Final report for GW22-233
Project Information
Plant pathogens pose a serious threat to food security, and growers in Hawaii are especially challenged by pest pressure all year round. Rising cost of agriculture production inputs is calling food producers in Hawaii to adopt sustainable pest management tactics using local resources. Papaya seeds are considered as an agricultural waste in Hawaii. This project aims to evaluate the use of ground papaya seed (PGS) to suppress plant-parasitic nematodes and soil-borne fungi, as the hydrolysis of PGS produces a biofumigant, benzyl isothiocyanate (BITC), which is suppressive to many soil-borne pathogens. The novelty of this project is to explore the innate immune response of treated crops to the microbiome associated with PGS treatment. Specific objectives are to 1) examine if PGS can induce systemic resistance (ISR) of crops against broad spectrum of pests and pathogens by examining expression of ISR genes and related phytohormones, 2) evaluate the efficacy of using PGS on kai choi, onion, and zucchini seedlings through seedling media amendment to protect these seedlings at transplanting, 3) examine the adequate frequency of post-plant foliar spray vs soil drenching of transplants in fields naturally infested with Fusarium spp. or Meloidogyne incognita. We will work with three commercial farmers in Hawaii challenged by the above-mentioned pathogens, while collaborating with extension agents to disseminate research findings with vegetable crop producers in Hawaii through workshops and online extension newsletters or publications. We expect successful adoption of this local farms’ waste-based biofumigation approach to help Hawaii farmers to combat a year-round pathogen infestation problem.
Research objectives:
Objective 1: Examine if PGS can induce systemic resistance (ISR) of crops against broad spectrum of pests and pathogens by examining expression of ISR genes.
Objective 2: Evaluate the efficacy of using PGS crude extract on kai choi, onion, and zucchini seedlings through drenching of seedling media.
Objective 3: Examine the adequate frequency of post-plant foliar spray vs soil drenching of transplants in fields naturally infested with Fusarium spp. or Meloidogyne incognita.
Cooperators
- (Researcher)
- - Producer
- - Producer
- - Producer
- (Researcher)
Research
Hypothesis:
Our central hypothesis was that PGS can be used to induce plant immune responses against targeted pathogens of interest. Additionally, a secondary hypothesis was that the use of a crude extract from PGS may be more effective in inducing the immune response by delivering BITC more effectively. These hypotheses are supported in part by data collected in our preliminary study as well as associated literature. Thus, our research plan was to assist local vegetable food crop producers in Hawai`i to overcome the overarching challenge of soil-borne diseases by using ground papaya seed (PGS) to induce plant systemic resistance against the targeted pathogens. Our overall goal was to work with local growers and extension agents to develop a protocol for farmers to use PGS effectively.
Materials and methods:
During our first year, we collected data pertaining to (1) kai choi greenhouse trial using papaya ground seed (PGS) and papaya seed crude extract (CE) treatments to induce systemic resistance and (2) a field trial of PGS and CE on Rhizoctonia- and Fusarium-infested soil on a collaborating kai choi farmer’s farm (Owen Kaneshiro Farm, Waianae, HI).
In Year 2, while we were designing and validating real-time PCR assay protocol for Fusarium isolated from Owen Kaneshiro Farm, we detected a new Fusarium species in this soil. While the PI’s lab is working on verifying this is the first report of Fusarium commune in Hawaii, PI Braley modified the study to include the testing on PGS effects on F. commune. Thus, we requested one year’s no-cost extension of this project. Overall, in Year 2 we: 1) designed and validated a real-time, quantitative PCR (RT-qPCR) assay for detection of R. solani in Hawaii’s clay-like soil, 2) isolated and sequenced four Fusarium commune isolates from diseased kai choi roots with Fusarium wilt-like symptoms, 3) designed a preliminary RT-qPCR assay for detection of F. commune in Hawaii soils, 4) improved PGS+CE drenching methods in two field trials of kai choi with F. commune infested soil at Owen Kaneshiro Farm, Waianae, HI.
During our final year of testing PGS biofumigation as a tool to trigger ISR against a wide range of soil-borne plant pathogens, we expanded our target pest range to include the burrowing nematode, Radopholus similis, with which we conducted (1) in vitro assay experiment and (2) anthurium shade house pot trials. In addition, we conducted (2) tomato split-root greenhouse trials and (2) tomato field pot trials for root-knot nematodes (Meloidogyne spp.) and reniform nematodes (Rotylenchulus reniformis). Experiments were conducted at the University of Hawaii at Manoa in Honolulu, Hawaii unless stated otherwise.
Objective 1: Examine if PGS can induce systemic resistance (ISR) of crops against broad spectrum of pests and pathogens by examining expression of ISR genes.
Ho: Drenching of plants with PGS crude extract or amending soil with PGS prior to transplanting will induce ISR to achieve soil-borne pathogen suppression.
Kai choi greenhouse trial
One greenhouse pot trial was conducted using field soil collected from Owen Kaneshiro Farm with a history of R. solani and F. oxysporum infestation. ‘Hirayama’ kai choi seedlings prepared in sterile potting media were grown for 2 weeks prior to transplanting into the test soil. Round, 10-cm diameter pots were filled with 300g field soil or sterile potting media. A total of 8 treatments were tested: 1-3) field soil amended with papaya ground seed (PGS) at 0.1, 0.5, 1.0 % (dry weight equivalent of amendment to soil), 4-6) field soil drenched with a papaya seed crude extract (CE) at 0.1, 0.5, 1.0 % concentration, 7) untreated control with water only, and 8) autoclaved field soil as control. For treatments 4-6, PGS was mixed with a 0.1% Tween80 solution at 1:20 rate, then stirred constantly for 30-minutes at 60°C. The mixture was then centrifuged to separate the lipid and water layer from the PGS residue, and the liquid was diluted in order for crude extract to be added at a rate of 50 ml per pot. Each treatment had 4 replications. Plant tissue from kai choi was collected using a 0.5-cm cork borer, sampling from the last fully matured leaves at 0, 2, 4, 6 and 8 days after soil treatment. CE drenching was repeated once per week over a 6-week growing period to equal the treatment final concentration. Disease incidence of Rhizoctonia rot/Fusarium wilt and biomass of kai choi was recorded at the end of the experiment. Root colonization by R. solani and Fusarium spp. was tested by plating three root pieces per root system on Rhizoctonia-selective KH media and Fusarium-selective Komada media and incubated at room temperature (Ko and Hora, 1971; Komada, 1976). The plated roots were monitored for suspected R. solani and F. oxysporum colonies and the percent of roots colonized were recorded.
---------------
Year 2
Rhizoctonia solani qPCR development
Pure culture of R. solani mycelium was extracted for DNA using a modified CTAB method with ceramic beads and a bead homogenizer, centrifuged, pelletized, and DNA was extracted from soil using the E.Z.N.A. Soil DNA kit from Omega Bio-Tek (Norcross, GA, U.S.A.).
Primer/probe design
Primers RsAG2-1F (5’-ATCTCTTGGCTCTCGCATCG-3’) and RsAG2-1R (5’-AACAGGCATGCTCCAAGGAA-3’), and the flurogenic probe used for Rhizoctonia solani targeted the internal transcribed spacer 1-2 (ITS 1-2) region (DeShields, 2018). Probe RsAG2-1Pro was designed to be labeled with flurophore reporter (FAM) and Black Hole Quencher-1 (BHQ) as 5’ reporter and 3’ quencher respectively (5’-[FAM]ATCTTTGAACGCACCTTGCG[BHQ]-3’).
qPCR reaction protocol
qPCR reactions were completed using the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA) following the program protocol: (1) denaturation at 95°C for 15 seconds, (2) primer annealing at 60°C for 30 seconds, and (3) repeat reaction cycle 39 times for a total of 40 cycles. Each reaction tube contained 10 µL of SsoAdvanced Universal Probes Supermix (Bio-Rad), 0.6 µL of 10 mM forward primer, 0.6 µL of 10mM reverse primer, 0.3 µL of 10 mM fluorescent probe, 6.5 µL of sterile water, and 2 µL of template DNA, for a total reaction volume of 20 µL.
Fusarium commune qPCR development
Low detection of R. solani from the field soil using the above qPCR method led us to suspect that R. solani might not be the main causing agent of kai choi wilt. However, the Komada media detected different color of Fusarium colonies from this soil. Suspected Fusarium spp. isolated from diseased kai choi roots were sent for Sanger sequencing to the University of Hawai`i Advanced Studies in Genomics, Proteomics and Bioinformatics (ASGPB) facility, where sequencing was performed using an Applied Biosystems 3730XL DNA Analyzer (Thermo Fischer Scientific, Waltham, MA, U.S.A.). Pure DNA of these isolates were prepared. PCR amplification was performed using previously designed primers, FusariumEF1 (5’-ATGGGTAAGGARGACAAGAC-3’) and FusariumEF2 (5’- GGARGTACCAGTSATCATG ), for the Fusarium translation elongation factor 1a (TEF-1a) region (O’Donnell et al., 2010).
Reactions were run using the following protocol: (1) denaturation at 98°C for 10 seconds, (2) primer annealing at 57°C for 15 seconds, (3) extension at 72°C for 1 minute, and (3) repeat reaction cycle 34 times for a total of 35 cycles. Each reaction tube contained 10 uL of 5X Phusion High-Fidelity buffer (New England Biolabs, Ipswich, MA, U.S.A.), 2uL of 10 mM forward primer, 2 uL of 10mM reverse primer, 1 uL of 10 mM dNTPs, 0.5 uL of Phusion High-Fidelity DNA polymerase (New England Biolabs), 33.5 uL of sterile ddH2O, and 1 uL of template DNA, for a total reaction volume of 50 uL.
PCR product cleanup was performed using the ExoSAP-IT PCR Product Cleanup reagent from Thermo Fischer Scientific. Reaction tubes contained 5 uL PCR product and 2 uL ExoSAP-IT reagent for 7 uL total volume. Tubes were incubated at 37°C for 15 minutes, followed by 80°C for 15 minutes.
SYBR Green qPCR Primer design: Primers used were previously designed and validated for use in fluorescent dye-based qPCR, targeting the mitochondrial small subunit (mtSSU) rDNA region: FO1 (5’- GCCATAGGTCAGATAACCAGTT-3’) and FO2 (5’- TCACTACTGGTGTCAGAAACGG-3’) (Zhu et al., 2016).
qPCR reaction protocol
qPCR reactions were completed using the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Reactions were run with the following steps: (1) denaturation at 95°C for 15 seconds, (2) primer annealing at 58°C for 20 seconds, and (3) extension at 72°C for 20s, then 77°C for 5s, and (4) repeating the reaction cycle 39 times for a total of 40 cycles. Melting curve data was obtained after reaction runs by cooling to 65°C for 5s and heating to 95°C at 0.5°C increments, with collection of fluorescent data. Each reaction tube contained 12.5 µL of SsoAdvanced Universal SYBR Green Supermix (Bio-Rad), 3.25 µL of 0.1 mM forward primer, 3.25 µL of 0.1 mM reverse primer and 1 µL of template DNA, for a total reaction volume of 20 µL.
Objective 2: Evaluate the efficacy of using PGS crude extract on kai choi and zucchini seedlings through soil drenching.
Ho: Drenching of plants with PGS crude extract (CE) can achieve soil-borne pathogen suppression equivalent to amending soil with PGS.
Kai choi field trial
In Summer of 2023, a field trial was conducted in a Fusarium- and Rhizoctonia-infested commercial farm (Owen Kaneshiro Farm, LLC). Twenty-four, 6.5x1.5 ft2 plots were arranged in a randomized complete block design and established according to farmer’s practice. Six soil treatments with 4 replications each were included: 1-2) amendment with PGS at 0.5 and 1.0% (dry weight equivalent of amendment to soil), 3) split amendment application of PGS at 1.0% (½ amount applied pre-plant and ½ amount applied at halfway point in cropping cycle), 4-5) drenching with papaya seed CE at 0.5 and 1.0 % concentration, and 6) an untreated, bare ground control. For treatments 4-5, CE was prepared same as the greenhouse trial.
‘Hirayama’ kai choi was first seeded in potting media in 128-cell seedling trays for 1 week prior to transplanting. Treatments 1-2, the first half of treatment 3, and the first portions of 4-5 were applied 48 hours prior to transplanting seedlings. Post-transplanting, the remaining PGS CE treatments were evenly divided and drenched weekly (to be the equivalent of the final PGS amendment rate used) using a standard watering can, with the final drench being applied one week prior to harvest. Incidence of Rhizoctonia rot/Fusarium wilt was recorded after 2 weeks and at termination. Soil samples were collected for downstream analysis 1 week after plot treatments and at termination.
Data analysis
Data collected from all field and greenhouse trials was subjected to analysis of variance using PROC GLM in SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Wherever necessary, data was normalized using appropriate transformation. Means were separated using Waller-Duncan k-ratio (k = 100) t-test.
References cited:
Komada, H. (1976). A new selective medium for isolating Fusarium from natural soil. Proceedings of the American Phytopathological Society 3:221.
