Final report for LNE14-336
Project Information
In this project we have worked with three Maine oyster farms testing methods for reducing the settlement and colonization of oysters the pest polychaete worm species known as blister worm. We found that periodic air-drying of oysters substantially reduced, but did not eliminate blister worm outbreaks and that washing oysters prior to air-drying resulted in further, modest, decreases in blister worm abundance and the number of oysters impacted by this pest. More intensive research was conducted at one farm where blister worm has been particularly problematic; this additional work has supported a more detailed analysis of the efficacy of our methods as a function of seasonal and annual levels of pest pressure.
In conjunction with our research efforts, we distributed a survey to oyster growers throughout the Northeast assessing the impact of blister worm on their farms. Based on responses from 56 growers, we found that blister worm is a problem throughout the region. Few of the respondents reported having lost sales or farm reputation due to blister worm; however, the vast majority of those responding indicated that are concerned about this pest. Through the survey we also documented culture methods and environmental conditions associated with reduced impact of blister worm, as well as ways in which growers feel they can reliably control the abundance of adult blister worms on their farms.
We have communicated the findings from both the research project and the survey to industry participants through several means, including local workshops. These workshops included discussion of the development of pest management plans and the nformation growers need for such plans. At these meetings, growers were encouraged to create biosecurity plans (for reducing risk) and making sure that protocols for minimizing the inadvertent movement of blister worm needed to be part of those biosecurity plans.
We are currently in the process of assessing how many participants have adopted these recommendations and begun to develop pest management plans.
Twenty northeastern oyster farms with annual aggregate sales of about $4 million will each implement a comprehensive polychaete pest management plan. This will reduce pest prevalence and improve crop quality compared to prior years, avoiding an estimated $4 million aggregate loss in annual sales.
Blister worm is a polychaete worm that burrows into the shells of oysters and other shellfish. Infestations create visible pockets or blisters of mud and fecal waste on the inside surface of the oyster shell. These blisters mar the oyster’s appearance, particularly when oysters are served on the half-shell and breakage of the blisters can result in off-flavors, both of which may seriously undermine the market value of oysters from an impacted farm. Our research and education program targeted the onset of pest infestation during larval settlement to eliminate the need for costly and inefficient treatment of oysters infested with adult blister worms. Through workshops communicating the results from our field research and our survey of growers' experience with blister worm, we anticipate growers developing and adopting blister worm management programs.
Cooperators
Research
Blister worm burrows will be substantially reduced in oysters that receive preventative treatments compared to oysters grown in standard surface cages without preventative treatments.
Blister worm burrows will be substantially reduced in oysters that receive preventative treatments once a week compared to those that receive treatments less frequently.
Blister worm burrows will be substantially reduced in oysters receive combined air-drying and pressure washing treatments compared to those that receive only single treatments.
The research portion of our project was encapsulated in milestones #3 and #6 under which we tested preventative treatments for reducing or  eliminating blister worm infestations on oyster farms. In the Northeast, oysters are grown using a variety of methodologies, including several that “plant” oysters directly on the bottom substrate of a lease site or in some form of caging system held near the bottom. In contrast, other methodologies hold oysters in surface cage systems. Our research, on reducing blister worm impacts, has focused on farms employing the latter type of culture system. One type of surface culture system common among oyster farms throughout the Northeast uses plastic mesh bags supported at the surface by small floats (Fig. R1). These can be flipped periodically to dry the bag surface in order to reduce the amount of fouling from tunicates, algae, mussels, barnacles and other sessile organisms. Fouling organisms can reduce water flow through the bag and many of the sessile invertebrates that are part of the fouling community compete with the oysters for suspended food. Thus, periodic flipping of the bag is necessary to dry and remove fouling organisms and sustain oyster growth.
eliminating blister worm infestations on oyster farms. In the Northeast, oysters are grown using a variety of methodologies, including several that “plant” oysters directly on the bottom substrate of a lease site or in some form of caging system held near the bottom. In contrast, other methodologies hold oysters in surface cage systems. Our research, on reducing blister worm impacts, has focused on farms employing the latter type of culture system. One type of surface culture system common among oyster farms throughout the Northeast uses plastic mesh bags supported at the surface by small floats (Fig. R1). These can be flipped periodically to dry the bag surface in order to reduce the amount of fouling from tunicates, algae, mussels, barnacles and other sessile organisms. Fouling organisms can reduce water flow through the bag and many of the sessile invertebrates that are part of the fouling community compete with the oysters for suspended food. Thus, periodic flipping of the bag is necessary to dry and remove fouling organisms and sustain oyster growth.
 The oysters inside the bag, however, are not dried and there is no opportunity to kill any fouling organisms that settle directly onto the oysters. Given that the life cycle of blister worms includes a dispersive larval stage, we hypothesized that periodic drying of oysters and washing of the oyster shell surface would disrupt blister worm larval settlement and reduce or eliminate infestations. We employed 4-bag Oyster-Ranch (Go-Deep International) cages in our experimental design. These cages consist of a wire mesh frame into which four individual plastic mesh bags containing oysters can be held near the surface suspended beneath two large plastic floats or pontoons. The majority of the time the oysters are submerged, but the cage system can be flipped exposing the wire frame, individual bags, and most importantly, the oysters (see Fig. R2). In comparison to oysters cultured in a typical flip bag, oysters in an Oyster-Ranch (or similar Oyster-GroTM) system can be air-dried and washed during periods of exposure. We used these caging systems to address the research hypotheses given, above.
