Progress report for GW22-233
Project Information
Plant pathogens pose a serious threat to food security, and growers in Hawaii are especially challenged by pest pressure all year round. Rising cost of agriculture production inputs is calling food producers in Hawaii to adopt sustainable pest management tactics using local resources. Papaya seeds are considered as an agricultural waste in Hawaii. This project aims to evaluate the use of ground papaya seed (PGS) to suppress plant-parasitic nematodes and soil-borne fungi, as the hydrolysis of PGS produces a biofumigant, benzyl isothiocyanate (BITC), which is suppressive to many soil-borne pathogens. The novelty of this project is to explore the innate immune response of treated crops to the microbiome associated with PGS treatment. Specific objectives are to 1) examine if PGS can induce systemic resistance (ISR) of crops against broad spectrum of pests and pathogens by examining expression of ISR genes and related phytohormones, 2) evaluate the efficacy of using PGS on kai choi, onion, and zucchini seedlings through seedling media amendment to protect these seedlings at transplanting, 3) examine the adequate frequency of post-plant foliar spray vs soil drenching of transplants in fields naturally infested with Fusarium spp. or Meloidogyne incognita. We will work with three commercial farmers in Hawaii challenged by the above-mentioned pathogens, while collaborating with extension agents to disseminate research findings with vegetable crop producers in Hawaii through workshops and online extension newsletters or publications. We expect successful adoption of this local farms’ waste-based biofumigation approach to help Hawaii farmers to combat a year-round pathogen infestation problem.
Research objectives:
Objective 1: Examine if PGS can induce systemic resistance (ISR) of crops against broad spectrum of pests and pathogens by examining expression of ISR genes.
Objective 2: Evaluate the efficacy of using PGS crude extract on kai choi, onion, and zucchini seedlings through drenching of seedling media.
Objective 3: Examine the adequate frequency of post-plant foliar spray vs soil drenching of transplants in fields naturally infested with Fusarium spp. or Meloidogyne incognita.
Cooperators
- (Researcher)
- - Producer
- - Producer
- - Producer
- (Researcher)
Research
Hypothesis:
Our central hypothesis is that PGS can be used to induce plant immune responses against targeted pathogens of interest. Additionally, a secondary hypothesis is that the use of a crude extract from PGS may be more effective in inducing the immune response by delivering BITC more effectively. These hypotheses are supported in part by data collected in our preliminary study as well as associated literature. Thus, our research plan is to assist local vegetable food crop producers in Hawai`i to overcome the overarching challenge of soil-borne diseases by using ground papaya seed (PGS) to induce plant systemic resistance against the targeted pathogens. Our overall goal is to work with local growers and extension agents to develop a protocol for farmers to use PGS effectively.
Materials and methods:
During our first year, we collected data pertaining to (1) kai choi greenhouse trial using PGS and CE treatments to induce systemic resistance and (2) a field trial of PGS and CE on Rhizoctonia- and Fusarium-infested soil on a collaborating kai choi farmer’s farm (Owen Kaneshiro Farm, Waianae, HI).
Objective 1: Examine if PGS can induce systemic resistance (ISR) of crops against broad spectrum of pests and pathogens by examining expression of ISR genes.
Ho: Drenching of plants with PGS crude extract or amending soil with PGS prior to transplanting will induce ISR to achieve soil-borne pathogen suppression.
Kai choi greenhouse trial
One greenhouse pot trial was conducted using field soil collected from Owen Kaneshiro Farm with a history of R. solani and F. oxysporum infestation. ‘Hirayama’ kai choi seedlings prepared in sterile potting media were grown for 2 weeks prior to transplanting into the test soil. Round, 10-cm diameter pots were filled with 300g field soil or sterile potting media. A total of 8 treatments were tested: 1-3) field soil amended with papaya ground seed (PGS) at 0.1, 0.5, 1.0 % (dry weight equivalent of amendment to soil), 4-6) field soil drenched with a papaya seed crude extract (CE) at 0.1, 0.5, 1.0 % concentration, 7) untreated control with water only, and 8) autoclaved field soil as control. For treatments 4-6, PGS was mixed with a 0.1% Tween80 solution at 1:20 rate, then stirred constantly for 30-minutes at 60°C. The mixture was then centrifuged to separate the lipid and water layer from the PGS residue, and the liquid was diluted in order for crude extract to be added at a rate of 50 ml per pot. Each treatment had 4 replications. Plant tissue from kai choi was collected using a 0.5-cm cork borer, sampling from the last fully matured leaves at 0, 2, 4, 6 and 8 days after soil treatment. CE drenching was repeated once per week over a 6-week growing period to equal the treatment final concentration. Disease incidence of Rhizoctonia rot/Fusarium wilt and biomass of kai choi was recorded at the end of the experiment. Root colonization by R. solani and Fusarium spp. was tested by plating three root pieces per root system on Rhizoctonia-selective KH media and Fusarium-selective Komada media and incubated at room temperature (Ko and Hora, 1971; Komada, 1976). The plated roots were monitored for suspected R. solani and F. oxysporum colonies and the percent of roots colonized were recorded.