Ko, W.-H. and Hora, F. K. (1971). A Selective Medium for the Quantitative Determination of Rhizoctonia solani in Soil. Phytopathology, 61(6), 707. https://doi.org/10.1094/Phyto-61-707
Year 2
Learning from the field trial conducted in the summer of 2023, we repeated two more field trials at Owen Kaneshiro Farm with field sites naturally infested with Fusarium commune. Since the last field trial showed that PGS crude extract (CE) performed better than PGS as a soil biofumigant, field trials conducted in the Fall and Winter of 2023 tested the best PGS CE treatment and compared to a sorghum cover crop (which produce hydrogen cyanide as another natural fumigant). We were unable to test PGS CE against Vapam because the farmer had some challenges in applying Vapam this year.

Three treatments tested were 1) drenching with papaya seed CE at 0.5% concentration twice (2-week intervals), 2) ‘NX-D-61’ energy sorghum grown for 2 months prior to soil incorporation, and 3) an untreated, bare ground control. The experiment was arranged in randomize complete block design with 4 replications. ‘Hirayama’ kai choi seedlings were transplanted. Soon after transplanting, PGS CE was drenched on the seedlings using a watering can (pictured above). PGS CE was reapplied again 2 weeks after transplanting. Incidence of Fusarium wilt and disease index in a scale of 0-4 was recorded at 2 and 4 weeks after transplanting. Soil samples were collected for downstream analysis 1 week after plot treatments and at termination.
Final year
In vitro bioassays comparing PGS CE to BITC
Toxicities of PGS CE and BITC to vermiform stages of R. similis were tested in 50-mL beakers in the laboratory. Pure, synthetic BITC from Sigma-Aldrich (Merck KGAaA, Darmstadt, Germany) was dissolved in dimethylsulfoxide (DMSO) with 15 mL sterile water to prepare 0.5 mM or 0.1 mM BITC. Papaya ground seed crude extract 2% stock solution was prepared, and diluted with sterile water to 1.0% or 0.5% in 15-mL final volume. AzaGuard® (a.i. 3% azadirachtin, BioSafe Systems, East Hartford, Connecticut) at 2 µl/ml was included as a reference control (labeled as Neem). Sterile water was included as the untreated control. Each treatment was replicated in 4 beakers, with 100 vermiform stages of R. similis added per beaker. Thus, a total of 24 beakers with 6 treatments (0.5 mM BITC, 0.1 mM BITC, 1% PGS CE, 0.5% PGS CE, 0.2% AzaGuard® and untreated control) were covered with parafilm and incubated at room temperature (23°C) for 24 hours in a hood. Proportions of dead (straight and unmoving when poked) versus live nematodes were then counted under a Leica DM1L inverted microscope to determine % mortality. To factor out nematostatic effects, counted samples were then rinsed with water on a 20-um-mesh sieve and transferred into clean beakers, each with no more than 20 mL water. Beakers were parafilmed and incubated again at room temperature (23°C) for 24 hours. At 48 hours, counting was repeated as previously described.
Tomato split-root greenhouse trials
Two greenhouse split-root trials were conducted using artificial inoculation of Meloidogyne incognita on tomato. Six-week-old ‘Orange Pixie’ tomato seedlings were transplanted with roots split between two, 10-cm diameter pots containing field soil collected from the University of Hawaii Poamoho Research Station in Waialua, Hawaii. The collected soil was frozen for one week prior to use in order to kill any natural populations of nematodes present. Two tomato seedlings were transplanted per replicate. Ten days after transplanting, tomato roots were checked to confirm root growth establishment within both pots. Roots established in the right-side pot were then drenched with 100 ml of either 0.5% or 1% concentration PGS crude extract (CE) or water as a negative application (NA) control. Tomato seedlings transplanted into untreated, autoclaved (Auto) field soil were used as an uninoculated positive control. Each treatment was replicated four times.
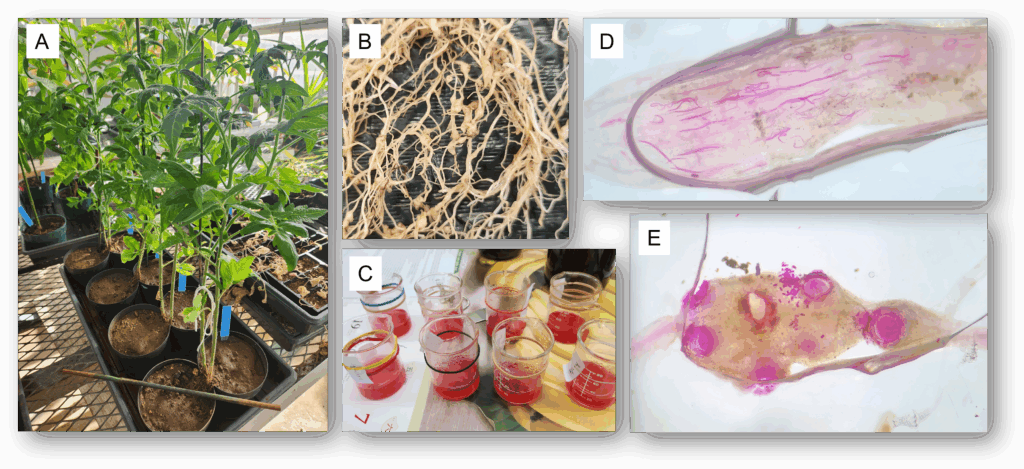
Twenty-four hours after drenching, roots in the left-side pot were inoculated with 200 second-stage M. incognita juveniles. The same drenching treatments were repeated every 2 weeks until experiment termination after 2 months. For downstream use in ISR-associated gene expression assays, root tissue was collected from each replicate at 24 h, 48 h, and 7 days. At termination of the experiment, plant height was measured, and root and shoot biomass was collected and recorded from the inoculated-side pot, and subsamples of 1 g of roots per pot were stained using an acid fuchsin stain method, then counted under a dissecting microscope to determine the number of M. incognita females, juveniles, and egg masses present in roots. Plant disease index ratings were assigned based on a scale of 0 to 4 (Fig. 1).

Tomato field microplot trials
Two microplot field trials were conducted at the University of Hawaii Poamoho Research Station in Waialua, Hawaii using soil collected on site that was naturally infested with a mixed population of Meloidogyne spp. and Rotylenchulus reniformis nematodes. Six-week-old ‘Orange Pixie’ tomato seedlings were transplanted into 38-L fabric pots filled with field soil with one seedling per pot. Each pot was then drenched with either 0.5% or 1% concentration PGS CE or water only as a no application (NA) control. Each treatment was replicated four times, and treatment drenching was repeated every 2 weeks until experiment termination at 3 months.

Soil samples were collected at experiment start, midway point (6 wks), and termination (12 wks). Soil microbial respiration was measured at day 0 and 6 weeks using the Solvita® CO2-burst test (Woods End Laboratories, LLC, Augusta, ME). Nematode populations were extracted from soil samples using elutriation followed by centrifugal sugar flotation. All nematodes were counted to the genus level using an inverted microscope, and data was subjected to nematode community analysis (Ferris et al., 2001). At experiment termination, the number of tomato fruit produced, plant height, and shoot and root biomass was recorded. Overall root-knot rating was assigned based on a scale of 0 to 10 (Bridge and Page, 1980).
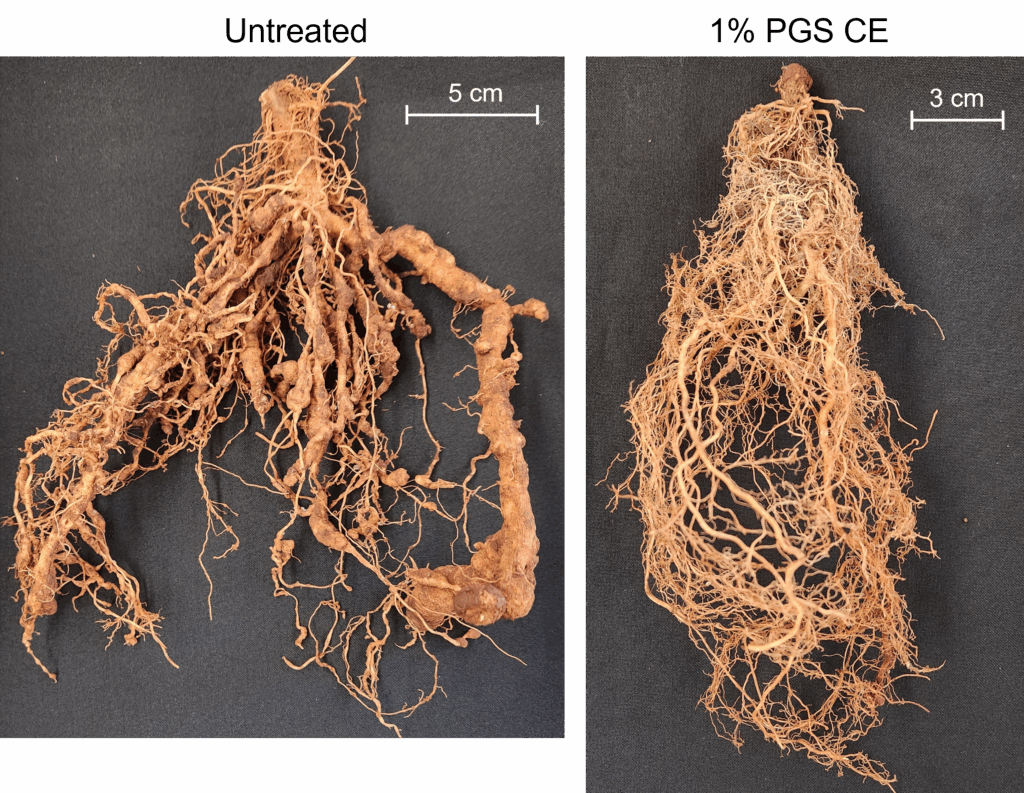
Anthurium shade house pot trials
Two greenhouse pot trials were conducted using artificial inoculation of Radopholus similis on anthurium at the USDA-ARS Daniel K. Inouye U.S. Pacific Basin Agricultural Research Center in Hilo, Hawai‘i. ‘Leilani’ anthurium plants (4 to 5 leaves/plant) were transplanted into clean cinder potting media in 10-cm square pots. A total of 16 pots were inoculated with 500 vermiform stages of R. similis per pot, whereas 4 pots were uninoculated. Five treatments tested were pots drenched with 1) 1.0% PGS CE, 2) 0.5 % PGS CE, 3) commercial neem oil, 0.2% AzaGuard® (a.i. 3% azadirachtin, BioSafe Systems, East Hartford, Connecticut), 4) no application control with water only (NA), and 5) untreated and uninoculated control with water only (UI). The experiment was arranged in randomized complete block design with 4 replications, terminated at 6 months after R. similis inoculation. Each pot was drenched with 71 mL/pot of the designated solution. Drenching was repeated once per month. The experiment was replicated once with the exact experimental design.
At termination of the experiment, the number of flowers and leaves produced, plant height, and root and shoot biomass was recorded for every pot. Overall root lesion rating was recorded based on a scale of 0 to 10, with 0 equal to no root lesions present (0%) and 10 equating to brown, necrotic lesions present on all roots (100%). To determine treatment effects on R. similis, nematodes were extracted from a 25 g subsample of roots and crown of anthurium tissues, and 85 g of potting media per pot over 72 hours using a Baermann funnel method (Walker and Wilson, 1960). Nematodes (R. similis and bacterivorous nematodes naturally found in the cinder media) were then counted under a Leica DM1L inverted microscope.
References cited
Bridge, J., & Page, S. L. J. (1980). Estimation of Root-knot Nematode Infestation Levels on Roots Using a Rating Chart. Tropical Pest Management, 26(3), 296–298. https://doi.org/10.1080/09670878009414416
Ferris, H., Bongers, T., & de Goede, R. G. M. 2001. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Applied Soil Ecology, 18(1), 13–29.
Walker, J. T. and Wilson, J. D. 1960. The separation of nematodes from soil by a modified Baermann funnel technique. Plant Disease Reporter 44:94-97.
Results and Discussion
This project started in fall 2022. During this first reporting period, there were no results produced using green onion in collaboration with Katsu Farm. It was found that the disease issue the farmer faced with green onion was not due to field soil-borne disease, but due to contaminated potting media used for seedlings on raised benches. After switching the potting media brand used, the grower’s disease issue was resolved. However, we were able to collaborate and work with one of our participating farmers, Owen Kaneshiro, to design greenhouse trials using field soil collected in his fields showing high disease pressure. We were also able to work with him to set-up an initial field trial to determine is PGS and/or PGS CE can induce systemic resistance against Fusarium oxysporum and Rhizoctonia solani. For these experiments, kai choi was used as an experimental crop.