The oysters inside the bag, however, are not dried and there is no opportunity to kill any fouling organisms that settle directly onto the oysters. Given that the life cycle of blister worms includes a dispersive larval stage, we hypothesized that periodic drying of oysters and washing of the oyster shell surface would disrupt blister worm larval settlement and reduce or eliminate infestations. We employed 4-bag Oyster-Ranch (Go-Deep International) cages in our experimental design. These cages consist of a wire mesh frame into which four individual plastic mesh bags containing oysters can be held near the surface suspended beneath two large plastic floats or pontoons. The majority of the time the oysters are submerged, but the cage system can be flipped exposing the wire frame, individual bags, and most importantly, the oysters (see Fig. R2). In comparison to oysters cultured in a typical flip bag, oysters in an Oyster-Ranch (or similar Oyster-GroTM) system can be air-dried and washed during periods of exposure. We used these caging systems to address the research hypotheses given, above.
Initially, we proposed to conduct this research at two oyster farms in Maine during the late spring to early fall (growing season) of 2014. Due to a delay in funding from NESARE, we obtained outside resources to start this research on one farm on the Bagaduce River during the 2014 growing season. The research was subsequently expanded to three farms in 2015, one farm on each of the Bagaduce, Weskaeg, and Damariscotta Rivers. We also continued this research for two additional seasons in 2016 and 2017 at the farm on the Bagaduce River (see Table R1).
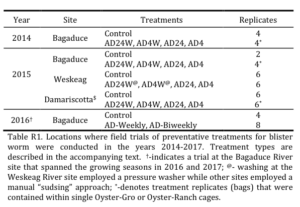 Bagaduce River Farm (2014 to 2017) - During the growing season of 2014, we compared the frequency of new blister worm burrows in the shells of oysters cultured in “flip-bags” to those cultured in bags held within Oyster-Ranch cage systems. Using oysters provided by the host farm, we placed ~250 oysters (35-40 mm shell height) in each of four replicate flip bags like those typically employed at the farm (Control treatment). We also placed oysters (~250; 35-40 mm SH) in individual bags that were then placed inside one of the four slots of an Oyster-Ranch cage. We set up four such Oyster-Ranch cages; one cage was flipped every two weeks to provide 24 hours of air-drying (AD24) and one cage was flipped every two weeks to provide 4 hours of air-drying (AD4). The bags in one cage system were removed every other week and washed to remove built up sediment and detritus, after replacing the bags in the Oyster-Ranch cage the bags were then dried for 24 hours (AD24W). The bags the fourth cage system were removed every other week, washed, and then dried for 4 hours (AD4W). Washing the oysters can reduce the buildup of sediments and dead organic material (detritus) on the shell surface that provides material for blister worm larvae to build an initial mud tube. From this mud tube the blister worms excavate a burrow into the oyster shell by chemical means. Although our initial proposal included pressure-washing of the oysters in the latter two treatments, we did not have access to a suitable portable power-washer that could be used on the Bagaduce farm. Instead of power-washing, we “sudsed” the each bag by lifting the bag out of the water and vigorously plunging the bag back into the water up to a dozen times, as is often done by the farm operator. In addition to removing sediment from the surface of the oysters, “sudsing” has the added benefit of dulling the sharp edges of the oyster shells (leading to better oyster shape), reduces fouling, and breaks up oysters that have attached to one another (doubles).
Bagaduce River Farm (2014 to 2017) - During the growing season of 2014, we compared the frequency of new blister worm burrows in the shells of oysters cultured in “flip-bags” to those cultured in bags held within Oyster-Ranch cage systems. Using oysters provided by the host farm, we placed ~250 oysters (35-40 mm shell height) in each of four replicate flip bags like those typically employed at the farm (Control treatment). We also placed oysters (~250; 35-40 mm SH) in individual bags that were then placed inside one of the four slots of an Oyster-Ranch cage. We set up four such Oyster-Ranch cages; one cage was flipped every two weeks to provide 24 hours of air-drying (AD24) and one cage was flipped every two weeks to provide 4 hours of air-drying (AD4). The bags in one cage system were removed every other week and washed to remove built up sediment and detritus, after replacing the bags in the Oyster-Ranch cage the bags were then dried for 24 hours (AD24W). The bags the fourth cage system were removed every other week, washed, and then dried for 4 hours (AD4W). Washing the oysters can reduce the buildup of sediments and dead organic material (detritus) on the shell surface that provides material for blister worm larvae to build an initial mud tube. From this mud tube the blister worms excavate a burrow into the oyster shell by chemical means. Although our initial proposal included pressure-washing of the oysters in the latter two treatments, we did not have access to a suitable portable power-washer that could be used on the Bagaduce farm. Instead of power-washing, we “sudsed” the each bag by lifting the bag out of the water and vigorously plunging the bag back into the water up to a dozen times, as is often done by the farm operator. In addition to removing sediment from the surface of the oysters, “sudsing” has the added benefit of dulling the sharp edges of the oyster shells (leading to better oyster shape), reduces fouling, and breaks up oysters that have attached to one another (doubles).
 The bags and cage systems were deployed on May 27, 2014. Starting on July 10 and continuing until November 8, 2014, we haphazardly sampled five oysters from each replicate bag in each treatment approximately every two weeks. At each sampling, individual oysters were brought to the University of Maine in Orono shucked, the meats discarded and the shells were scored for the presence of new worm burrows that contained worms (see Fig. R3). Although regular sampling reduced the number of oysters in each bag, we further reduced the number of oysters in each bag as they grew following standard practice employed at the Bagaduce River farm so that the final density on November 8 was ~125 per bag. Thinned oysters were returned to the host farm.