Objective 2: Evaluate the efficacy of using PGS crude extract on kai choi and zucchini seedlings through soil drenching.
Ho: Drenching of plants with PGS crude extract (CE) can achieve soil-borne pathogen suppression equivalent to amending soil with PGS.
Kai choi field trial
In Summer of 2023, a field trial was conducted in a Fusarium- and Rhizoctonia-infested commercial farm (Owen Kaneshiro Farm, LLC). Twenty-four, 6.5x1.5 ft2 plots were arranged in a randomized complete block design and established according to farmer’s practice. Six soil treatments with 4 replications each were included: 1-2) amendment with PGS at 0.5 and 1.0% (dry weight equivalent of amendment to soil), 3) split amendment application of PGS at 1.0% (½ amount applied pre-plant and ½ amount applied at halfway point in cropping cycle), 4-5) drenching with papaya seed CE at 0.5 and 1.0 % concentration, and 6) an untreated, bare ground control. For treatments 4-5, CE was prepared same as the greenhouse trial.
‘Hirayama’ kai choi was first seeded in potting media in 128-cell seedling trays for 1 week prior to transplanting. Treatments 1-2, the first half of treatment 3, and the first portions of 4-5 were applied 48 hours prior to transplanting seedlings. Post-transplanting, the remaining PGS CE treatments were evenly divided and drenched weekly (to be the equivalent of the final PGS amendment rate used) using a standard watering can, with the final drench being applied one week prior to harvest. Incidence of Rhizoctonia rot/Fusarium wilt was recorded after 2 weeks and at termination. Soil samples were collected for downstream analysis 1 week after plot treatments and at termination.
Data analysis
Data collected from all field and greenhouse trials was subjected to analysis of variance using PROC GLM in SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Wherever necessary, data was normalized using appropriate transformation. Means were separated using Waller-Duncan k-ratio (k = 100) t-test.
References cited:
Komada, H. (1976). A new selective medium for isolating Fusarium from natural soil. Proceedings of the American Phytopathological Society 3:221.
Ko, W.-H. and Hora, F. K. (1971). A Selective Medium for the Quantitative Determination of Rhizoctonia solani in Soil. Phytopathology, 61(6), 707. https://doi.org/10.1094/Phyto-61-707
Results and Discussion
This project started in fall 2022. During this first reporting period, there were no results produced using green onion in collaboration with Katsu Farm. It was found that the disease issue the farmer faced with green onion was not due to field soil-borne disease, but due to contaminated potting media used for seedlings on raised benches. After switching the potting media brand used, the grower’s disease issue was resolved. However, we were able to collaborate and work with one of our participating farmers, Owen Kaneshiro, to design greenhouse trials using field soil collected in his fields showing high disease pressure. We were also able to work with him to set-up an initial field trial to determine is PGS and/or PGS CE can induce systemic resistance against Fusarium oxysporum and Rhizoctonia solani. For these experiments, kai choi was used as an experimental crop.
Kai choi greenhouse trial
A. Disease Index
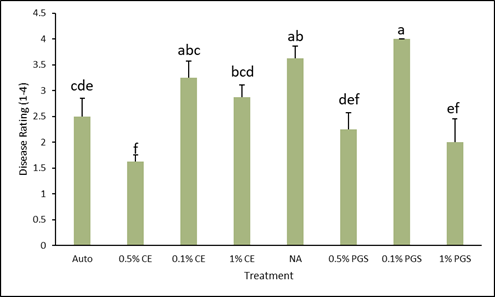
At experiment termination, level of disease for each treatment was rated on a scale of 1 to 4 (Fig.1). As demonstrated by the data in Figure 2, both the 0.1% PGS and NA treatments were comparable and both significantly higher in disease than the autoclaved control. However, the 0.5% CE treatment had the lowest level of disease, followed by the 1% PGS and 0.5% PGS treatments respectively, all of which were significantly lower than the NA control. Overall, PGS treatments performed consistently better, aside from the 0.5% CE treatment. Both 0.1% amendment rates did not reduce disease, indicating the dose is likely too low. Autoclave treatment results showed some disease symptoms, but this is likely due to overall lack of nutrients in the autoclaved soil, which could be remedied by including a standard fertilizer treatment.