Kai choi greenhouse trial
A. Disease Index
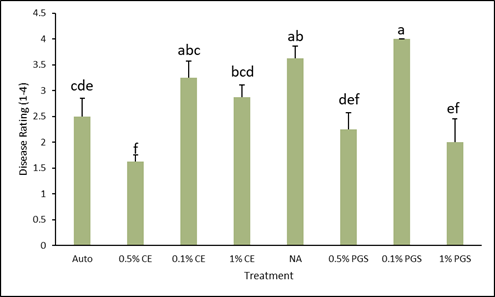
At experiment termination, level of disease for each treatment was rated on a scale of 1 to 4 (Fig.1). As demonstrated by the data in Figure 2, both the 0.1% PGS and NA treatments were comparable and both significantly higher in disease than the autoclaved control. However, the 0.5% CE treatment had the lowest level of disease, followed by the 1% PGS and 0.5% PGS treatments respectively, all of which were significantly lower than the NA control. Overall, PGS treatments performed consistently better, aside from the 0.5% CE treatment. Both 0.1% amendment rates did not reduce disease, indicating the dose is likely too low. Autoclave treatment results showed some disease symptoms, but this is likely due to overall lack of nutrients in the autoclaved soil, which could be remedied by including a standard fertilizer treatment.

B. Biomass & Yield
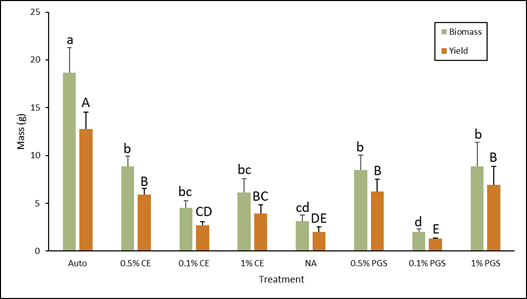
As expected, the autoclaved control treatment had the highest overall biomass and yield (Fig. 3). The 0.5% PGS, 1% PGS, and 0.5% CE treatments were all comparable and lower than the autoclave treatment, but still significantly higher than the NA treatment in both biomass and yield (Fig. 3). This positively reflects and is consistent with the decreased level of disease observed in these treatments (Fig. 2). 0.1% and 1% CE biomass did not differ greatly from NA, but the 1% CE yield was greater than NA yield (Fig.3). While it was not a significant difference, there was a small decrease in disease observed in the 1% CE treated pots compared to the NA treated pots, which can explain these yield results. Again, as seen in Figure 3, the 0.1% PGS treatment performed poorly, showing significantly lower biomass and yield than the NA treatment, which leads to speculating that it may somehow promote disease.
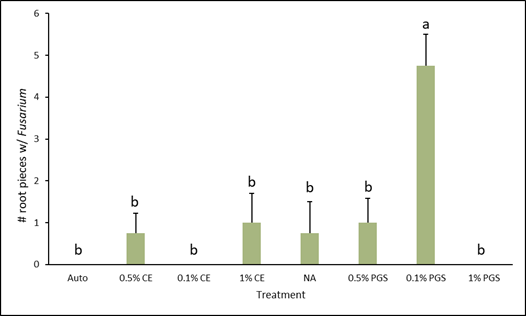
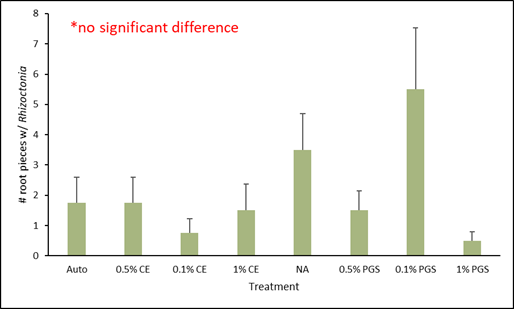
C. Fusarium and Rhizoctonia colonization of kai choi roots
Of the roots plated on the Fusarium- and Rhizoctonia-selective media, only those from the 0.1% PGS treatment were significantly different, with higher colonization in the Fusarium assay (Fig. 4), while all treatments from the Rhizoctonia assay were not significantly different from each other (Fig. 5). However, while not statistically significant, the 1% PGS and 0.1% CE treatments did reduce Fusarium root colonization to zero (Fig. 4). The low presence of Fusarium emergence in the NA treated roots was likely due to roots being overall rotted and reduced.
Kai choi field trial
A. Disease Incidence
A field plot at a commercial kai choi farm in Waianae, HI was treated 48 hours prior to transplanting 2-week old kai choi seedlings, then observed for 4 weeks.
A general observation was made 1 week after transplanting that plants were healthy and growing well (Fig. 6), however at 2 weeks, disease appeared in nearly all plots. As pictured in Figure 7 and through data collected, it was found that the 0.5% PGS and 1% PGS treatments still had significantly less disease present than the untreated, control plots whereas the CE treated plots were still comparable to the untreated plots (Fig. 8). By 4 weeks, nearly all kai choi plants died (Fig. 9). For this reason, numerical data for disease incidence at 4 weeks was not collected, nor was biomass or root colonization data, as there was no plant material to harvest.



Based on data obtained from 2 weeks of growth, it is apparent that the PGS treatments are showing to be effective until a certain growing point. It is speculated that the crops roots are going deeper in the soil than the depth of amendment for PGS, meaning the roots are reaching soil that BITC released from the PGS was unable to reach. Additionally, while the PGS treatments do decrease the level of disease incidence to some degree, there is still over 50% disease in the PGS plots. Higher concentration of PGS may be needed to further decrease this level of disease.
As stated previously, the CE treated plots did not show to be effective, but similar to the greenhouse pot experiment, the treatment was spread out over 4 weeks rather than the full concentration being applied at the start of the experiment. Thus, CE may show different results if the full treatment concentration is added from the start.
Year 2
Kai choi field trials (Fall and Winter of 2023)
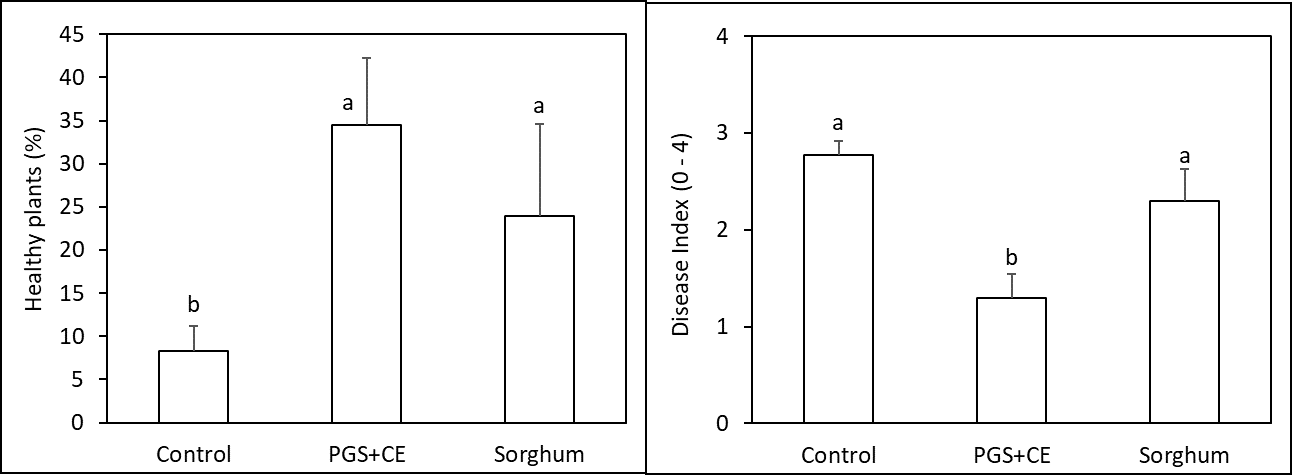
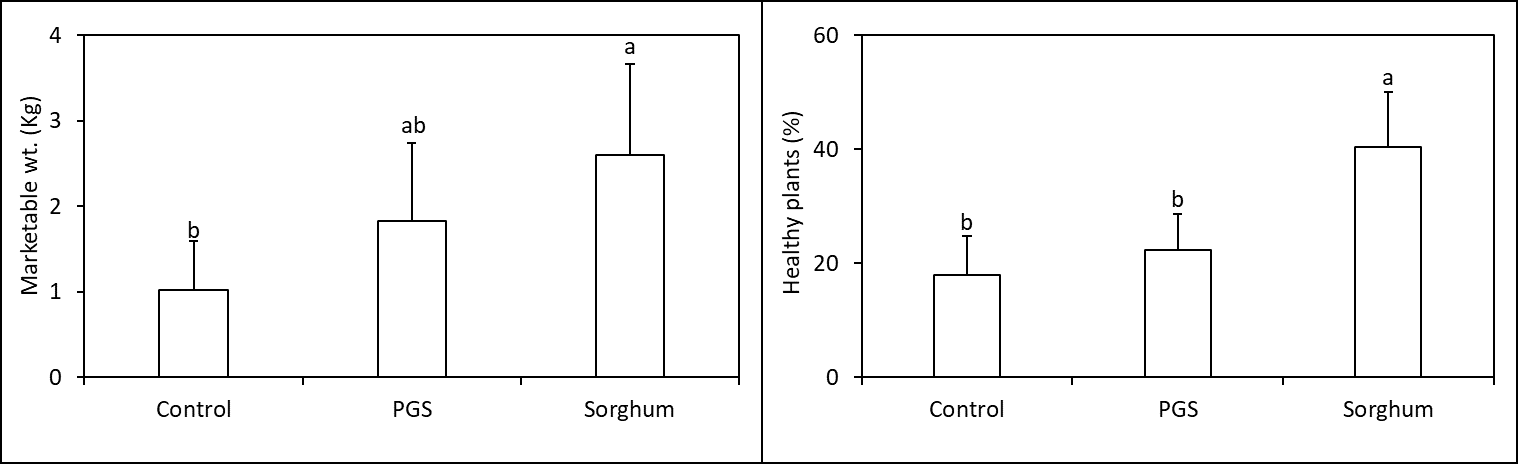
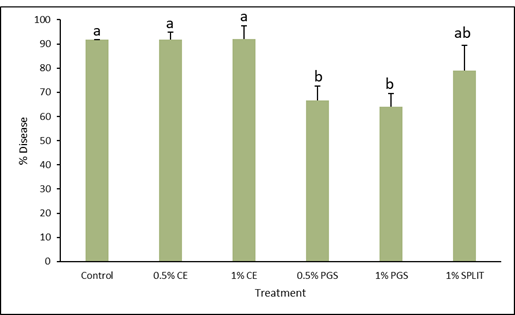
Results from the Fall 2023 Trial were promising, where PGS CE significantly increased number of healthy plants at harvest (P ≤ 0.05) (by approximately 5 times higher than the control) while reducing Fusarium wilt symptom index by 50% compared to the control (P ≤ 0.05, Figure 10). However, results in the Winter 2023 Trial were less promising for PGS CE where it slightly increased kai choi yield but not enough to increase percent of plant surviving Fusarium wilt (P > 0.05, Fig. 11). PGS CE slightly outperformed sorghum cover crop in the Fall Trial but underperformed from sorghum cover crop in the Winter Trial. None-the-less, more improvement is needed for PGS CE method against Fusarium wilt of kai choi as kai choi still suffering < 35% or 18% of survival rates in both trials, which is not economical for the farmers.
As we had hypothesized before, the ability of PGS to induce ISR would rely on soil health conditions. Field sites tested here were known to have rather poor soil health conditions due to the history of Vapam treatment. In the following year, we hope to examine the performance of PGS in a healthier soil environment in order to integrate PGS CE suppression efficacy with its ability to induce ISR.
Rhizoctonia solani qPCR protocol developed
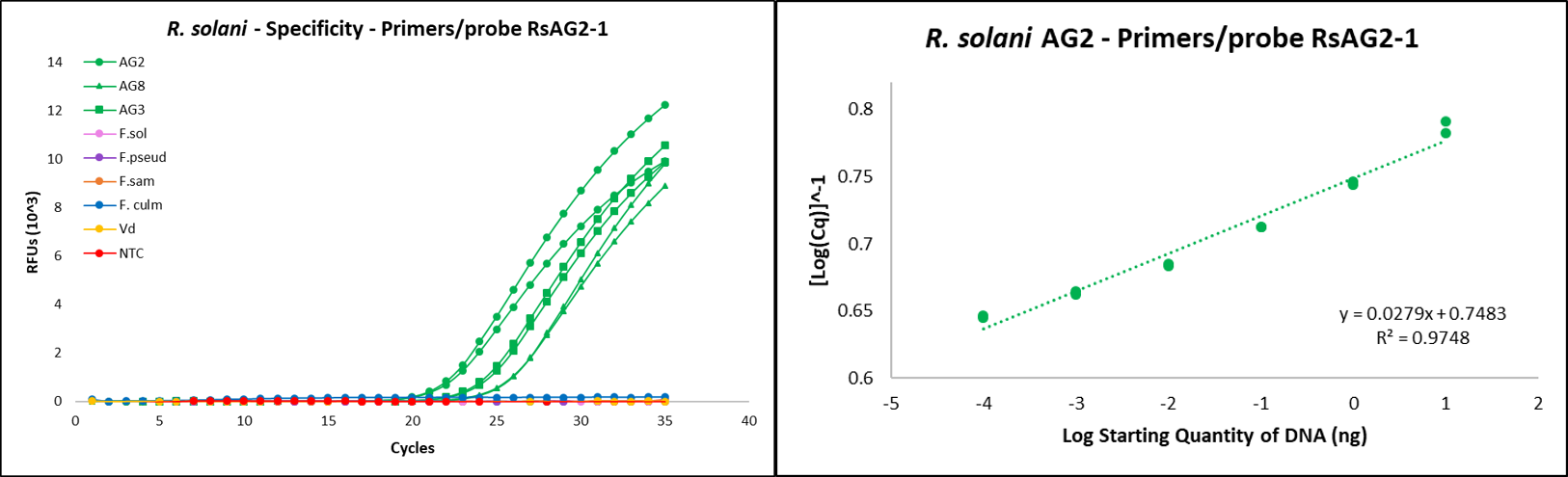
In order to accurately measure levels of R. solani in agricultural fields and soil, and diseased kai choi root tissue suspected to be Rhizoctonia-infested, a real-time quantitative PCR assay (Figure 12) was designed for use as a quantitative research tool.
