26 a |
30.88 a |
31.33 a |
29.45 a |
25.96 a |
26.11 a |
|
Fruit wt (kg) |
0.83 abc |
0.86 ab |
0.71 bc |
0.93 a |
0.94 a |
0.94 ab |
0.89 ab |
0.69 c |
|
Fruit no. |
7 a |
8 a |
7 a |
8 a |
8 a |
8 a |
8 a |
7 a |
|
Root wt (kg) |
0.14 a |
0.12 a |
0.12 a |
0.14 a |
0.14 a |
0.17 a |
0.12 a |
0.13 a |
|
RGI (0–10) z |
0.75 a |
1.25 a |
2 a |
0.75 a |
1.25 a |
1 a |
0.5 a |
0.75 a |
y Means (n = 60) are from repeated measures over 15 sampling dates for total fruit number/plot and total fruit weight/plot. Means (n = 4) for root-gall index, root weight, and plant height per plant/plot. Values followed by the same letter(s) in a row are not different based on Waller–Duncan k-ratio (k = 100) t-test. z RGI = Root-gall index on a 0–10 scale.
Objective 3
Field trial I (No-till system)
- At time of SSgH cover crop termination, total phospholipid fatty acids (TPLFA) analysis indicated that microbial biomass was increased (P ≤ 0.05) by 6 SSgH treatments except for ‘NX1’ (Table 8). Among the SSgH, ‘LA’ and ‘NX2’ were most promising with 92% and 60% higher microbial biomass, respectively, than the bare ground (BG) control. Energy sorghum ‘NX2’ significantly increased populations of non-arbuscular mycorrhizal fungi and eukaryotes compared to BG.
- Three months after eggplant planting, ‘LA’ still increase (P ≤ 0.05) microbial biomass with 87% more than the control. Although not statistically significant, ‘CV’ and ‘NX2’ showed a 45% and 38% increase in microbial biomass, respectively, compared to BG.
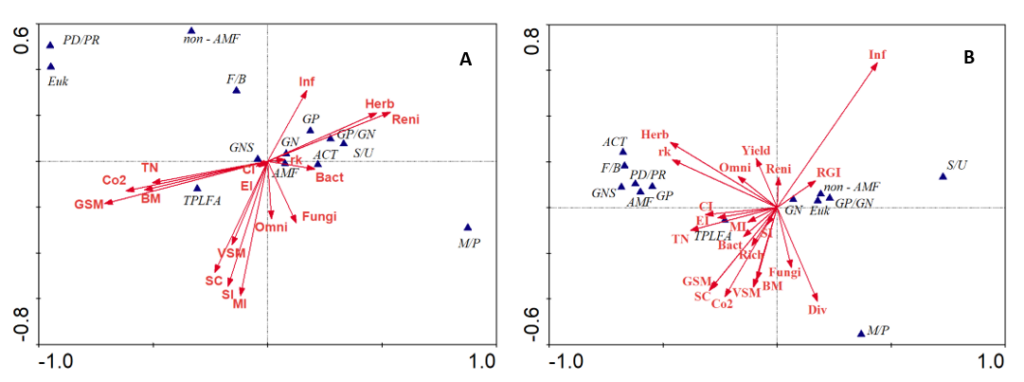
- Canonical Correspondence Analysis (CCA) at the time of termination of SSgH cover crop showed that most of the soil health indicators including total microbial biomass, soil microbial respiration rates, soil moisture, soil carbon, nematode enrichment index, maturity index, structure index, and abundance of omnivorous nematodes were negatively related to the abundance of plant-parasitic nematodes and reniform nematodes (Figure 2A). At 3 months after eggplant planting, a negative relationship between root-gall index on eggplant with the above-mentioned soil health indicators was also observed (Figure 2B).