B. Biomass & Yield
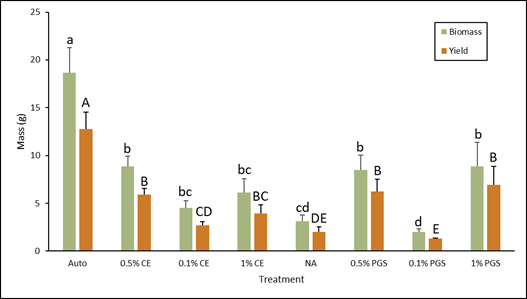
As expected, the autoclaved control treatment had the highest overall biomass and yield (Fig. 3). The 0.5% PGS, 1% PGS, and 0.5% CE treatments were all comparable and lower than the autoclave treatment, but still significantly higher than the NA treatment in both biomass and yield (Fig. 3). This positively reflects and is consistent with the decreased level of disease observed in these treatments (Fig. 2). 0.1% and 1% CE biomass did not differ greatly from NA, but the 1% CE yield was greater than NA yield (Fig.3). While it was not a significant difference, there was a small decrease in disease observed in the 1% CE treated pots compared to the NA treated pots, which can explain these yield results. Again, as seen in Figure 3, the 0.1% PGS treatment performed poorly, showing significantly lower biomass and yield than the NA treatment, which leads to speculating that it may somehow promote disease.
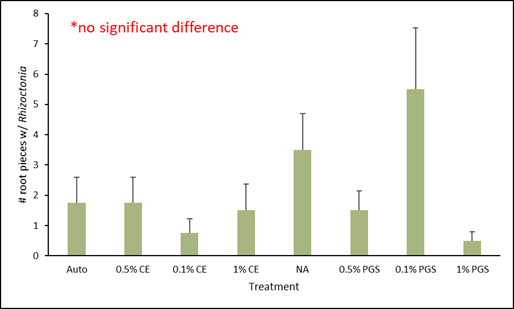
C. Fusarium and Rhizoctonia colonization of kai choi roots
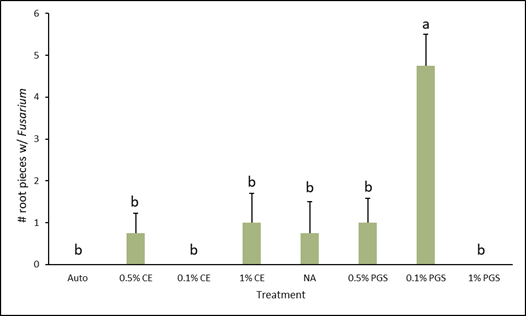
Of the roots plated on the Fusarium- and Rhizoctonia-selective media, only those from the 0.1% PGS treatment were significantly different, with higher colonization in the Fusarium assay (Fig. 4), while all treatments from the Rhizoctonia assay were not significantly different from each other (Fig. 5). However, while not statistically significant, the 1% PGS and 0.1% CE treatments did reduce Fusarium root colonization to zero (Fig. 4). The low presence of Fusarium emergence in the NA treated roots was likely due to roots being overall rotted and reduced.
Kai choi field trial
A. Disease Incidence
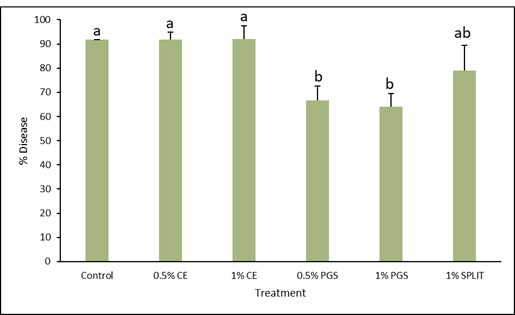
A field plot at a commercial kai choi farm in Waianae, HI was treated 48 hours prior to transplanting 2-week old kai choi seedlings, then observed for 4 weeks.