R. solani qPCR validation
To validate that the primers/probe used for the detection of R. solani would amplify only the targeted pathogen, R. solani pure culture isolates from anastomosis groups (AGs) 2, 3, and 8 were included as positive controls, and non-target pure culture isolates Fusarium solani, F. pseudograminearum, F. sambucinum, F. culmorum, and Verticillium dahliae were included as negative controls. Nuclease-free water was used as a no template control. To assess the overall sensitivity of the primers/probe, a 10-fold serial dilution of R. solani AG-2 was performed.
Amplification curve results demonstrated that the assay targeted and amplified various R. solani AGs while avoiding amplification of non-specific targets (Fig. 12, left). Additionally, the assay showed consistent detection sensitivity down to 0.1 pg of DNA (Fig. 12, right). Both assessments show high sensitivity and specificity of the assay, highlighting its potential as an accurate and sensitive detection tool for R. solani in future experiments.
Fusarium commune sequencing and qPCR

In order to determine the causal pathogen of disease in kai choi, after low levels of R. solani was detected in diseased field soil samples, Fusarium single-spore isolates were obtained from diseased kai choi roots and sequenced. After BLAST analysis, four Fusarium isolates returned with a 100% match for Fusarium commune, a known species involved in the Fusarium wilt and root rot disease complex. Based on the obtained sequence data, it was suspected that F. commune was a causal pathogen for disease in the kai choi plants rather than R. solani (Fig. 13).
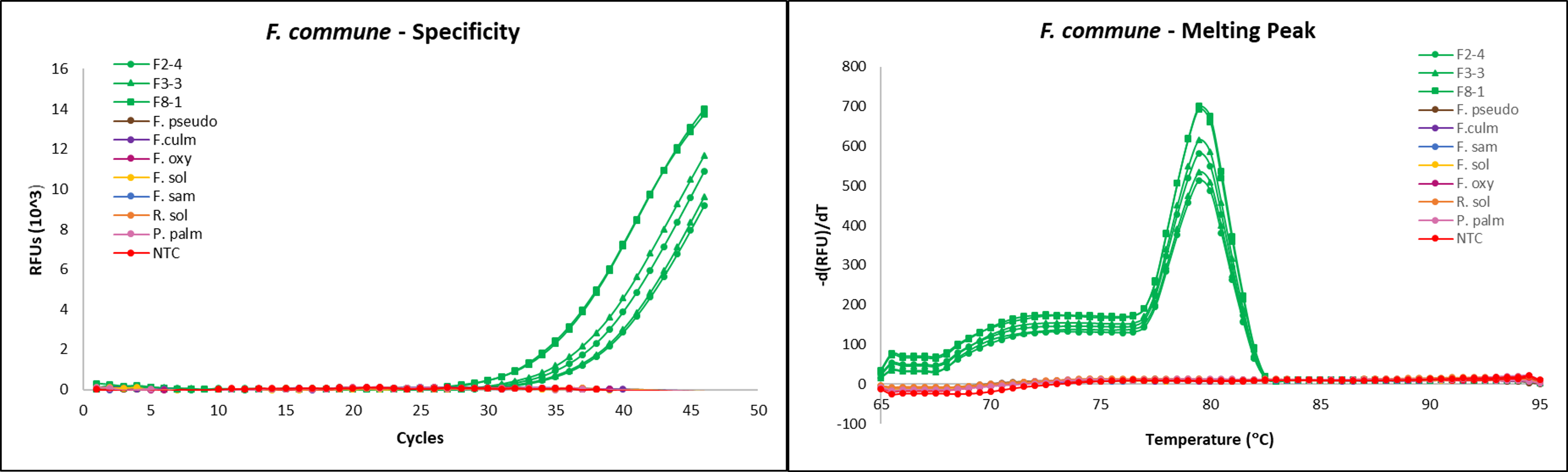
To test for and accurately measure levels of F. commune in agricultural soil and diseased kai choi root tissue alongside R. solani, a preliminary, SYBR green-based RT-qPCR assay was designed for use. To validate that primers were specific to amplifying only F. commune and not other Fusarium species, three sequenced Fusarium isolates with matches for F. commune were included. Non-target, pure culture isolates of Fusarium pseudograminearum, F. culmorum, F. oxysporum f.sp. cubense, F. solani, F. sambucinum, Rhizoctonia solani, and Phytophthora palmivora were included as negative controls, and nuclease-free water was used as a no template control.
We were able to demonstrate that the designed assay could amplify several F. commune isolates, while also being specific enough to avoid amplifying non-target species, including other pathogenic Fusarium (Fig. 14, left). Additionally, melting curves for the amplified targets reached their peaks at the same temperature, further confirming all sequenced F. commune isolates used in this assay are of the same species (Fig. 14, right).
However, initial amplification results showed late amplification of pure F. commune isolates. qPCR assays frequently face low or no amplification signals during reactions due to residual inhibitors present in the DNA template, including phenols, detergents, proteases, or other residuals specific to source material or tissue. Thus, this is one factor that may be contributing to the late amplification of the F. commune template used.
Final year
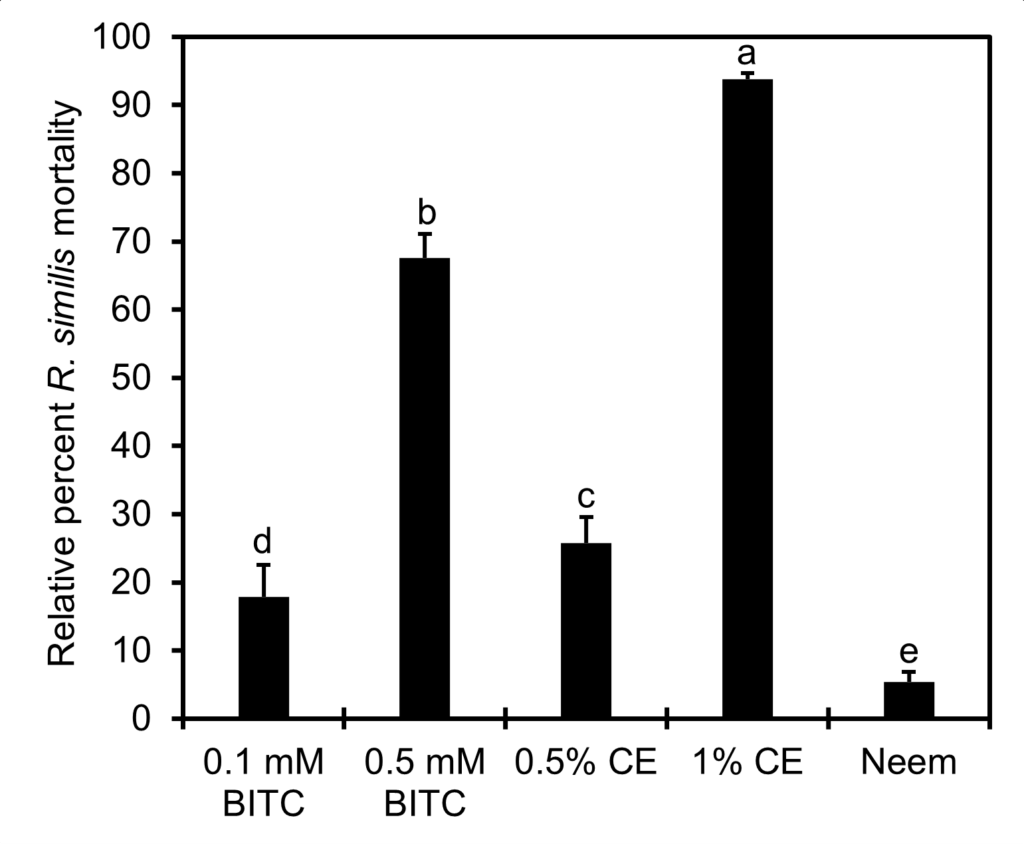
In vitro bioassays comparing PGS CE to BITC
No significant interaction between reading time (mortality at 24 hours versus 48 hours) and treatment was detected. In fact, no reduction of mortality was observed at 48-hour readings (after nematode rinsing) compared to at 24-hour reading (before nematode rinsing) (P > 0.05). Thus, only nematode mortality data from 24-hour incubation were presented (Fig. 15). The Neem reference control performed the poorest in killing R. similis with < 5% mortality at 24 hours after incubation. PGS CE at 1% outperformed both BITC concentrations tested (P ≤ 0.05), achieving close to 95% mortality within 24 hours of incubation, with no sign of recovery after rinsing in clear water and incubating in water for another 24 hours. However, mortality of R. similis in 0.5% PGS CE were only around 25%, though better than that in 0.1 mM BITC (P ≤ 0.05). Nonetheless, this study confirmed that R. similis was sensitive to BITC fumigation achieving ~70% mortality at 0.5 mM concentration.
Anthurium greenhouse trials
Effects of PGS CE on anthurium plant growth: At 6 months after initiation of the pot experiment, 0.5% PGS CE increased the number of flowers produced and plant height numerically (P > 0.05) compared to no application (NA) control (Fig. 16A, B), whereas both 0.5% and 1% PGS CE increased (P ≤ 0.05) number of leaves per pot compared to the no application control (Fig. 16C).
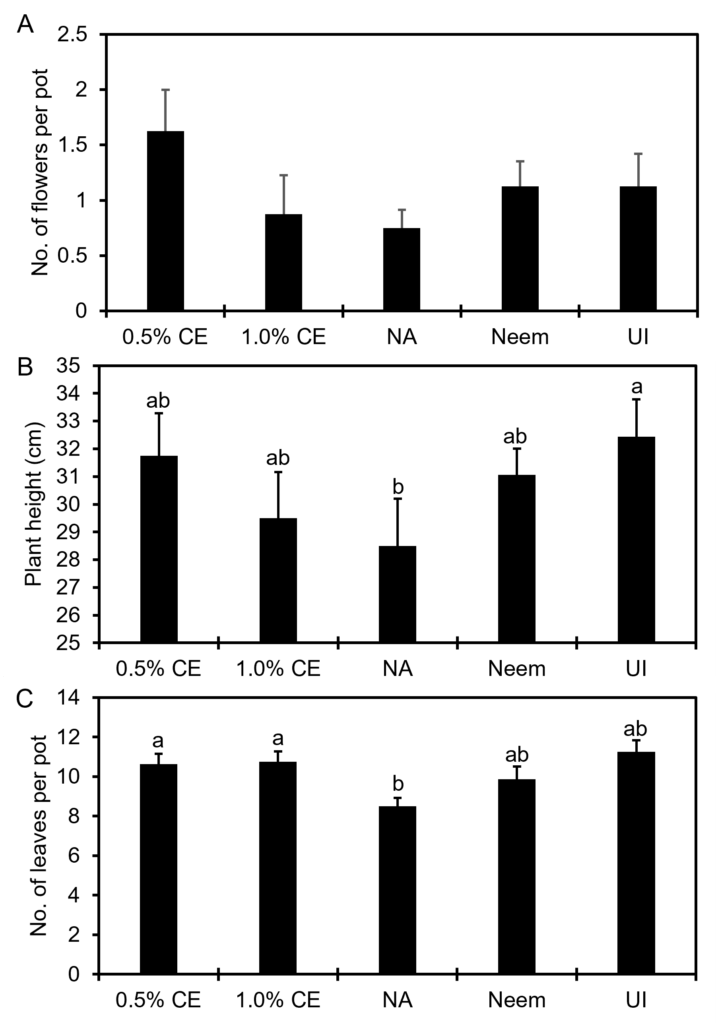
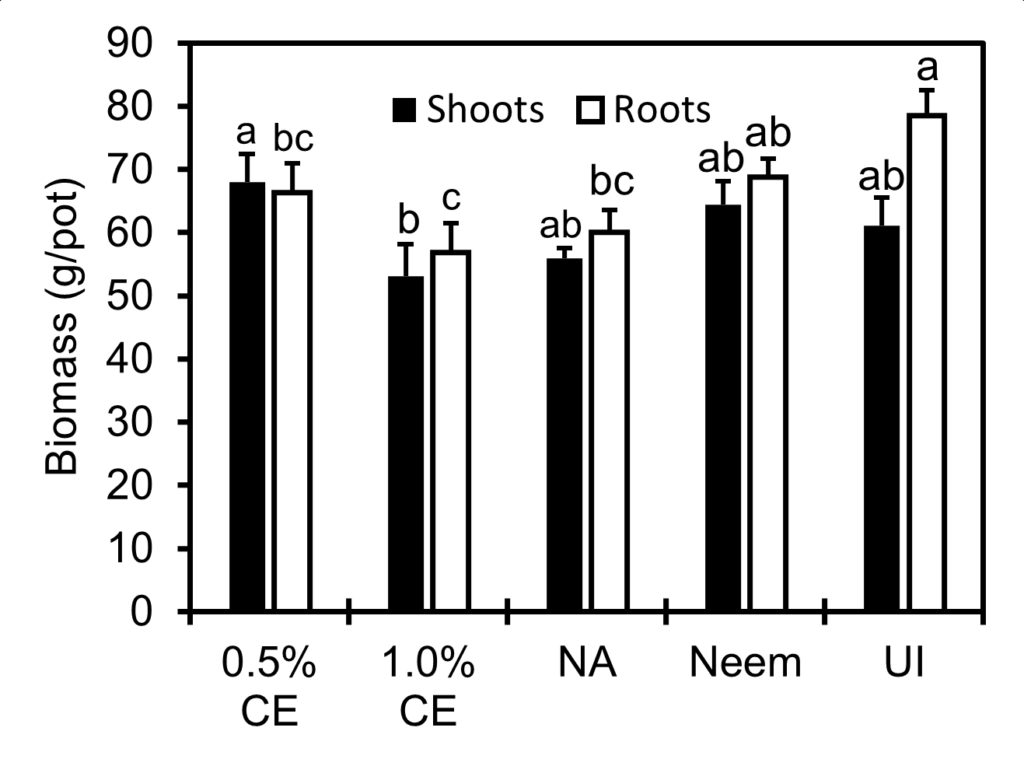
Shoot and root biomass in 1% PGS CE-treated pots was lower than that in the 0.5% PGS CE treatment, and numerically lower than NA (P > 0.05, Fig. 17). While shoot biomass in all treatments was not different from the uninoculated (UI) control, NA, 0.5% and 1.0% CE resulted in lower root biomass than UI (P ≤ 0.05). While no significant difference in shoot and root biomass was observed between Neem and NA, Neem treatment maintained a similar level of shoot and root biomass as well as numbers of flowers, leaves and plant height as the UI (Fig.16 and 17).