The bags and cage systems were deployed on May 27, 2014. Starting on July 10 and continuing until November 8, 2014, we haphazardly sampled five oysters from each replicate bag in each treatment approximately every two weeks. At each sampling, individual oysters were brought to the University of Maine in Orono shucked, the meats discarded and the shells were scored for the presence of new worm burrows that contained worms (see Fig. R3). Although regular sampling reduced the number of oysters in each bag, we further reduced the number of oysters in each bag as they grew following standard practice employed at the Bagaduce River farm so that the final density on November 8 was ~125 per bag. Thinned oysters were returned to the host farm.
We employed the same basic experimental design at the Bagaduce River farm during the 2015 growing season. Notable changes to our protocol included starting with larger oysters (mean shell height 70.9 ± 0.82 mm) and the oysters provided by the farm had been exposed to blister worm the previous year. Thus, when we set up the experiment on June 11, 2015, we sampled 90 oysters and estimated the size of existing worm burrows (from edge of the oyster shell to where the burrow looped back to the shell edge – see Fig. R3). From these measurements we developed a size frequency histogram for the existing “older burrows” (Fig. R4); given this distribution we set a threshold of < 3 mm for determining whether burrows observed in subsequently sampled oysters represented a “new” versus “older” burrow. We visited the Bagaduce River farm approximately biweekly from June 24 to November 1, 2015. At each visit we employed the same strategy as we did in 2014, sampling five oysters haphazardly from each replicate bag, shucking the oyster and scoring both valves of each oyster for the presence of new burrows.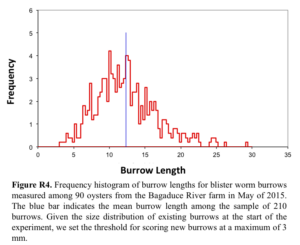
During the first two years of our work at the Bagaduce River farm we specified the dates on which the oysters were to be dried, or washed and dried in the experimental treatments. This likely reduced the effectiveness of drying, particularly on days when the weather was not suitable. For example, during mid-September in 2015 there was a period of unseasonably cool and rainy weather that was followed by a sharp increase in the number of new burrows not only in the oysters in the control treatment, but also in all four of the experimental treatments. Thus, in 2016 we requested that the farmer dry the oysters on warm, sunny days with low humidity and good airflow. In addition, because we observed only a minor reduction in blister worm settlement in the treatments that included washing during 2014 and 2015, our experimental design in 2016 focused on air-drying alone. Thus, in 2016 our experiment included oysters in eight plastic mesh bags distributed between two Oyster-Ranch cages that were dried weekly and oysters in another eight bags within two Oyster-Ranch cages that we dried every other week. We eliminated the washing step, and more importantly the cages were exposed on days that were most conducive to thorough drying of the surface of the oysters’ shells.
On May 12, 2016 we placed ~1000 oysters (30.3 ± 1.4 mm SH) into four replicate surface flip bags and ~500 oysters into each of 16 plastic mesh bags. The latter were subsequently deployed on the Bagaduce River in four separate Oyster-Ranch cages. The flip bags were turned weekly to control fouling without emersion of the oysters while the bags in the Oyster-Ranch cages were exposed, as described above. We visited the Bagaduce River farm every two weeks and haphazardly sampled five oysters from each replicate bag in each treatment. The oysters were processed and presence of new blister worm burrows (< 3 mm) was scored as in 2015.
We continued monitoring the oysters from 2016 into the 2017 growing season at the Bagaduce River farm. Oysters from the 2016 experiment were overwintered in one of two ways. The oysters in one control flip-bag were submerged at the Bagaduce River for the duration of the winter and redeployed on the surface on May 10, 2017. Oysters from the remaining three control flip-bags and all 16 of the Experimental treatment replicates were held in cold, damp storage (~38°F) for 3-4 weeks in a refrigerated trailer. Previous NESARE-funded research has shown that such treatment is generally successful at reducing the load of adult blister worms in infested oysters. Both control and experimental oysters were redeployed in their original mesh bags in their original Oyster-Ranch cage on May 10, 2017. We haphazardly sampled five oysters from each replicate bag on this date and monthly, thereafter, until mid-October. As in previous years, we estimated the number of new blister worm burrows less than 3 mm in length among the oysters in this sample. From May 10 to August 31 we also estimated the number of live worms present in older, established burrows.
Weskeag & Damariscotta River Farms (2015 Research) - We tested the efficacy of drying and washing treatments at lease sites on two other rivers during the 2015 growing season. The depth underneath the Weskeag River farm at low tide (~1 m) precluded the use of Oyster-Ranch or Oyster-Gro style cages. At this site, we relied on the use of 30 plastic mesh flip-bags into which approximately 250 oysters (72.8 ± 0.64 mm SH), supplied by the farm owner, were placed. Five of the 30 bags constituted our control treatment; these bags were flipped biweekly to manage fouling on the bag surface, but the oysters inside were not exposed at any time during the experiment. Four other sets of 5 bags constituted our experimental treatments. One of the four sets was hauled out of the water and onto a floating dock located on the oyster farm and dried for 4 h every other week while a second set was dried on the dock for 24 h every other week. A third set of bags was hauled out onto the dock, washed with a pressure washer and dried from 24 h every other week and the last set was pressure washed and dried for 4 h. Because the oysters used in this experiment had previously been exposed to blister worm, we measured the length of >500 blister worm burrows in the shells of a preliminary set of 90 oysters collected on May 24, 2015. From these measurements we developed a size-frequency histogram, as described above for the 2015 Bagaduce River experiment. Based on this histogram we set a maximum threshold of 3 mm in length for scoring new blister worm burrows (data not shown). We revisited the farm approximately every two weeks from June 17 to Nov 5, 2015. As at the Bagaduce River farm, at each visit we haphazardly sampled 5 oysters from each replicate bag, returned the sampled oysters to the University of Maine in Orono where they were shucked and the frequency of new blister worm burrows in each valve were promptly recorded.