Table 8: Effect of SSgH on soil microbial profile based on PLFA in no-till field trial/Field trial I.
|
Parameters |
Treatments |
|||||||
|
BG |
512 |
LA |
BK |
BKP |
CV |
NX1 |
NX2 |
|
|
Abundance |
---27 August 2020--- | |||||||
|
TPLFA (nmole/g) z |
40.7c y |
55.64b |
78.1 a |
55.1 b |
60.4 b |
60.1 b |
53.4 bc |
65.4 ab |
|
GN (%) |
34.6 b |
36.3 ab |
36.7 ab |
36.8 ab |
35.2 b |
34.7 b |
38 a |
36.1 ab |
|
GP (%) |
38.7 a |
36.9 a |
36.8 a |
39.4 a |
38 a |
37.1 a |
38.5 a |
35.5 a |
|
AMF (%) |
3.9 a |
3.9 a |
4.2 a |
4 a |
4.1 a |
3.9 a |
4.2 a |
4 a |
|
non-AMF (%) |
2.9 b |
3.9 ab |
4 ab |
3.7 ab |
4.1 ab |
5.4 a |
3.7 ab |
5.4 a |
|
EUK (%) |
0.9 b |
1.4 ab |
2.3 ab |
0.9 b |
1.6 ab |
1.7 ab |
1.4 ab |
2.5 a |
|
Ratios |
||||||||
|
GP/GN |
1.5 a |
1.3 b |
1.2 b |
1.3 b |
1.3 b |
1.3 b |
1.2 b |
1.2 b |
|
F/B |
0.09 b |
0.11 ab |
0.11 ab |
0.11 ab |
0.12 ab |
0.13 a |
0.11 ab |
0.13 a |
|
S/U |
1.7 a |
1.4 ab |
1.3 b |
1.5 ab |
1.4 b |
1.4 ab |
1.4 ab |
1.4 b |
|
M/P |
12. a |
7.2 ab |
5.8 b |
7.6 ab |
6.7 ab |
5.1 b |
7.3 ab |
4.9 b |
|
PD/PR |
0.01 a |
0.02 a |
0.04 a |
0.02 a |
0.03 a |
0.03 a |
0.02 a |
0.04 a |
|
Abundance |
---10 December 2020--- | |||||||
|
TPLFA (nmole/g) |
41.5 b |
51.7 ab |
77.6 a |
52.6 abc |
54.2 abc |
60.1 ab |
34.8 c |
57 abc |
|
GN (%) |
33.6 a |
33.2 a |
33.7 a |
33.3 a |
33.7 a |
33.9 a |
32.7 a |
35.6 a |
|
GP (%) |
47.4 a |
39 d |
35 e |
39.7 cd |
39.4 d |
37.4 de |
45.8 ab |
43.2 bc |
|
AMF (%) |
4.9 a |
2.8 bcd |
3.6 ab |
1.6 d |
3.1 bc |
3.3 bc |
3.8 ab |
2.1 cd |
|
non-AMF (%) |
9.7 a |
4.5 a |
6.1 a |
3.5 a |
5.2 a |
4.6 a |
17.2 a |
3.5 b |
|
EUK (%) |
1.8 c |
4 abc |
8 a |
6.1 ab |
5.5 ab |
4.4 abc |
1.1 d |
3.3 bc |
|
Ratios |
||||||||
|
GP/GN |
1.9 a |
1.5 bc |
1.3 c |
1.6 b |
1.6 b |
1.5 bc |
2 a |
1.6 b |
|
F/B |
0.2 a |
0.1 abc |
0.1 ab |
0.1 bc |
0.1 abc |
0.1 abc |
0.4 a |
0.1 c |
|
S/U |
7.8 ab |
2.4 de |
1.5 e |
2.9 cd |
4.9 cd |
2 de |
9 a |
5.4 bc |
|
M/P |
6.6 b |
3.1 bc |
2.5 c |
2.5 c |
2.7 c |
3.1 bc |
10.6 a |
4.6 bc |
|
PD/PR |
0.02 b |
0.1 ab |
0.1 a |
0.1 a |
0.1 a |
0.1 ab |
0.02 c |
0.1 ab |
Means (n = 4) followed by the same letter(s) in a row are not different based on Waller–Duncan k-ratio (k = 100) t-test.