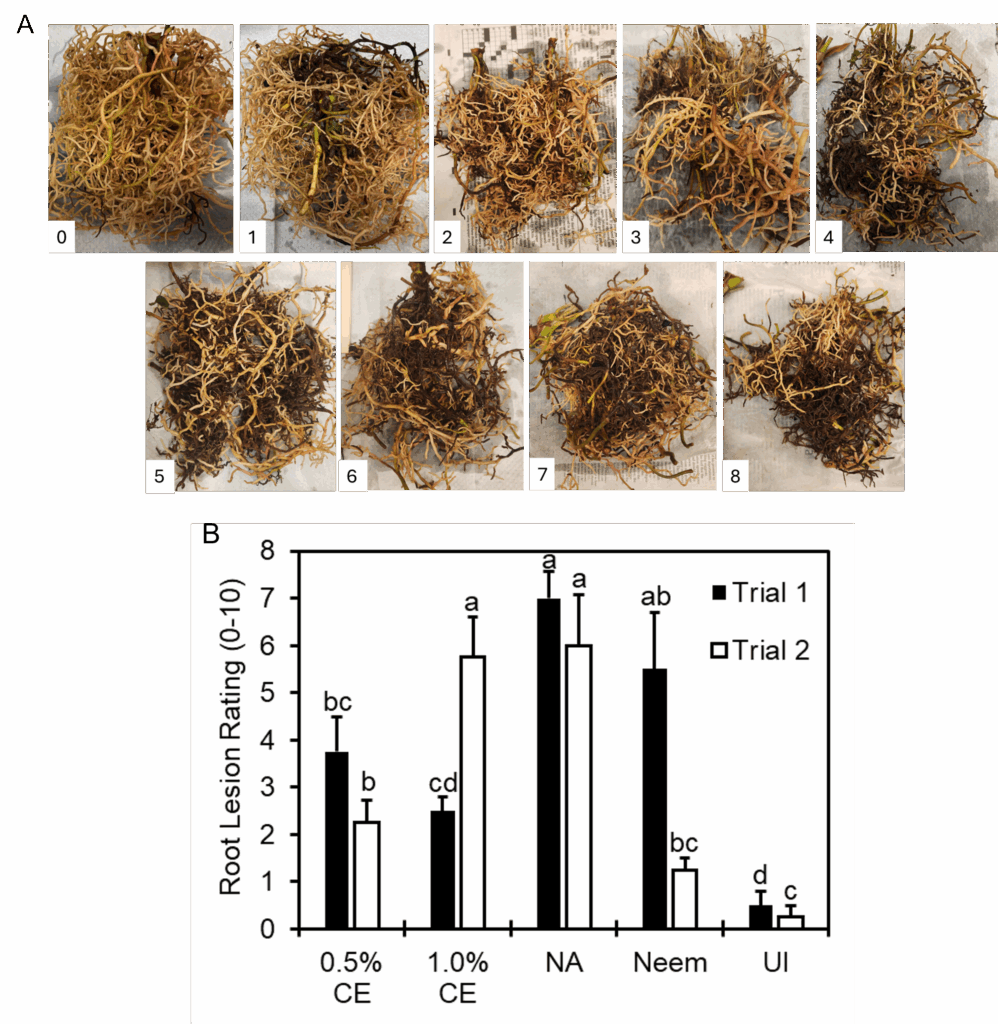
Effects of PGS CE biofumigation on root lesion caused by R. similis: Image of root lesion rating caused by R. similis from 0-8 were shown in Fig 18A. No rating 9-10 were observed in this experiment. Since significant interaction between trial and treatment effects occurred, data were presented by trial for this parameter. In both trials, 0.5% PGS CE reduced root lesion damage compared to NA (no application) consistently, whereas performance of 1% PGS CE on root lesion was inconsistent, where it had significantly less root lesion damage than NA in Trial 1 but not in Trial 2 (Fig. 18). Inversely, Neem-treated roots had root lesion damage comparable to NA in Trial 1, but significantly less root damage than NA in Trial 2 (Fig. 18).
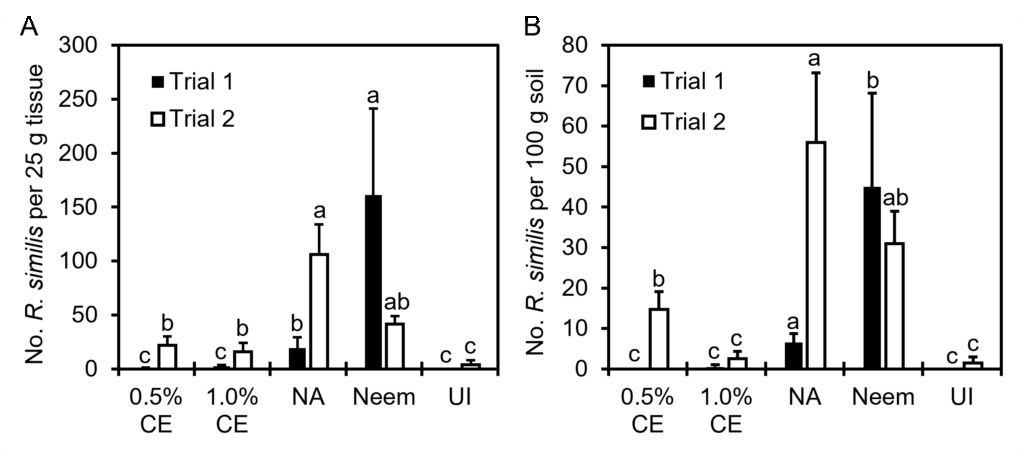
Effects of PGS CE biofumigation on R. similis population densities: Since significant interaction between trial and treatment occurred, data were presented by trial for this parameter. Both 0.5% and 1% PGS CE significantly reduced the number of R. similis from anthurium roots/basal stem tissue (Fig. 19A) and potting media (Fig. 19B) compared to NA consistently in both trials. In contrast, Neem failed to suppress R. similis in both trials, with significantly more nematodes present in both the plant tissue and soil than NA in Trial 1 (Fig. 19).
Effects of PGS CE biofumigation on non-target bacterivorous nematodes: PGS CE maintained comparable number of free-living bacteriovorous nematodes to the uninoculated control in root and basal stem tissue, whereas Neem-treated and no application control (NA) revealed a significant increase in bacteriovores compared to the uninoculated (Fig. 20A). In terms of the number of free-living bacteriovorous nematodes recovered from cinder soil media, though they were not significantly different from the UI control, both PGS CE and the NA control had more bacteriovorous nematodes compared to UI (Fig. 20B).

Tomato split-pot greenhouse trials
Disease index rating across treatments was comparable. Application of PGS CE appears to have no significant effect on the level of disease observed in tomato plants, however overall disease ratings were low (Fig. 21). Considering the split-root experiments were run for 2 months, a longer trial period may be needed in order to see more nematode disease symptoms.
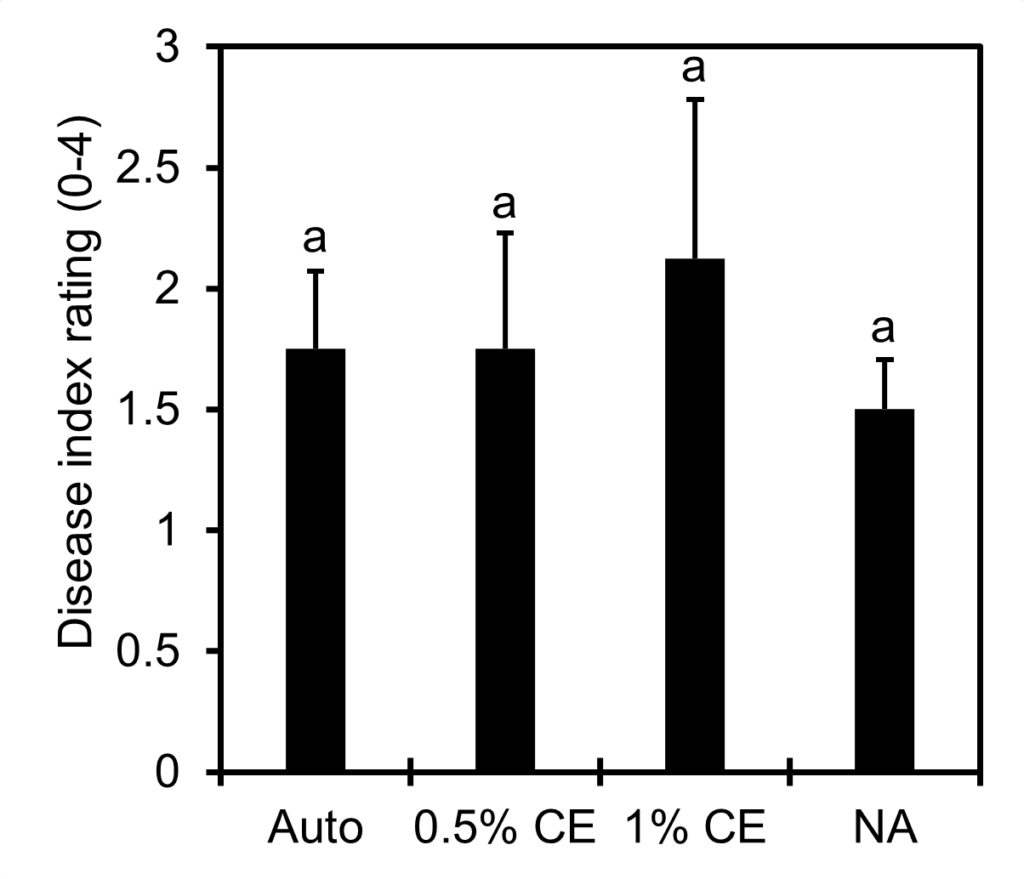
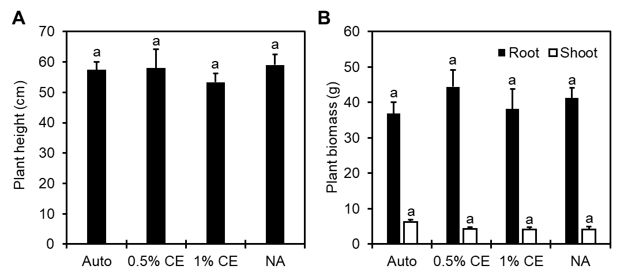
Final plant height as well as the root and shoot biomass recorded demonstrated similar results across treatment groups, with no significant changes observed (Fig.22). However, there was a small numerical increase (p>0.05) in the average root mass in NA and 0.5% CE treated pots compared to Auto and 1% CE treated pots in Trial 1 (Fig. 22B).