The lease-holder at the Damariscotta River farm provided us with a set of four Oyster-Gro cages which were each capable of holding six plastic mesh bags and also provided six regular surface flip bags. These 36 bags were stocked with roughly 500 oysters (~40-50 mm SH) on May 11th, 2015. The experimental protocol mirrored that employed at the Bagaduce River farm in 2015; the flip cages were turned biweekly to control external fouling without drying the oysters, the oysters in one Oyster-Gro were dried for 24 h, the oysters in a second Oyster-Gro cage were dried for 4 h, the oysters in a third were both washed by “sudsing” and dried for 24 h, while those in a fourth cage were washed by “sudsing” and dried for 4 h. Each treatment was applied every other week. We visited the farm every other week from May 26 to October 9, at which time five oysters were haphazardly sampled from each replicate bag. These oysters were taken to the University of Maine’s Darling Marine Center in Walpole, ME where they were shucked and both valves of each oyster were scored for the presence of new blister worm burrows less than 3 mm in length.
Oyster Growth as a Function of Blister Worm Treatment - We explored whether the drying and washing treatments applied to control blister worm settlement affected the growth of oysters at the Bagaduce River farm and the Weskeag River farm in 2015. At the Bagaduce River farm, we individually measured the shell height, shell width and shell inflation on 90 oysters prior to marking each with colored and numbered “bee dots”. These 90 oysters were then distributed among the replicate bags belonging to each of our five experimental treatments on June 24, 2015. Similarly, at the Weskeag River farm 150 individually measured and tagged 150 oysters were deployed among the replicate bags in the five experimental treatments on June 17, 2015. If these oysters were inadvertently sampled during our biweekly sampling trips they were immediately returned to their respective bags. We recovered 71 of the oysters deployed at the Bagaduce River farm on November 11, 2015 and 92 of the tagged oysters deployed at the Weskeag River farm on November 5, 2015. Tagged oysters were missing at the final sampling due to both mortality during the experiment (and we could not be sure when they died) or because tags were no longer readable. We calculated specific growth for each recovered oysters using the formula ((ln[final shell height]-ln[initial shell height])/time elapsed) and then used a one-way ANOVA to determine whether mean specific growth rates varied among treatments at each farm.
In addition, we estimated the change in the mean size of oysters during each sampling interval at the Bagaduce River farm in 2016. Among the five oysters we collected from each replicate at each sampling, we measured individual shell height, shell width and shell inflation. From these measurements we calculated the mean shell height for the oysters from each treatment (n=20 per time point in Controls and n=40 in Experimental oysters) at each sampling. We then conducted a one-way ANOVA at each time point to determine whether there were significant differences in mean shell height over time.
Identification of Blister Worm Larvae - As part of milestone #3, we also conducted plankton tows near the Bagaduce River farm in 2014 with the intention of developing a guide for growers on the identification and monitoring of blister worm larval abundance on their farms. Our expectation was that, once trained, farmers could better time the application of preventative treatments to reduce blister worm outbreaks. Based on the literature, we anticipated that the life cycle of blister worms includes a dispersive larval stage that may last up to 45 or more days after which point they settle onto shellfish hosts and excavate a burrow in the host shell. Each time we visited the Bagaduce River Farm we took two plankton tows with an 80 μm net, one on either side of the rows of longlines constituting the central part of the oyster farm. From each tow we typically recovered 50-100 ml of concentrated plankton. After thorough mixing, several replicates of approximately 10-15 ml of concentrated plankton was preserved in 35-40 ml of 95% ethanol and returned to the University of Maine. Processing of the samples included re-sieving to remove the ethanol, resuspending the sample in distilled water, and transferring polychaete larvae into a separate 15 ml petri dish with distilled water. Each of the isolated polychaete larvae were measured using a stage micrometer on a dissecting microscope, photographed, and cataloged. We relied on the key in Blake (1969) to discriminate P. websteri larvae from those of closely related species. In addition, we isolated DNA from individual larvae and amplified a portion of the mitochondrial cytochrome oxidase I gene (mtCO1) for each using conserved primers. The PCR products were subsequently sequenced or used in a restriction fragment length assay diagnostic for P. websteri.