z TPLFA = Total phospholipid fatty acid representing total microbial biomass in nanomoles per gram of soil; Microbial groups such as GN = Gram-negative bacteria; GP = Gram-positive bacteria; AMF = Arbuscular mycorrhizal fungi; non-AMF = non-arbuscular mycorrhizal fungi; EUK = Eukaryotes are percentage of signature fatty acid peaks; GP/GN = ratio of Gram + ve bacteria to Gram − ve bacteria; F/B = ratio of fungi to bacteria ; S/U = ratio of saturated to unsaturated fatty acids; M/P = ratio of monounsaturated to polyunsaturated fatty acids; PD/PR = ratio of predator to prey (Protozoa/bacteria).
Figure 2. Canonical correspondence analysis (CCA) biplots showing the relationships among species variables (blue triangles) and environmental variables (red arrows) at (A) the time of cover crop termination (B) at 3 months after eggplant planting in no-till field trial/Field trial I. The first two axes explained 87.4% and 86% of the variation in the data in A and B, respectively. Species variables include total phospholipid fatty acid (TPLFA), arbuscular mycorrhizal fungi (AMF), Gram-negative bacteria (GN), Gram-positive bacteria (GP), actinomyces (ACT), eukaryotes (EUK), non-arbuscular mycorrhizal fungi (non-AMF), fungi/bacteria (F/B), predator/prey (PD/PR), GP/GN, saturated fatty acids/unsaturated fatty acids (S/U), monounsaturated fatty acids/polyunsaturated fatty acids (M/P), and Gram-negative stress (GNS). Environmental variables include reniform nematode (Reni), root-knot nematode (rk), herbivores (Herb), bacterivores (Bact), fungivores (Fungi), omnivores (Omni), nematode richness (Rich), nematode diversity (div), maturity index (MI), enrichment index (EI), structure index (SI), channel index (CI), soil infiltration rate (Inf), soil carbon (SC), eggplant yield (Yield), soil respiration (Co2), volumetric soil moisture (VSM), gravimetric soil moisture (GSM), SSgH tissue nitrogen (TN), SSgH biomass (BM), and root-gall index (RGI).
Field trial II (Low-till system)
- At SSgH cover crop termination, TPLFA was increased (P ≤ 0.05) by all SSgH treatments excluding ‘5355’ sorghum (Table 9). Total microbial biomass was 42.7% and 35.9% higher in ‘NX2’ and ‘Piper’ sorghum compared to BG. ‘NX2’ and ‘Piper’ increased Gram-positive (GP) and Gram-negative (GN) bacteria compared to the BG control. The abundance of actinomyces (ACT) was significantly lower in ‘NX2’ and ‘Piper’ sorghum (P ≤ 0.05).
- Three months after eggplant planting, ‘5355’ sorghum resulted in the highest TPLFA, although ‘512’ and ‘LA’ supported similar amount of TPLFA. Gram-positive (GP), gram-negative (GN) bacteria and arbuscular mycorrhizal fungi (AMF) were all increased (P ≤ 0.05) by "LA" and "5355" sorghum, whereas the abundance of actinomyces was only increased by ‘LA’ compared to BG.