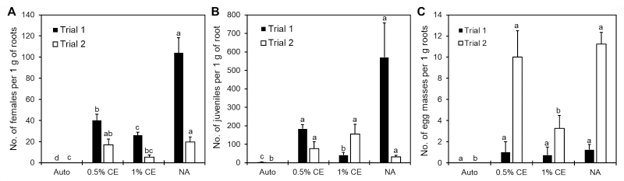
Notably, in Trial 1, NA and 0.5% CE treated pots also had significantly higher numbers of M. incognita adult females within the roots, with NA having the highest number out of all treatments (Fig. 23A). With a higher population of female root-knot nematodes (RKN) present, higher gall formation was likely starting to occur, which can cause an increase in root weight as well. While the differences in number of females was not significant (p>0.05) in Trial 2, data collected still followed the same numerical trend observed in Trial 1, with NA and 0.5% CE treated plants having higher female RKN counts (Fig. 23A).
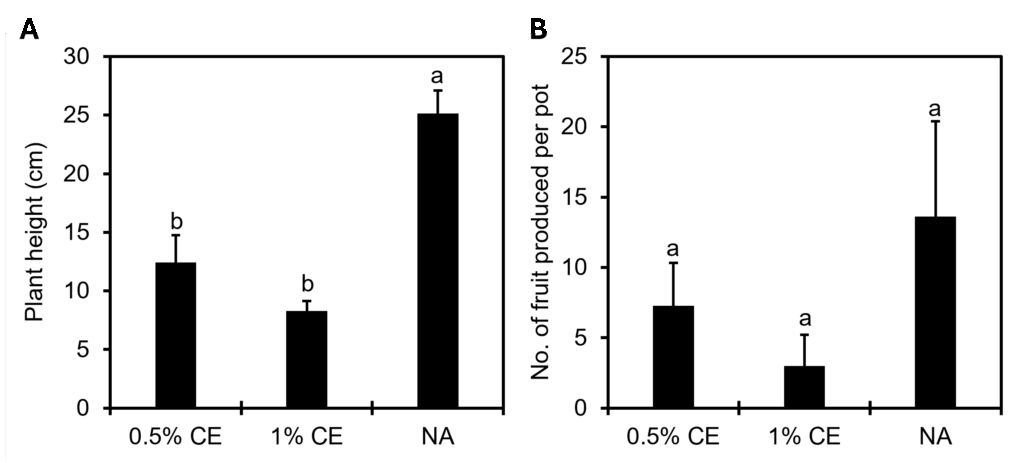
Tomato field pot trials
For overall plant growth, there was a significant (p<0.05) negative difference measure in plant height in field pots treated with PGS CE versus NA treated pots (Fig. 24A), and while not significant (p≥0.05), there was a numerical decrease in the number of fruits produced when using PGS CE versus without (Fig. 24B).
At the start of the experiment, due to drenching with PGS CE right after transplanting, some PGS CE treated seedlings died in the first week likely due to a combination of transplant shock and phytotoxic stress from the BITC within PGS CE, so height and yield may be lower due to delayed growth from replacing dead seedlings one week later. Drenching PGS CE prior to transplanting seedlings or allowing seedlings to first establish roots after transplanting prior to drenching (as was done in the split-root greenhouse trials) could reduce this. However, a decrease in plant growth can also be an indicator that ISR is being activated, as the plant reallocates resources to increase defenses, which takes away from resources that would otherwise be put into growth.
A similar growth pattern was observed in a small decrease in shoot biomass in PGS CE treated replicates versus NA (Fig. 25A), whereas root biomass was more variable. In Trial 2, root biomass was recorded to be numerically greater (p≥0.05) in NA treated pots compared to PGS CE treatment groups (Fig. 25B).
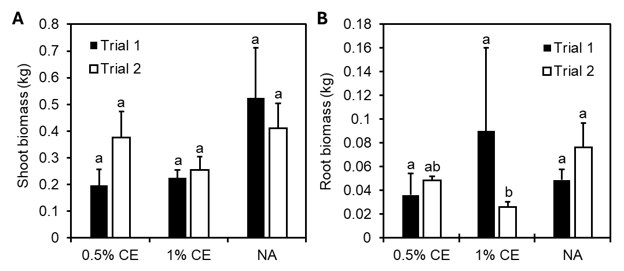
The greater root mass observed in Trial 2 NA treatments appeared to correspond with an increase in root-knot galls found on NA-treated plant roots in the Trial 2 as well (Fig. 26A). Additionally, when looking at the number of root-knot nematodes (RKN) found in the soil, NA treatment also led to significantly greater RKN population numbers compared to both PGS CE concentrations (Fig. 26B). While population differences were not significant in Trial 1 due to a much lower starting population of RKN present in the soil in Trial 1 (Pi=37) compared to Trial 2 (Pi=402), the same population trend was still observed (Fig. 26B).
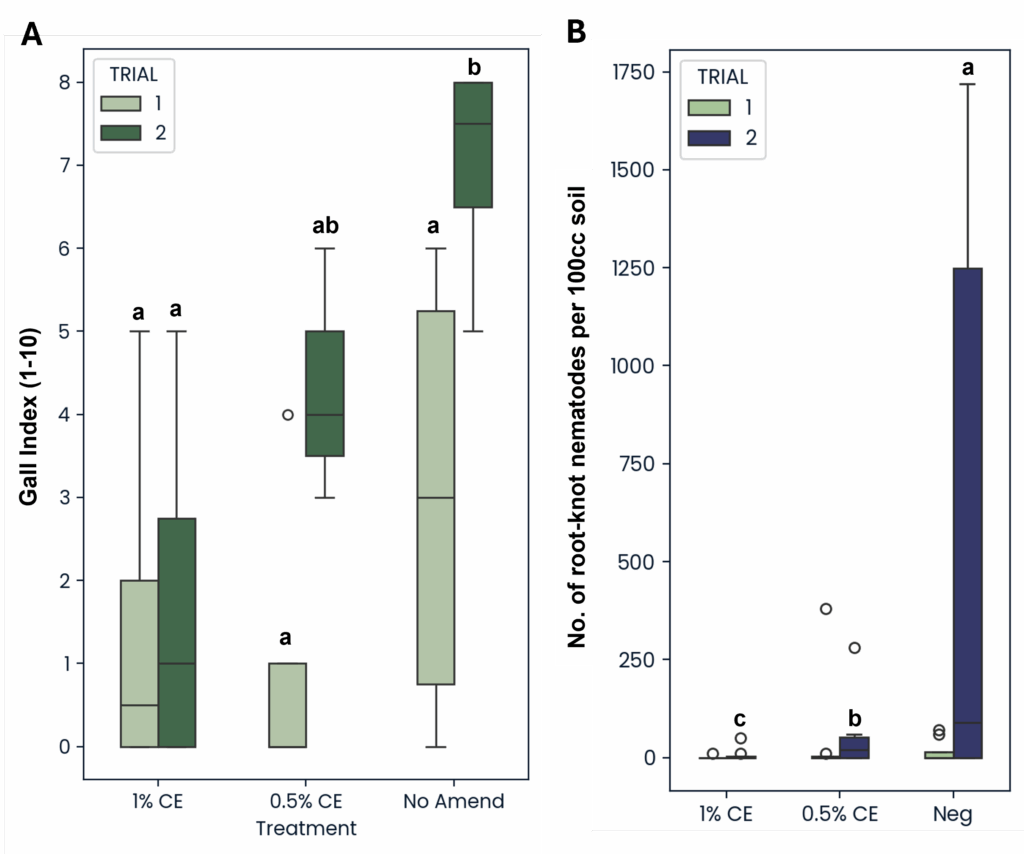
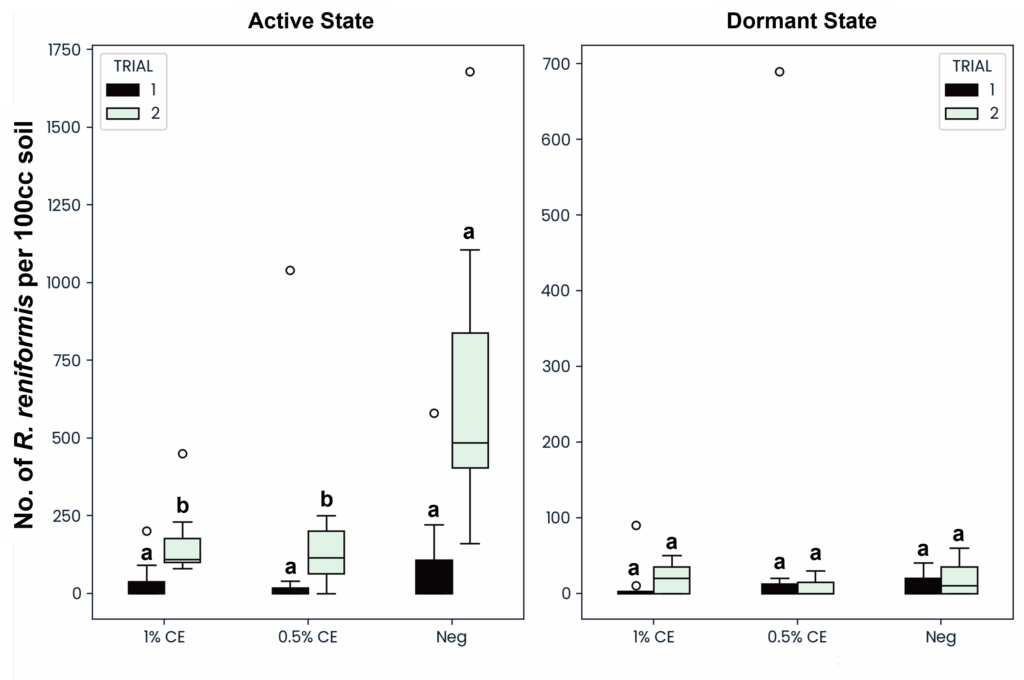
Similarly, the total populations of reniform nematodes (Rotylenchulus reniformis) as well as the total number of all plant-parasite nematodes (Meloidogyne, R. reniformis, Tylenchus, Paratylenchus, Helicotylenchus, Pratylenchus, Paratrichodorus, and Xiphinema) from soil were decreased in field pots treated with PGS CE versus without (Fig. 27). This suggests that PGS CE is not only effective at managing RKN, but that PGS CE applications are also broad spectrum when managing plant-parasitic nematodes.
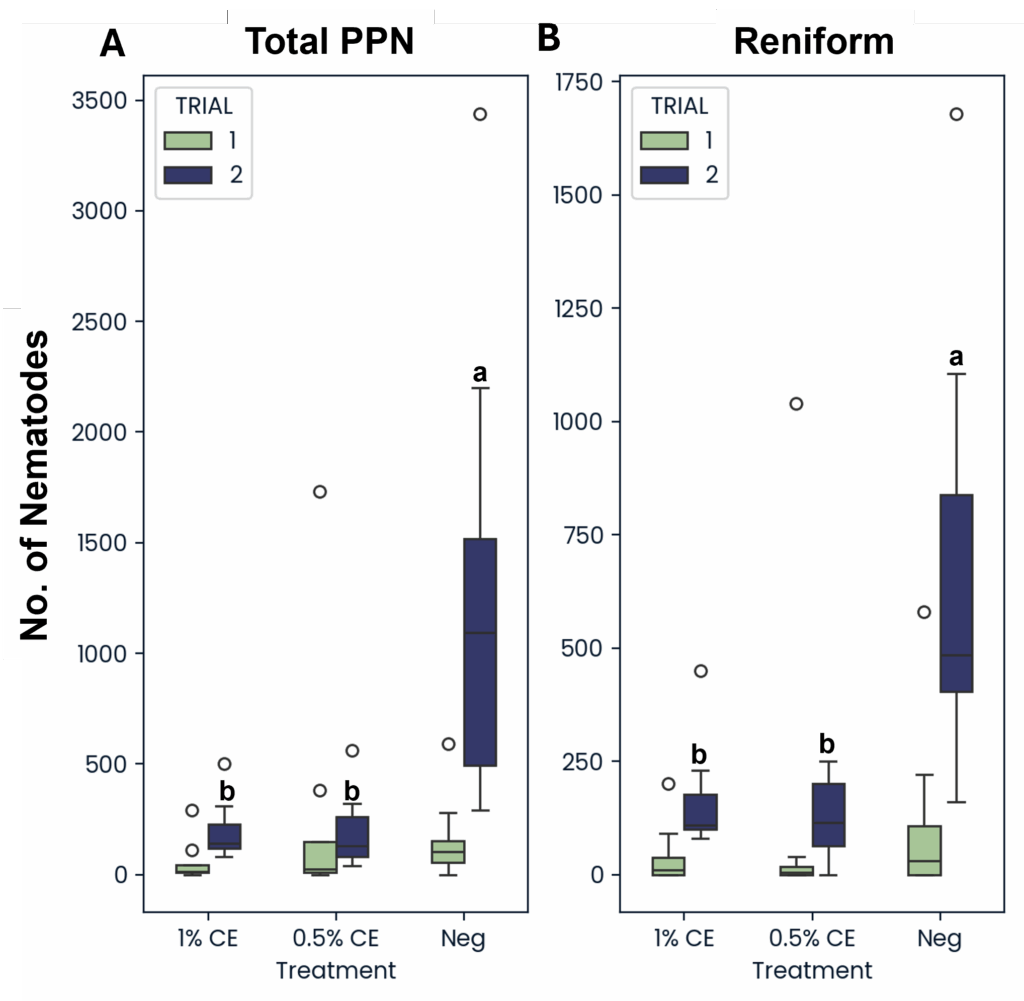
To determine if PGS CE drenching would be effective against both the active and dormant (anhydrobiotic) states of reniform nematodes, both states were counted and recorded separately. When treated with PGS CE at either 0.5% or 1% PGS CE, the active state of reniform was significantly reduced compared to NA treated pots, however no difference or decrease was seen in the number of dormant anhydrobiotic reniform (Fig. 28). This suggests that the BITC from PGS CE is unable to penetrate and kill reniform nematodes in their survival state.