Bagaduce River Farm (2014 to 2017) - We observed new blister worm burrows in oysters from the control and treatment bags as early as mid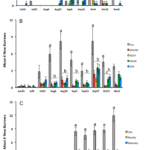 -July in 2014 (Fig. R5A). However, the greatest increase in new burrows occurred from late September through to November. Overall, the level of blister worm infestation was relatively light (< 1 burrow/oyster) and there was a high degree of variance in the “load” of new blister worm burrows within each treatment. Even so, by the mid-October and November sampling oysters in the control treatment had, on average, nearly 8-fold more new blister worm burrows than did the experimental treatments. Generally, the oysters that were dried for longer (24 h versus 4 h) had fewer new burrows and washing resulted in still fewer burrows per oyster. Thus, by the end of the season, control oysters had the highest number of new burrows while oysters that were washed and then dried for 24 h ever other week had the fewest. Because the individual bags that were held within each Oyster-Ranch cage did not represent true “statistical replicates”, we pooled all individuals within each treatment and compared means using a one-way analysis of variance. When a significant treatment effect was observed, we used multiple pair-wise comparisons (Tukey-Kramer) to test for differences in the mean number of burrows among each type of treatment while keeping the experiment-wise error rate at p<0.05. The ANOVA results suggest that at the October 16 and November 8 sampling there were significantly more new burrows among oysters in the control bags relative to oysters in the experimental treatments. We also examined whether the proportion of oysters affected by blister worm (prevalence) varied among treatments. For this analysis, we computed a running total of oysters in each treatment that had new burrows versus those that did not. When totaled across all sampling dates we found that nearly 15% of oysters in the Control treatment we sampled had new blister worm burrows while less than 1% of those in the AD24W treatment had signs of new burrows (Fig. R6A). An R x C contingency analysis indicated that a lower proportion of oysters had new burrows in the AD4 treatment relative to the Control treatment and there was an even lower prevalence observed among the oysters in the other three experimental treatments.
-July in 2014 (Fig. R5A). However, the greatest increase in new burrows occurred from late September through to November. Overall, the level of blister worm infestation was relatively light (< 1 burrow/oyster) and there was a high degree of variance in the “load” of new blister worm burrows within each treatment. Even so, by the mid-October and November sampling oysters in the control treatment had, on average, nearly 8-fold more new blister worm burrows than did the experimental treatments. Generally, the oysters that were dried for longer (24 h versus 4 h) had fewer new burrows and washing resulted in still fewer burrows per oyster. Thus, by the end of the season, control oysters had the highest number of new burrows while oysters that were washed and then dried for 24 h ever other week had the fewest. Because the individual bags that were held within each Oyster-Ranch cage did not represent true “statistical replicates”, we pooled all individuals within each treatment and compared means using a one-way analysis of variance. When a significant treatment effect was observed, we used multiple pair-wise comparisons (Tukey-Kramer) to test for differences in the mean number of burrows among each type of treatment while keeping the experiment-wise error rate at p<0.05. The ANOVA results suggest that at the October 16 and November 8 sampling there were significantly more new burrows among oysters in the control bags relative to oysters in the experimental treatments. We also examined whether the proportion of oysters affected by blister worm (prevalence) varied among treatments. For this analysis, we computed a running total of oysters in each treatment that had new burrows versus those that did not. When totaled across all sampling dates we found that nearly 15% of oysters in the Control treatment we sampled had new blister worm burrows while less than 1% of those in the AD24W treatment had signs of new burrows (Fig. R6A). An R x C contingency analysis indicated that a lower proportion of oysters had new burrows in the AD4 treatment relative to the Control treatment and there was an even lower prevalence observed among the oysters in the other three experimental treatments.
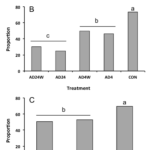 We observed new burrows as early as late July and a substantial increase in the number of new burrows by mid August during the 2015 season at the Bagaduce River farm (Fig. R5B). The level of blister worm settlement was nearly was several times higher in 2015 compared to 2014; overall there was an average of > 4 new burrows per oyster across all treatments, although some oysters in the control treatment had as many as 27 new burrows when totaled across both valves. From August 18 to October 11, we observed roughly 3 to 4-fold more new burrows in the control oysters relative to the next highest treatment. Statistical analysis indicated that the frequency of new burrows was significantly higher in oysters from the control treatment relative to the oysters in all of the experimental treatments. The mean number of new burrows for treatments that included washing and drying was similar to the comparable treatments that included only drying. As in 2014, drying for 24 h compared to 4 h reduced the load of new blister worms. This effect was statistically significant at the October 11 sampling, although these results have to be viewed with caution due to the degree of pseudoreplication in our experimental design (noted above). Even so, when viewed across years we observed a consistent effect of drying on reducing the load of new blister worms impacting oysters at the Bagaduce River farm. Compared to what we observed in 2014, however, there was a higher prevalence of new burrow among oysters in all of the treatments. For the control oysters, prevalence increased from < 15 % to over 70% (Fig. R6B). Similar dramatic increases occurred in each of the experimental treatments with the lowest value seen (~30%) among oysters dried for 24 h every other week.
We observed new burrows as early as late July and a substantial increase in the number of new burrows by mid August during the 2015 season at the Bagaduce River farm (Fig. R5B). The level of blister worm settlement was nearly was several times higher in 2015 compared to 2014; overall there was an average of > 4 new burrows per oyster across all treatments, although some oysters in the control treatment had as many as 27 new burrows when totaled across both valves. From August 18 to October 11, we observed roughly 3 to 4-fold more new burrows in the control oysters relative to the next highest treatment. Statistical analysis indicated that the frequency of new burrows was significantly higher in oysters from the control treatment relative to the oysters in all of the experimental treatments. The mean number of new burrows for treatments that included washing and drying was similar to the comparable treatments that included only drying. As in 2014, drying for 24 h compared to 4 h reduced the load of new blister worms. This effect was statistically significant at the October 11 sampling, although these results have to be viewed with caution due to the degree of pseudoreplication in our experimental design (noted above). Even so, when viewed across years we observed a consistent effect of drying on reducing the load of new blister worms impacting oysters at the Bagaduce River farm. Compared to what we observed in 2014, however, there was a higher prevalence of new burrow among oysters in all of the treatments. For the control oysters, prevalence increased from < 15 % to over 70% (Fig. R6B). Similar dramatic increases occurred in each of the experimental treatments with the lowest value seen (~30%) among oysters dried for 24 h every other week.