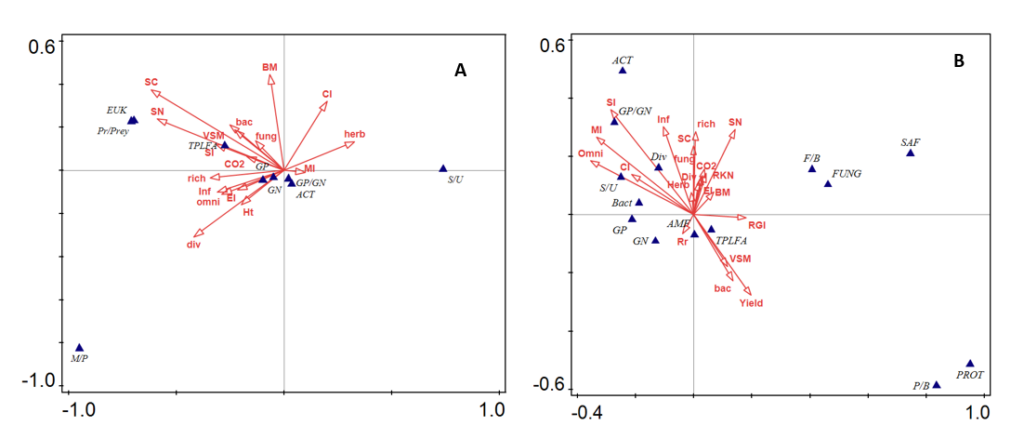
- Multivariate analysis among all parameters at time of SSgH cover crop termination showed positive relationships among soil health indicators: structure index (SI), soil microbial respiration rates (CO2), soil carbon content (SC), volumetric soil moisture (VSM) (Figure 3A). The negative relationship between the abundance of plant-parasitic nematodes (herb) with the above-mentioned variables reassure the fact that improvement in soil health could lead to reduction of nematode pest infestation. At 3 months after eggplant planting, multivariate analysis performed showed positive relationships among soil health indicators: structure index (SI), soil microbial respiration rates (CO2), soil carbon content (SC), nematode richness (rich), diversity (div), and water infiltration rate (Infiltration) (Figure 3B). However, eggplant yield (Yield) was only positively related to volumetric soil moisture (VSM), abundance of bacterivorous nematodes (bact), total microbial biomass, (TPLFA) and arbuscular mycorrhizal fungi biomass (AMF).
Table 9: Effect of SSgH on soil microbial profile based on PLFA in low-till field trial/Field trial II.
|
Parameters |
Treatments |
|||||||
|
BG |
512 |
5355 |
542 |
LA |
BK |
NX2 |
Piper |
|
|
Abundance ---9 September 2021--- |
||||||||
|
TPLFA (nmole/g)z |
35.3 b y |
48.6 a |
40.8 b |
47.3 a |
47.7 a |
45.4 a |
50.4 a |
48.0 a |
|
GP (%) |
51.6 b |
51.8 ab |
52.1 ab |
52.1 ab |
53.3 ab |
52.7 ab |
53.8 a |
51.1 b |
|
GN (%) |
24.3b |
25.0ab |
24.5ab |
24.4ab |
24.3b |
24.3b |
23.7b |
26.2a |
|
ACT (%) |
23.1a |
21.5ab |
22.1ab |
21.6ab |
21.3ab |
21.6ab |
20.9b |
20.8b |
|
Abundance ---9 December 2021--- |
||||||||
|
TPLFA(ng/g)x |
5007.0 bc |
6469.8 ab |
7446.5 a |
4964.4 bc |
6677.5 ab |
4350.3 c |
4279.9 c |
3793.7 c |
|
GP (ng/g) |
912.2 cd |
1054.3 bc |
1264.8 ab |
1022.0 bcd |
1446.5 a |
848.4 cd |
893.8 cd |
770.7 d |
|
GN (ng/g) |
633.5 bc |
939.2 ab |
1201.0 a |
762.1 bc |
1125.6 a |
639.0 bc |
644.2 bc |
568.1 c |
|
ACT (ng/g) |
359.6 ab |
400.9 ab |
465.7 ab |
291.7 bc |
480.0 a |
356.8 ab |
366.2 ab |
184.9 c |
|
AMF (ng/g) |
164.0 cd |
214.4 bc |
314.4 a |
192.0 cd |
264.9 ab |
179.9 cd |
161.0 cd |
132.5 d |
Means (n = 4) followed by the same letter(s) in a row are not different based on Waller–Duncan k-ratio (k = 100) t-test.
z TPLFA = Total phospholipid fatty acid representing total microbial biomass in nanomoles per gram of soil; Microbial groups such as GN = Gram-negative bacteria; GP = Gram-positive bacteria; ACT = Actinomyces are percentage of signature fatty acid peaks.
xTPLFA = Total phospholipid fatty acid representing total microbial biomass, GN = Gram-negative bacteria; GP = Gram-positive bacteria; ACT = Actinomyces; AMF = Arbuscular mycorrhizal fungi in nanogram per gram of soil.