A general observation was made 1 week after transplanting that plants were healthy and growing well (Fig. 6), however at 2 weeks, disease appeared in nearly all plots. As pictured in Figure 7 and through data collected, it was found that the 0.5% PGS and 1% PGS treatments still had significantly less disease present than the untreated, control plots whereas the CE treated plots were still comparable to the untreated plots (Fig. 8). By 4 weeks, nearly all kai choi plants died (Fig. 9). For this reason, numerical data for disease incidence at 4 weeks was not collected, nor was biomass or root colonization data, as there was no plant material to harvest.



Based on data obtained from 2 weeks of growth, it is apparent that the PGS treatments are showing to be effective until a certain growing point. It is speculated that the crops roots are going deeper in the soil than the depth of amendment for PGS, meaning the roots are reaching soil that BITC released from the PGS was unable to reach. Additionally, while the PGS treatments do decrease the level of disease incidence to some degree, there is still over 50% disease in the PGS plots. Higher concentration of PGS may be needed to further decrease this level of disease.
As stated previously, the CE treated plots did not show to be effective, but similar to the greenhouse pot experiment, the treatment was spread out over 4 weeks rather than the full concentration being applied at the start of the experiment. Thus, CE may show different results if the full treatment concentration is added from the start.
Concluding remarks
Dry PGS amendment presently shows some initial greater effect at helping reduce disease than the PGS CE drenching, however, with the amendment only being applied at 5-10 cm deep in the soil profile, PGS appears to lose its effectiveness at the growing point in which the kai choi roots grow past this zone and into untreated soil (i.e about 3 weeks after planting). Thus, it would be helpful to assess the root zone size of kai choi and other target crops in order to accurately and effectively apply PGS amendment.
Our hypothesis that the use of PGS CE may transport BITC more effectively and deeper into the soil than dry PGS was not supported in both the greenhouse pot and field trials. In fact, our field trial showed that PGS soil amendment was more potent than PGS CE drenching. Changing how CE is applied by rate and frequency of application would be our next step for exploration. On the other hand, applying PGS to deeper soil depth where root zone of kai choi would grow into could resolve this issue. Knowing the fact that biofumigation of PGS occurred soon after hydrolysis happened, future research would also consider split application of PGS over time instead of one time at preplant. We will use a flex-tine weeder to assist in post-plant PGS applications in the future. Additionally, as increasing the PGS concentrations from 0.5 to 1.0% did not show a desired disease reduction in the field for both PGS and CE treatments, it is indicated that a higher concentration of treatment is necessary.
Research Outcomes
The key expected outcome of this project is to demonstrate the use of a locally-sourced and environmentally friendly agricultural waste product as a means of disease management in Hawai’i fields, by inducing an increase in overall crop resistance. Development and adoption of this technology will help reduce the use of unsustainable and harmful chemical pesticides in crops where resistant cultivars are either unavailable or commercially undesired.
During our first year reporting period, it was demonstrated that 1) water-activated PGS is promising and effective against soil-borne fungi in contained, greenhouse pots, but only somewhat effective in field settings, and 2) at the current rate and frequency of application (regardless of whether in the greenhouse or field) PGS CE is mostly ineffective at this time and requires rate and treatment frequency adjustments. In addition to knowledge gained, new professional collaborations came from this project in order to source culled papaya material for obtaining seeds, including Kamiya Papaya farm and Kahuku farms. We completed one kai choi greenhouse trial and one kai choi field trial, during which we saw protection of kai choi in greenhouse pots by PGS and early protection of kai choi by PGS in the field. We saw very limited protection by PGS CE in both settings.
Recommendations
In year 2 of the project, increased amendment rates of PGS and PGS CE will be evaluated for optimization in addition to performing greenhouse microplot experiments. The microplot experiments will be able to mimic a mock field setting for assessing adequate treatment rate and method prior to reattempting a full field experiment. Within the microplots, kai choi lateral root growth can be measured to determine the best amendment depth with PGS. The potential efficacy of the PGS CE treatment may also improve if CE is applied all at once pre-transplanting rather than weekly, over the growing period, as performed in the first year trials. Additionally, we are also planning to further examine the effects of applying PGS using split application approach at post plant to assess if allowing more post-plant biofumigation to occur can further protect new roots from the fungal pathogens. With samples collected and as more are obtained, we will continue with developing assays for assessing the upregulation of resistance associated genes and production of jasmonic acid (JA) in order to connect phenotypic results with genetic and molecular results.