An increase in the frequency of new burrows was evident in all three treatments by the sampling on July 27, during the 2016 season (Fig. R5C). The mean number of new burrows in the oysters dried weekly or biweekly increased to ~2 worms/oyster by August 15 and remained at the level till the end of the experiment on November 11. In contrast, the mean number of new burrows for oysters in the control bags was over 3-fold higher than in the drying treatments by August 15 and almost 4-fold higher by November 11. Although we had replication at the cage-level in the experimental design for 2016, the nesting of replicates within treatment differed between control and experimental treatments. Thus, as in previous years, we decided to pool individuals, ignored replication within each treatment, and tested for differences among treatment means using a one-way ANOVA. This analysis indicated that the mean number of burrows for oysters in the control treatments exceeded those in both Experimental treatments from July 27 through to November 11. In contrast, there was no evidence of any difference in the mean number of new burrows among the two experimental treatments. Similarly, there was a statistically significant difference in the prevalence of new burrows between the oysters in control versus the two experimental treatments, but not between the two experiment treatments, themselves (Fig. R6C). Our results suggest that ensuring oysters are regularly exposed to conditions that promote drying at the shell surface reduces, but may not completely eliminate, the settlement of blister worms.
Among the oysters redeployed in 2017, new burrows began appearing in July and reached a peak in August in all treatments, although the number 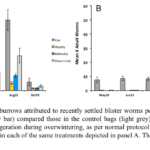 of new burrows in the control bags was 2 to 4-fold higher than in bags dried weekly or biweekly (Fig. R7A). These results suggest that drying treatments can substantially decrease the settlement of new blister worms even among oysters that were exposed to blister worms in previous years. We detected an average of 7.6 to 8.2 live adult worms per oyster in the control bag that was held submerged on the Bagaduce River from late 2016 till May 2017 (Fig. R7B). These data clearly show that blister worms are capable of surviving the cold water temperatures common in Maine rivers over the winter. The abundance of live adult worms in oysters from the bag overwintered in the river increased over 4-fold in August suggesting that new worms recruiting in July rapidly grew and extended their burrows to over 10 mm in length. We currently do not have solid estimates of the growth rate of individual worms, but these results suggest that adult worms can extend the size of their burrows several millimeters per month during the growing season.
of new burrows in the control bags was 2 to 4-fold higher than in bags dried weekly or biweekly (Fig. R7A). These results suggest that drying treatments can substantially decrease the settlement of new blister worms even among oysters that were exposed to blister worms in previous years. We detected an average of 7.6 to 8.2 live adult worms per oyster in the control bag that was held submerged on the Bagaduce River from late 2016 till May 2017 (Fig. R7B). These data clearly show that blister worms are capable of surviving the cold water temperatures common in Maine rivers over the winter. The abundance of live adult worms in oysters from the bag overwintered in the river increased over 4-fold in August suggesting that new worms recruiting in July rapidly grew and extended their burrows to over 10 mm in length. We currently do not have solid estimates of the growth rate of individual worms, but these results suggest that adult worms can extend the size of their burrows several millimeters per month during the growing season.
Weskeag and Damariscotta River Farms (2015) - A low level (< 0.5 new burrows per oyster) was evident from mid-June to mid-August at the Weskeag River farm in 2015 (Fig. R8A). There was a 4 to 6-fold increase in new blister worm burrows from late August through November. During this time period, the mean number of burrows in control oysters was higher than in the oysters from the drying and washing treatments. The average number of new burrows decreased with increasing drying time, but washing did not result in any consistent decrease in the mean number of new burrows observed. Given the full replication in all treatments in this experiment, we conducted a nested ANOVA (bag nested within treatment) to test for differences in means at each sampling date. These analyses indicated that there were no statistically significant differences among any of our treatments at any of our sampling dates. We also analyzed the prevalence of new burrows among all of the oysters sampled as a function of treatment type. Prevalence was highest in the control oysters and lowest among those dried biweekly for 24 h, although the differences in prevalence 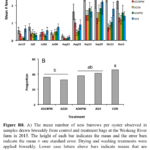 was much lower at the Weskeag River farm compared to what we observed at the Bagaduce River farm in 2014 and 2015 (Fig. R8B).
was much lower at the Weskeag River farm compared to what we observed at the Bagaduce River farm in 2014 and 2015 (Fig. R8B).
Due to corruption of the electronic file containing the data from the Damariscotta River farm experiments we are unable to present an analysis of our observations. Unfortunately, we were unable to re-enter all of the data from the original notebooks by the deadline for this report. We plan to submit an amended report as soon as we can rebuild the dataset.
Oyster Growth as a Function of Blister Worm Treatment - In general, the drying and washing treatments we applied to control blister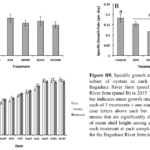 worm settlement had little impact on oyster growth (Fig. R9). Growth rates over the summer were very similar among oysters in the control and experimental treatments at the Bagaduce River farm in 2015. There was more variability in growth rate among oysters in the different treatments at the Weskeag River farm in 2015. However, the only significantly different difference in mean growth rate that we observed was between that for oysters washed and dried for 24 h every other week relative to the mean growth rate for oysters in the control bags. Similarly, we found no statistical difference in the average shell height of oysters in the control oysters compared to those air-dried on a weekly or biweekly basis at the Bagaduce River farm in 2016.
worm settlement had little impact on oyster growth (Fig. R9). Growth rates over the summer were very similar among oysters in the control and experimental treatments at the Bagaduce River farm in 2015. There was more variability in growth rate among oysters in the different treatments at the Weskeag River farm in 2015. However, the only significantly different difference in mean growth rate that we observed was between that for oysters washed and dried for 24 h every other week relative to the mean growth rate for oysters in the control bags. Similarly, we found no statistical difference in the average shell height of oysters in the control oysters compared to those air-dried on a weekly or biweekly basis at the Bagaduce River farm in 2016.