Figure 3. Canonical correspondence analysis (CCA) biplots showing the relationships among species variables (blue triangles) and environmental variables (red arrows) at (A) the time of cover crop termination (B) at 3 months after eggplant planting in low-till field trial/Field trial II. The first two axes explained 94.47% and 76.59% of the variation in the data in (A,B), respectively. The species variables include total phospholipid fatty acid (TPLFA), arbuscular mycorrhizal fungi (AMF), Gram-negative bacteria (GN), Gram-positive bacteria (GP), actinomyces (ACT), eukaryotes (EUK), saprophytic fungi (FUNG), fungi/bacteria (F/B), predator/prey (Pr/Prey), protozoa/bacteria (P/B), Gram-positive bacteria/Gram-negative bacteria (GP/GN), saturated fatty acids/unsaturated fatty acids (S/U), monounsaturated fatty acids/polyunsaturated fatty acids (M/P). Environmental variables include root-knot nematode (RKN), plant-parasitic nematode/herbivore (herb), bacterivores (bac), fungivores (fung), omnivores (Omni), nematode richness (rich), nematode diversity (div), maturity index (MI), enrichment index (EI), structure index (SI), channel index (CI), soil infiltration rate (Inf), soil carbon (SC), eggplant yield (Yield), soil respiration (Co2), volumetric soil moisture (VSM), soil nitrogen (SN), SSgH biomass (BM), and root-gall index (RGI).
Research Outcomes
Education and Outreach
Participation Summary:
Workshops/field days
-
Regenerative Agriculture using sorghum as a cover crop in a low till system. Poamoho Cover Crop Field Day. 2022. Poamoho Experiment Station (50 participants).
- GoFarm Hawaii New Farmers Training Program: Cover crop and Nematode Management. GoFarm at Hilo. Aug 16, 2022 (12 participants, Farm Coach:Danny Randerson).
-
Establishing Long-Term Ground Cover vs Mulching with Long-Lasting Cover Crop Residues of sorghum. Healthy Soil, Healthy Orchard Workshop: from Planning to Practices. Island Harvest, Kohala, Hawaii Island. July 15, 2022 (20 participants).
- GoFarm Hawaii New Farmers Training Program: Cover crop and IPM. GoFarm at Waimanalo. June 29, 2022 (12 participants, Farm Coach: Rachel Ladrig).
- GoFarm Hawaii New Farmers Training Program: Sustainable nematode and other pest management in agroecosystems through cover cropping, biological derived products and field sanitation. GoFarm Hawaii at Kauai June 23, 2022 (9 participants, Farm Coach: Jin-Wah Lau).
- Sorghum soil health research updates. “Poamoho Orchard Field Day”. Poamoho Experiment Station. April 30, 2022. (42 participants: 28 in person, 15 on zoom).
- GoFarm Hawaii New Farmers Training Program: Integrating cover crops, crop rotation, and biodiversity into agroecosystems. GoFarm Hawaii at Kauai. Nov 9, 2021 (12 participants, Farm Coach: Eric Hanssen).
-
Benefits of cover crops for water conservation. Sustainable and Organic Agriculture Program (SOAP) Mini-Conference. Oct 28, 2021 (37 participants).
- Ecological & Sustainable Nematode Management. NRCS Conversations on Soil Health: Nematode Management and Cover Crops (Adobe Acrobat on-line event), June 17, 2021 (75 participants-NRCS Staff), Organized by Rachel Seman-Varner, Ph.D.
- Integrating cover crops and organic fertilizers into your nutrient management regime to meet your farm’s soil health goals. Hawaii Women Farmer’s Network: Soil Health Workshop Four-Part Series. Kahumana Organic Farm, June 15, 2021 (12 participants), organized by India Clark, Oahu Resource Conservation and Development Council.