Education and Outreach
Participation Summary:
PI Braley attended one scientific conference and presented an informational poster about the use of PGS technology to combat plant-parasitic nematodes, in which the idea for using PGS to induce systemic resistance in crop plants was introduced to a broad audience. Additionally, PI Braley also gave one oral presentation on PGS as a biofumigant tool and trigger for induced systemic resistance at the ARCS Foundation Inc., Honolulu chapter, scholar symposium, in honor of being awarded the Dr. Jacqueline Maly Award in Tropical Agriculture.
PI Wang also shared preliminary outcomes from our PGS amendment study with local farmers through the GoFarm Hawaii New Farmers’ training program. Three guest lectures were delivered to new farmers in the GoFarm training program related to sustainable nematode and soil-borne disease management since the inception of this project. In addition, one educational tutorial video on the preparation of PGS CE was filmed by and is currently undergoing editing and production by co-PI Silva, and a field day organized by co-PI Uyeda on Sustainable Nematode Management will also allow us to showcase the use of PGS for soil-borne pathogen management, in particular against plant-parasitic nematodes.
As more data is obtained and interpreted, we will begin to share more about PGS ISR technology in outreach activities in year 2 of the project. We will aim to engage further with local farmers, producers, and agriculture professionals through lecture and outreach events, such as the annual virtual IPM and Soil Health Mini-Conference, “IPM for GoFarm Hawaii” guest lectures, and field days and demonstrations. From these events, we will ask for direct feedback and suggestions from participants. Information will also be disseminated through electronic and paper publications. Research findings will be submitted for publication in peer-reviewed journals, and technical knowledge, factsheets, and tutorials will be published online via the CTAHR Sustainable and Organic Agriculture Program (SOAP), Hānai’Ai online newsletter.
Citations
Braley, L.B. and Wang, KH. Papaya ground seed as a biofumigant against root-knot and reniform nematodes in Hawai’i. Poster presentation at: Society of Nematologists 62nd Annual Meeting. 2023 July 12: Columbus, OH. (174 participants)
Braley, L.B. Papaya Ground Seed as a Biofumigant and Trigger for Induced Systemic Resistance against Soil-Borne Pathogens in Hawaii. Oral presentation at: ARCS Scholar Award Symposium for the Dr. Jacqueline Maly Award in Tropical Agriculture. 2023 April 15: Honolulu, HI.
Wang, K.-H. Soil health and soil-borne disease management. GoFarm at Waimanalo and Kauai. Farm Coach, Rachel Ladrig and Terry Lau, May 3, 2023 (20 participants).
Wang, K.-H. Soil health and soil-borne disease management. GoFarm at Hilo. Farm Coach, Danny Randerson. May 2, 2023 (12 participants).
Wang, K.-H. Soil health and soil-borne disease management. GoFarm at Waialua, Farm Coach, Dan Caroll, December 28, 2022 (12 participants).
Wang, K.-H. Soil health and soil-borne disease management. GoFarm at Hilo. Farm Coach, Danny Randerson. Aug 22, 2022 (12 participants).
Wang, K.-H., J. Uyeda, 2023. Sustainable nematode management. Aug 4, 2023. Poamoho Experiment Station, Waialua, HI (20 participants).
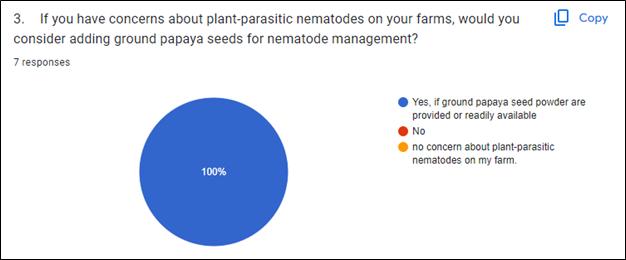
Based on a survey conducted following a GoFarm guest lecture where we presented different approaches of Sustainable Soil health and soil-borne disease management methods, it was found that the participating farmers are very receptive to using PGS for nematode pest management (Fig. 1). Most of the participating farmers have a preference for organic farming, and are actively looking for local resources to solve their pressing problem: plant-parasitic nematodes.