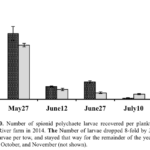 Identification of Blister Worm Larvae - We recovered over 250 larvae with morphological features resembling those of typical spionid (P. websteri is in the family Spionidae) larvae. The majority of these were recovered from the plankton tows taken in May 2014 (Fig. R10); as the season progressed the number of spionid larvae in the tows declined to well below ten per tow. In addition to P. websteri, larvae of the congener P. cornuta can be locally abundant in estuaries along the Atlantic coast. Differentiating the larvae of P. websteri and P. cornuta relies on subtle differences in shape and patterns of pigmentation. Unfortunately, patterns of pigmentation do not preserve well, particularly when using ethanol as the preservative, and we confidently identified < 5% to species using morphological analyses. We successfully isolated DNA and amplified a portion of the mtCO1 gene using conserved for roughly 70% (65 out of 91 attempts) of the larvae tested. The restriction fragment assay identified all 65 as the congener, P. cornuta.
Identification of Blister Worm Larvae - We recovered over 250 larvae with morphological features resembling those of typical spionid (P. websteri is in the family Spionidae) larvae. The majority of these were recovered from the plankton tows taken in May 2014 (Fig. R10); as the season progressed the number of spionid larvae in the tows declined to well below ten per tow. In addition to P. websteri, larvae of the congener P. cornuta can be locally abundant in estuaries along the Atlantic coast. Differentiating the larvae of P. websteri and P. cornuta relies on subtle differences in shape and patterns of pigmentation. Unfortunately, patterns of pigmentation do not preserve well, particularly when using ethanol as the preservative, and we confidently identified < 5% to species using morphological analyses. We successfully isolated DNA and amplified a portion of the mtCO1 gene using conserved for roughly 70% (65 out of 91 attempts) of the larvae tested. The restriction fragment assay identified all 65 as the congener, P. cornuta.
Preventative Treatments for Blister Worm - Our results indicate that air-drying is an effective way to reduce settlement of new blister worms in oysters and to also reduce the number of oysters infested (prevalence) on farms utilizing surface culture methodologies. Washing mud and detritus from the surface of the oysters on a regular basis also helped to reduce the load of blister worms, but the effect was relatively minor compared to just air-drying. We found only a weak effect of air-drying and washing treatments on oyster growth, even when drying conducted weekly. Even so, growers applying preventative measures will have to track the relative performance of oysters under the unique conditions that occur at individual farms. One key observation from our research is that to prevent the settlement of new blister worms oysters do not have to be exposed for long periods of time. Rather, the drying needs to be effective. This means drying oysters when air temperatures are worm, humidity is low and there is some wind to increase the rate of evaporation from the surface of the oyster shell. Given this combination, it is likely that drying is highly dependent on the density of oysters within each culture system. If the oysters are packed too tightly, those in the middle, surrounded by other oysters may not completely dry. Similarly, growers will need to carefully control the abundance of other forms of fouling which may impact the rate of drying. Anecdotally, we often observed heavy settlement of filamentous green algae (Enteromorpha sp.) and tunicates (Ciona intestinalis) at the same time that new blister worm burrows increased at the Bagaduce River farm. Whether they are just temporally correlated or the settlement and growth of the fouling community decreased the effectiveness of air-drying remains undetermined. Future work should focus on the effects of oyster size and density, and the timing of settlement by other components of the fouling community on the effectiveness of drying, along with ways to apply preventative treatments on farms not utilizing surface culture.
Identification of Blister Worm Larvae – Given the presence of multiple species and the subtle differences by which the larvae of these species are distinguished, we feel that it may be unrealistic for growers to track the abundance of blister worm larvae by plankton tows and morphological identification. We had hoped to be able to utilize DNA-based methods to identify larvae, as well. However, our PCR reactions had a higher than expected failure rate and only identified the congener, P. cornuta, in plankton samples.
There are several potential explanations for our results. First, the 26 PCR “failures” may be due to poor DNA recovery. While this is always a concern given the small amount of tissue available in larvae that were as small as 200 – 350 μm in length, the 30% failure rate is high when compared to our work with bivalve larvae. Second, the 26 PCR “failures” may have occurred at the PCR level. Other researchers have reported difficulty obtaining reliable PCR amplification of mtCO1 from spionid polychaetes when using conserved primer approaches. We, too, often see PCR failures even attempting to amplify mtCO1 from DNA isolated from adult P. websteri. To address this, we redesigned the PCR primers used in our assay based on an alignment of mtCO1 sequences from Genbank for several species in the genus Polydora. The new primers had a much higher success rate (~98%) compared to the conserved primers when used with adult P. websteri DNA. We then used the new primers to investigate the levels of genetic variation among P. websteri populations, worldwide. Our results, published in the journal Aquaculture Environment Interactions (https://doi.org/10.3354/aei00281), found very low levels of genetic variation at the mtCO1 gene, even when the worms were sampled from locations in different ocean basins. We, thus, feel confident that our new primers should have more success when amplifying the DNA from larvae if the failures were due to primer mismatch. As yet, we have not had a chance to revisit this possibility.