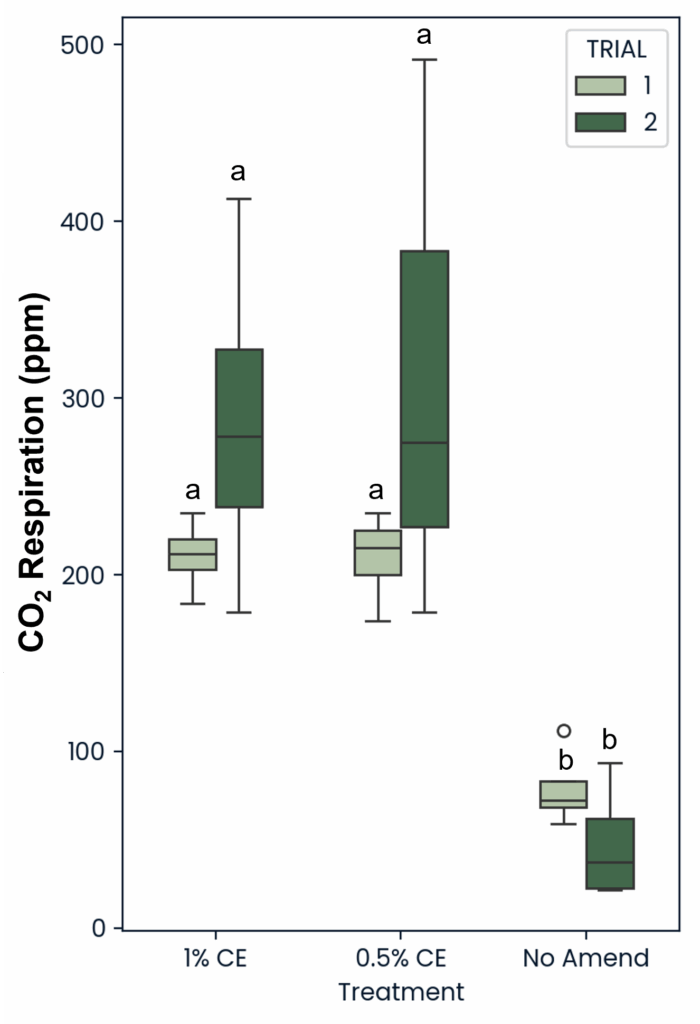
Across trials, both 0.5% and 1% CE saw a significant (p<0.05) increase in CO2 ppm present in the soil compared to the NA treated replicates (Fig. 29). This suggests that the use of PGS CE also increased soil microbial respiration.
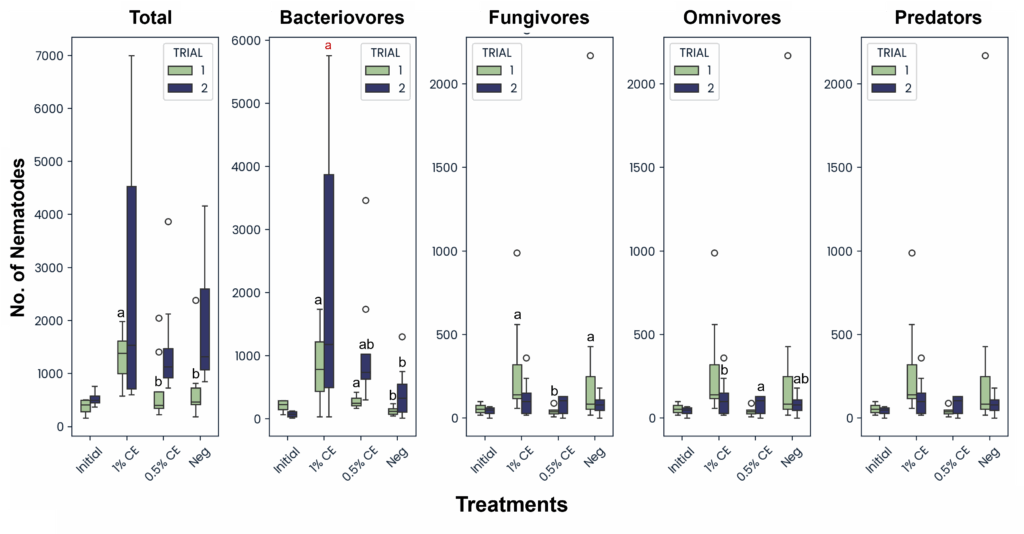
Across treatments and trials, the total abundance of free-living fungivore, omnivore, and predator nematodes were mostly comparable and unchanging, except for Trial 1 fungivores, in which significantly higher fungivores were present in the 1% CE and NA treatments versus 0.5% CE treated pots (Fig. 30). Trial 1 data also showed a significant increase in bacteriovore nematode populations as with pots treated with CE, with 1% CE having the highest difference. This was likely due to the PGS debris being added to the pots post-drenching, which provided an organic material source for bacteria to colonize during the decomposition process, leading to a larger available food source for bacterial-feeding nematodes. While not statistically significant (p≥0.05), bacterivore populations in Trial 2 also followed the same trend (Fig. 30).
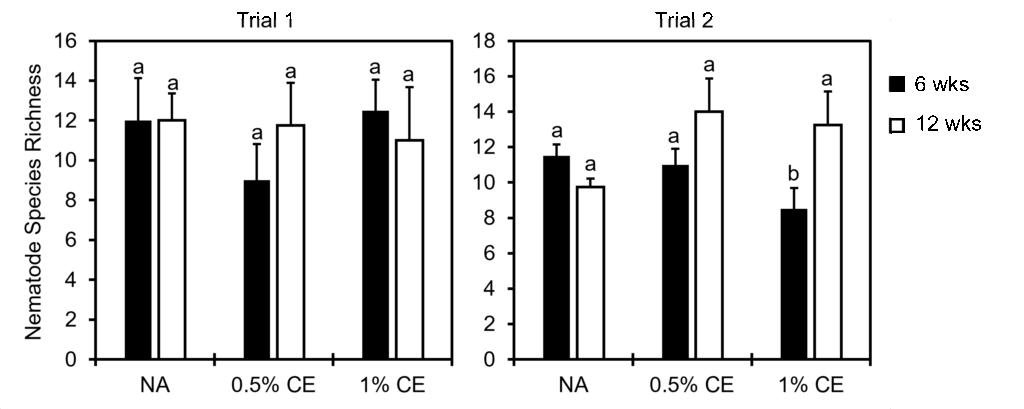
When looking at nematode community indices, there was a significant decrease in the nematode richness index observed in 1% CE treated pots at 6 weeks compared to NA and 0.5% CE treated pots, however at 12 weeks nematode richness recovered (Fig. 31). This suggests that the higher concentration of CE used may have been nematidical to other free-living nematodes present at first, but that due to added organic matter (PGS debris), the nematode community richness level was able to recover quickly compared to NA treatments.
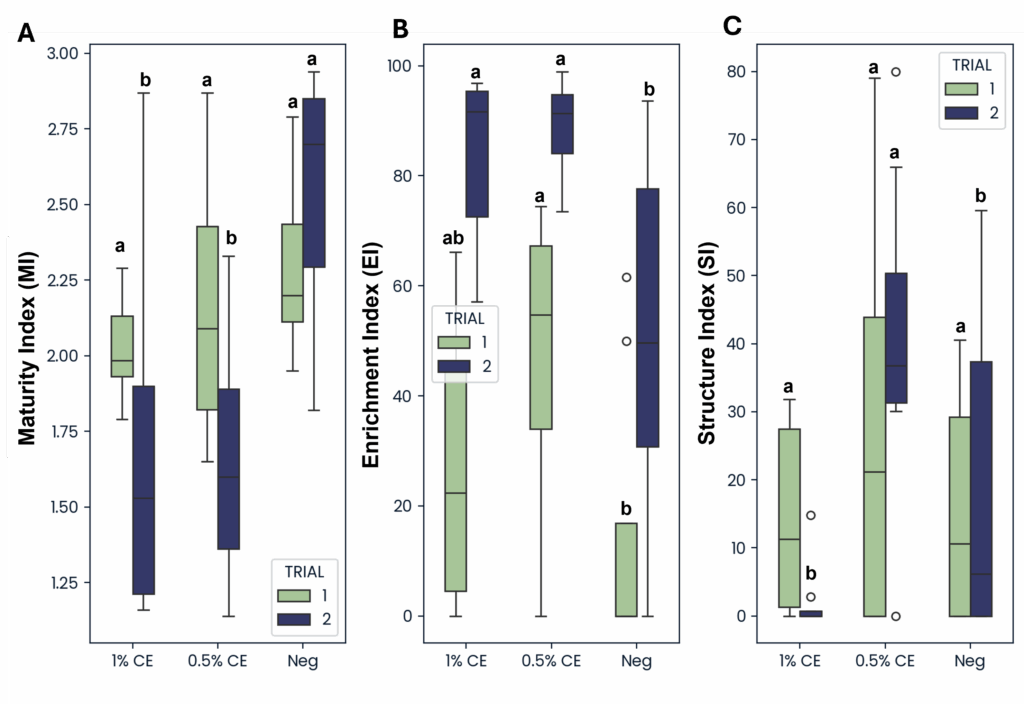
The enrichment index (EI) (characterized based on the presence of opportunistic bacterial and fungal feeding nematodes), was greater in both 0.5% and 1% CE treated groups compared to NA across both trials (Fig. 32), indicating that drenching with PGS CE is boosting microbial activity in the soil.
In Trial 1, maturity index (MI) and structure index (SI) were comparable across treatments. However, in Trial 2 MI was lower in both 0.5% and 1% CE treatment groups compared to NA, and SI was observed to be greater when treating with 0.5% CE, but was lower at 1% CE and comparable to the SI of NA treated pots (Fig. 32). This suggest that PGS CE drenching has benefits for nematode community structure, but with a concentration:benefit threshold somewhere between 0.5% and 1% CE.
Concluding remarks
Dry PGS amendment showed some initial greater effect at helping reduce disease than the PGS CE drenching, however, with the amendment only being applied at 5-10 cm deep in the soil profile, PGS appeared to lose its effectiveness at the growing point in which the kai choi roots grow past this zone and into untreated soil (i.e about 3 weeks after planting). Thus, we considered the root zone size of kai choi and other target crops in order to determine a better application method to have better long-term disease suppression in the soil.
Our hypothesis that the use of PGS CE may transport BITC more effectively and deeper into the soil than dry PGS was not supported in both the kai choi greenhouse pot and field trials when treating again F. commune. In fact, our field trial showed that PGS soil amendment was more potent than PGS CE drenching. Changing how we applied by rate and frequency of application was the next step we took. On the other hand, applying PGS to deeper soil depth where root zone of kai choi would grow into could resolve this issue. Knowing the fact that biofumigation of PGS occurred soon after hydrolysis happened, future research should also consider split application of PGS over time instead of one time at preplant. Additionally, as increasing the PGS concentrations from 0.5 to 1.0% did not show a desired disease reduction in the field for both PGS and CE treatments, we concluded that a higher concentration of treatment is necessary for managing Fusarium wilt in kai choi.
Year 2
Following the outcome of Year 2 studies, it became apparent that we needed to integrate the fumigation effect of PGS CE with its ability to induce ISR against soil-borne pathogens. While had yet to test gene expression related to ISR, results from this year confirmed that PGS crude extract is a better delivery method for applying PGS.
Final year
Based on our findings in our final year, it can be concluded that applying PGS as a crude extract through drenching was an effective method for managing and reducing disease pressure on tomato and anthurium caused by root-knot, reniform, and burrowing nematodes. Additionally, through the use of split-root greenhouse experiments, we were able to demonstrate phenotypically that drenching with PGS crude extract triggered an immune response in the plant through ISR. This is further supported by the increase of bacteriovores and fungivores observed in nematode communities in the field microplot trials, indicating an increase in microbes present. When ISR is triggered within the plant, the plant sends out signals to recruit beneficial bacteria and fungi in the soil to colonize and prime the root system for pathogen attack.
In summary, ground papaya seed offers a novel, sustainable, and locally sourced biofumigant created from existing agricultural waste. By effectively reducing a range of plant-parasitic nematode populations in edible crops and ornamentals, this product could improve economic viability of both papaya growers and other grower industries in Hawai‘i.
Research outcomes
The key expected outcome of this project was to demonstrate the use of a locally-sourced and environmentally friendly agricultural waste product as a means of disease management in Hawai’i fields, by inducing an increase in overall crop resistance. Development and adoption of this technology will help reduce the use of unsustainable and harmful chemical pesticides in crops where resistant cultivars are either unavailable or commercially undesired.