- The science behind cover cropping. Oahu County Cooperative Extension’s Research in the Garden Series. Urban Garden Center, May 27, 2021. Organized by J. Sugano (30 participants). A cover crop field day was conducted at the Urban garden center, in collaboration with CTAHR extension agents .The advantage of growing sorghum/sorghum-sudangrass hybrids (SSgH) was demonstrated through a series of activities including a soil slaking test to compare no-till cover crop vs tilled plots and a simulation rainfall test to compare the infiltration rate of 3 SSgH varieties against a bare ground control. Participants were encouraged and involved in performing each of these activities. In addition, a written copy of the benefits of SSgH cover cropping along with our recent research finding was made available to all participants. Seeds of best-performing sorghum 'NX-D-61 was distributed to the attendees.
-
GoFarm Hawaii New Farmers Training Program: Sustainable nematode and other pest management in agroecosystems through cover cropping or biological derived products (12 participants, Farm Coach: Eric Hanssen; March 24, 2021; 12 participants, Farm Coach: Jay Bost).
- Soil Health Lecture. Together We Farm Online Learning Platform. Oahu Oahu ACA.tovuti.io (Jan-March, 2021).
- Virtual soil health and IPM Mini-Conference. A presentation on 'Which sorghum/sorghum-sudangrass hybrids have higher allelopathic toxicity against soil-borne pests' was delivered to farmers and agricultural professionals through zoom on Aug 4, 2020 (30 participants).
Presentations
- Paudel, R. and K.-H Wang. Expediting soil health improvement effects of sorghum/sorghum-sudangrass hybrids through low-till practice in a Rotylenchulus reniformis infested soil. Society of Nematologists 61th Annual Conference, September 26-29, 2022, Anchorage, Alaska.
- Paudel, R. and K.-H. Wang, Screening sorghum/sorghum-sudangrass hybrids for allelopathic effects against root-knot nematodes and their potential for soil health management in a no-till agroecosystem. Society of Nematologists Annual Conference, September 12-15, 2021, Gulf Shores, Alabama.
- Paudel, R. and K. -H. Wang. 2021. University of Hawaii Plant Sciences Symposium, “Management of plant-parasitic nematodes and soil health using sorghum/sorghum-sudangrass hybrids as a cover crop”. Honolulu, HI.
- Paudel, R. and K. -H. Wang. and Waisen, P. 2020. Society of Nematologists virtual meeting, “Management of plant-parasitic nematodes and soil health using sorghum/sorghum-sudangrass hybrids as a cover crop”. Dec 14-18, 2020.
Extension videos
Two videos were created and uploaded to the PIs YouTube channel.
1. https://www.youtube.com/watch?v=XrdYbhQnVAc
This video demonstrates the importance of soil health management as the foundation of Plant Health Management. Keeping the ground cover 24/7 is important in 1) reducing soil erosion thus maintaining soils for crop roots to grow, 2) preventing water runoff and pollution, 3) maximizing water infiltration to conserve water and mitigate drought problems in crop production, and 4) sustaining soil organisms that are crucial for soil nutrient cycling.
2. https://www.youtube.com/watch?v=hbCSWttx8_A
This video showcases the multipurpose of sorghum as a cover crop in terms of suppressing plant-parasitic nematodes, adding soil carbon, improving water conservation and microbial activities in a vegetable agroecosystem in Hawaii.
Journal article
- Paudel, R., P. Waisen, and K.-H. Wang. 2021. Exploiting the innate potential of sorghum/sorghum–sudangrass cover crops to improve soil microbial profile that can lead to suppression of plant-parasitic nematodes. MDPI-Microorganisms 9:1831 https://doi.org/10.3390/microorganisms9091831
Extension article
- Paudel, R., L. Braley, Joshua Silva and K.-H. Wang. 2022. Subbing sunn hemp with sorghum in Fusarium soils. HānaiʻAi Newsletter December 2022 (in press).
- Paudel, R., S. Budhathoki, and K.-H. Wang. 2021. Revitalized degraded soil in the tropic with energy sorghum. https://myemail.constantcontact.com/The-Latest-H-nai-Ai-News---April---May---June-2021-Volume-42.html?soid=1102675671876&aid=F9Y1OK_qJKk.