During our first year reporting period, it was demonstrated that 1) water-activated PGS is promising and effective against soil-borne fungi in contained, greenhouse pots, but only somewhat effective in field settings, and 2) at the current rate and frequency of application (regardless of whether in the greenhouse or field) PGS CE is mostly ineffective at this time and requires rate and treatment frequency adjustments. In addition to knowledge gained, new professional collaborations came from this project in order to source culled papaya material for obtaining seeds, including Kamiya Papaya farm and Kahuku farms. We completed one kai choi greenhouse trial and one kai choi field trial, during which we saw protection of kai choi in greenhouse pots by PGS and early protection of kai choi by PGS in the field. We saw very limited protection by PGS CE in both settings.
During the 2nd year reporting period, it was promising to record that PGS CE at 0.5% rate showed some significant effects against Fusarium wilt caused by F. commune in one trial, but the effect was marginal in the 2nd trial. However, we are confident that drenching of PGS CE was a better delivery method than soil incorporating PGS powder into the soil.
During our final (3rd) year reporting period, we were pleased to find that drenching with PGS crude extract at both 0.5% and 1% CE showed significant reduction of root-knot, reniform, and burrowing nematode populations while having minimal effects on the beneficial, free-living soil nematodes present. In addition, we also found that PGS CE acts as a trigger for the host plant to induce an immune response, making PGS CE an effective control against plant-parasitic nematodes present, through both nematicidal effects and preventative effects. Lastly, we established two new working collaborations in our final year of this study, with (1) USDA-ARS nematology researcher and (1) commercial anthurium grower, with interest for collaboration with other growers also increasing.
Recommendations
In year 2 of the project, as recommended, increased amendment rates of PGS and PGS CE were evaluated for optimization through two kai choi field trials, in which PGS and PGS CE were applied all at once at the start of the trial, split application over time for the duration of the trial, or applied every two weeks during the trial at full rate. It was also found that the causal pathogen of the Fusarium wilt symptoms in kai choi was caused by Fusarium commune rather than Fusarium oxysporum, which was reported in a previous study to be highly virulent by comparison and difficult to manage (Stewart et al., 2012). Based on results obtained from the kai choi field trials, it was determined thus far that PGS biofumigation and ISR methods were proving to be inconsistent or overall ineffective against F. commune in a field setting, so it was recommended that the grower rotate non-host crops, like bok choy, in fields with high F. commune disease pressure. For year 3, the project team instead recommended to assess the effects PGS CE applications against plant-parasitic nematodes when applied at the start of the experiment followed by subsequent applications post-planting at regular intervals.
For future projects, we suggest conducting additional greenhouse trials that include a mid-point PGS CE concentration (e.g. 0.75% CE) to determine the effective concentration of PGS CE needed to effectively manage pest pressure while also maintaining a healthy soil system. We also recommend additional split-root experiments with other edible crops and ornamentals of interest to assess if PGS CE triggers ISR in other plant systems as well. Finding alternative methods to managing F. commune in kai choi field soil are still needed, so experiments focusing on the minimum needed concentration of PGS to be effective against F. commune should be revisited in vitro. Additionally, on a broader scale of disease management, an islands-wide survey for F. commune is recommended in order to determine the overall risk that F. commune poses for agriculture in Hawaii. In terms of the use of PGS CE to induce resistance and manage plant-parasitic nematodes, more field application research in collaboration with new interested growers is also needed to develop a means of upscaling PGS production for distribution to and use by growers (e.g. PGS CE application in raised beds for ornamental growers to test against burrowing nematode and in raised bench greenhouses to control against root-knot nematodes in basil).
Education and Outreach
Participation summary:
In year 1, PI Braley attended one scientific conference and presented an informational poster about the use of PGS technology to combat plant-parasitic nematodes, in which the idea for using PGS to induce systemic resistance in crop plants was introduced to a broad audience. Additionally, PI Braley also gave one oral presentation on PGS as a biofumigant tool and trigger for induced systemic resistance at the ARCS Foundation Inc., Honolulu chapter, scholar symposium, in honor of being awarded the Dr. Jacqueline Maly Award in Tropical Agriculture.
PI Wang also shared preliminary outcomes from our PGS amendment study with local farmers through the GoFarm Hawaii New Farmers’ training program. Three guest lectures were delivered to new farmers in the GoFarm training program related to sustainable nematode and soil-borne disease management since the inception of this project. In addition, one educational tutorial video on the preparation of PGS CE was filmed by and is currently undergoing editing and production by co-PI Silva, and a field day organized by co-PI Uyeda on Sustainable Nematode Management will also allow us to showcase the use of PGS for soil-borne pathogen management, in particular against plant-parasitic nematodes.
Citations
Braley, L.B. and Wang, KH. Papaya ground seed as a biofumigant against root-knot and reniform nematodes in Hawai’i. Poster presentation at: Society of Nematologists 62nd Annual Meeting. 2023 July 12: Columbus, OH. (174 participants)
Braley, L.B. Papaya Ground Seed as a Biofumigant and Trigger for Induced Systemic Resistance against Soil-Borne Pathogens in Hawaii. Oral presentation at: ARCS Scholar Award Symposium for the Dr. Jacqueline Maly Award in Tropical Agriculture. 2023 April 15: Honolulu, HI.
Wang, K.-H. Soil health and soil-borne disease management. GoFarm at Waimanalo and Kauai. Farm Coach, Rachel Ladrig and Terry Lau, May 3, 2023 (20 participants).
Wang, K.-H. Soil health and soil-borne disease management. GoFarm at Hilo. Farm Coach, Danny Randerson. May 2, 2023 (12 participants).
Wang, K.-H. Soil health and soil-borne disease management. GoFarm at Waialua, Farm Coach, Dan Caroll, December 28, 2022 (12 participants).
Wang, K.-H. Soil health and soil-borne disease management. GoFarm at Hilo. Farm Coach, Danny Randerson. Aug 22, 2022 (12 participants).
Wang, K.-H., J. Uyeda, 2023. Sustainable nematode management. Aug 4, 2023. Poamoho Experiment Station, Waialua, HI (20 participants).
During year 2, PI Braley gave one oral presentation on PGS as a biofumigant tool and trigger for induced systemic resistance at the 2024 CTAHR Student Research Symposium hosted at the University of Hawaii at Manoa, where other university students, faculty, and local farm producers were in attendance. PI Wang also shared project research updates with local farmers during a GoFarm Hawaii guest lecture.
Citations
Braley, L.B., W.-W. Su and K.-H. Wang. 2024. Papaya ground seed as biofumigant against soil-borne pathogens in Hawai’i. CTAHR Student Research Symposium, Honolulu, HI. April 5, 2024. (25 participants)
Wang, K.-H. Cover crop, nematode management and field sanitation. GoFarm at Waialua, Farm Coach, Dan Caroll, December 27, 2023. (12 participants)
During the final year reporting period, PI Braley gave two oral presentations: one for growers during the 2025 CTAHR IPM Mini Conference in Waimanalo, Hawaii to provide information on the use of PGS in managing fungal and nematode disease problems, and one during the 64th Annual Meeting of the Society of Nematologists to share results of using PGS CE for nematode management and for triggering ISR in plants. PI Braley also presented two scientific posters, one during the 2025 CTAHR Showcase and Research Symposium at the University of Hawaii at Manoa and one during the American Phytopathological Society (APS) Plant Health 2025 meeting in Honolulu, Hawaii.
In addition, during the APS Plant Health 2025, one field trip was held at the University of Hawaii’s Poamoho Research Extension Station, where PI Braley presented and shared information with attendees about papaya production in Hawaii and the use of papaya seed waste as PGS to manage various soil-borne fungi and nematodes through biofumigation and induced systemic resistance (ISR).
In collaboration with Dr. Roxana Myers at the Daniel K. Inouye U.S. Pacific Basin Agricultural Research Center, U.S. Department of Agriculture, Agricultural Research Service station in Hilo, Hawaii, results of PGS crude extract (CE) treatments on anthurium against burrowing nematodes were shared with Hawaii anthurium/floriculture growers during the Grower’s Research Update Seminar for the Hawaii Floriculture and Nursery Association (HFNA). One peer-reviewed journal article was also submitted for publication to the Journal of Nematology based on results obtained from the two anthurium screenhouse trials described previously. Lastly, two podcast interview episodes were produced in collaboration with extension specialist Ed Zaworski from Iowa State University and the I See Dead Plants podcast to reach a broader public audience, which featured discussion of the PGS ISR project in Hawaii.
Outputs
Braley, L., Myers, R., Paudel, R., Su, W.W., & Wang, K.-H. Papaya Seed Extract for Management of Radopholus similis on Anthurium. (2025). Journal of Nematology. Manuscript submitted for publication.
Lauren Braley, Koon-Hui Wang. 2025. Using papaya ground seed to induce systemic resistance against root-knot nematodes in Hawaiʻi. HānaiʻAi 58: June, 2025. https://gms.ctahr.hawaii.edu/gs/handler/getmedia.ashx?moid=74156&dt=3&g=12
Myers, R. Management of Burrowing Nematodes and Queensland Long-Horned Beetle in Ornamental Production. Oral presentation at: Grower’s Research Update Seminar, Hawaii Floriculture and Nursery Association. 2025 Sept 26. (25 online, 15 in-person participants)
Zaworski, E. (Host), Wang, K-.H., Paudel, R. Braley, L., and Wiseman, B. (Interviewees). S4:E35 (Podcast). Island IPM: CTAHR Sustainable Pest Management Part 2. 2025 Sept 24. In I See Dead Plants. Crop Protection Network. https://sites.libsyn.com/416264/island-ipm-ctahr-sustainable-pest-management-part-1
Zaworski, E. (Host), Wang, K-.H., Paudel, R. Braley, L., and Wiseman, B. (Interviewees). S4:E34 (Podcast). Island IPM: CTAHR Sustainable Pest Management Part 1. 2025 Sept 17. In I See Dead Plants. Crop Protection Network. https://sites.libsyn.com/416264/island-ipm-ctahr-sustainable-pest-management-part-2
Braley, L. & Wang, K.-H. In vitro toxicity of benzyl isothiocyanate from ground papaya seed biofumigation against soil-borne plant-pathogenic fungi in Hawaii. Poster presentation at: Plant Health 2025, American Phytopathological Society. 2025 Aug 2-5: Honolulu, HI.
Hawaiian Agriculture Up Close: A Field Tour of Crops, Pathogens, and Research Efforts. Field tour at: Plant Health 2025, American Phytopathological Society. 2025 Aug 2: Poamoho Research Station, Waialua, HI.
Braley, L., Su, W.W., & Wang, K.-H. Papaya ground seed for nematode and fungal pathogen management. Oral presentation at: CTAHR IPM Mini Conference. 2025 July 25: Waimanalo, HI.
Braley, L. & Wang, K.-H. Using papaya ground seed to induce systemic resistance against root-knot nematodes in Hawaii. Oral presentation at: Society of Nematologists 64th Annual Meeting. 2025 July 17: Victoria, BC, Canada.
Braley, L. & Wang, K.-H. Using Papaya Ground Seed to Induce Systemic Resistance Against Root-Knot Nematodes in Hawai`i. HānaiʻAi 58: Newsletter June 2025.
Braley, L. & Wang, K.-H. Using papaya ground seed to induce systemic resistance against root-knot nematodes in Hawaii. Poster presentation at: CTAHR Showcase and Research Symposium. 2025 April 4: Honolulu, HI.
Wang, K.-H. Cover crop and other nematode management updates. GoFarm Waimanalo and Waialua (virtual guest lecture). March 5, 2025 (20 participants).
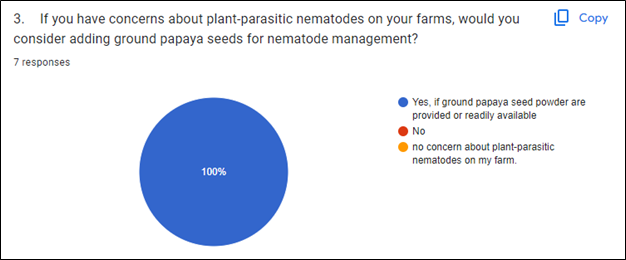
Based on a survey conducted following a GoFarm guest lecture where we presented different approaches of Sustainable Soil health and soil-borne disease management methods, it was found that the participating farmers are very receptive to using PGS for nematode pest management (Fig. 1). Most of the participating farmers have a preference for organic farming, and are actively looking for local resources to solve their pressing problem: plant-parasitic nematodes.
In year 2, during the CTAHR Student Research Symposium, PI Braley had the opportunity to discuss the development of PGS as a sustainable pest management option with local farm producers. During discussion, positive feedback was received and producers expressed support for and the need for this alternative control option in Hawaii compared to chemical controls.
During our final year reporting period, after the presentation given during the CTAHR IPM Mini Conference, one farmer approached and expressed interest in trying PGS applications on her farm to manage a root-knot nematode problem on kava trees. Based on a survey conducted after the IPM Mini Conference, an additional farmer also said yes to considering adding PGS extract to their nematode disease management if PGS were provided or readily available. In addition, following the HFNA research update seminar, 3 participating farmers expressed interest in using PGS crude extract to control burrowing nematodes in their fields, and said that they would like to participate in future field trials.