The third possibility is that the conserved PCR primers failed for 26 of the larval samples because they were neither P. websteri nor P. cornuta. In working with nearly a dozen oyster growers from Cape Cod, MA to eastern Maine we have found evidence of two other shell-boring polychaetes, tentatively identified as P. haswelli and P. onagawaensis, in the region. We first identified these species using burrow and whole worm morphology and sequencing of a portion of the nuclear 18S rRNA gene. However, we have had much more difficulty amplifying mtCO1 products from the DNA of these two species using either the conserved primers or our redesigned P. websteri primers. In order to amplify mtCO1 reliably from P. haswelli and P. onagawaensis we have undertaken yet another primer redesign. As yet we have not had an opportunity to explore the utility of these primers. Finally, there is the possibility that P. websteri larvae spend less time in the plankton than was originally reported in the literature. Several recent reports on blister worm species in other parts of the world (e.g., Simon 2015) have proposed that blister worm species may have a variable reproductive mode. These reports suggest that they switch from producing dispersive, planktotrophic larvae at low population densities to producing adelphophagic larvae that spend little time in the plankton before being competent to settle. As our project has come to a close, we have not resolved these questions.
Two recent advances in molecular biology may help with the identification of blister worm larvae in the field. These advances include real-time, on-site, hand-held PCR devices and the development of eDNA (environmental DNA) methods which identify the presence of DNA from target species in environmental samples (e.g. water or sediments). However, the resources needed to employ eDNA approaches to monitor larval abundance are beyond what would be required to conduct the basic PCR assays we have designed, which themselves are beyond what oyster growers are likely to have available.
There is, however, a simpler alternative by which growers can track the initial settlement of blister worms on their farms in order to better time the application of drying and washing treatments. As shown in figure R3, as recently settled blister worms begin to excavate a burrow in the host shell it quickly takes on a distinct u-shape that does not occur among other members of the fouling community on oyster shells. By shucking oysters and backlighting the shell, these new burrows are readily visible and farmers can track the timing of blister worm settlement. We have regularly employed this method in our research and found that the timing of the appearance of new burrows is fairly consistent from year to year on the Bagaduce River. Farmers can apply the same approach to determine the phenology of blister worm settlement on their farms to better focus the timing of preventative treatments.
Education
Our educational approach to increasing knowledge of and methods for controlling blister worm was multifaceted.
Fact Sheet – Our activities included communication of basic biological information on blister worm such as our blister worm factsheet (Milestone 5, distributed September 2015). Historically, there has been much misconception about blister worms, their biology, and their impact on oyster culture (and the culture of other shellfish). With support from the Maine Sea Grant College Program and the University of Maine Cooperative Extension Program, we produced an eight page color brochure to clarify and combat some of these misconceptions. Some of the key topics covered in this fact sheet were: how blister worms invade the host shell, a detailed description of the pest species and its life history, and a description of the multitude of attempts aquaculturists have used to combat blister worm outbreaks (mostly targeting adult worms). A key message conveyed in this Fact Sheet was the need to consider blister worm worthy of inclusion in biosecurity plans in order to minimize the inadvertent introduction of the pest to farms. This fact sheet was distributed to industry members on-line and is available at https://www.seagrant.umaine.edu/files/Dana%20Morse/PolydoraFactSheet_Web_101515.pdf.
Growers Survey - A major component of our educational approach was a survey of oyster growers in the Northeast assessing the impact of blister worm on their farms (Milestones 1 &2). We distributed this survey on-line to oyster producers from Maine to Virginia, using the list serve of the East Coast Shellfish Growers Association and email lists from extension contacts. The goals of the survey were to understand the breadth and types of problems caused by blister worm, including whether there are any associations between the occurrence and severity of blister worm infestations and culture methodologies and local environmental factors. We also gathered information on the success associated with any farm management measures growers have employed against blister worm. Over a series of 31 questions, we asked growers and producers for information in the following general areas:
- Farm location and physical attributes (such as salinity, temperature, depth, substrate type)
- Production systems employed by growers
- Grower observations of the frequency/intensity of blister worm infestations
- Producers’ level of concern about blister worm
- Potential or realized impacts of blister worm infestations
- Effectiveness of various remedies and their relative costs/benefits
- Information growers need to address the problem
The following is a shortened summary of the results from this survey:
Farm Site Characteristics: A total of fifty-six responses were received from growers in nine states with the bulk of responses (50%) coming from 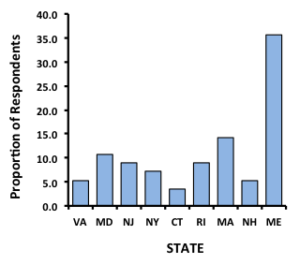 Maine and Massachusetts. Roughly 86% of growers categorized their farms as being located in tidal rivers or bays, with 5% or less indicating their operation was located on a salt pond, marsh or tidal creek. The median depth of farms ranged from 3 to 8 feet at low and high tide, respectively, with over 70% of farms reported as being subtidal. Salinity ranged from 12-13 to 32 ppt; generally, salinity increased with increasing latitude, although farms from CT to ME had very similar and high salinities. Average minimum winter water temperatures were relative similar across the nine states (range 31 to 36°F) while there was a noticeable gradient in average maximum summer water temperatures with highest values (86°F) among farms in New Jersey and lowest values (72°F) for Maine farms. The majority of growers described their farms as having intermediate to strong tidal exchange and tidal currents (79%) while fewer than 5% indicated their farms have weak or extremely strong exchange, indicating that growers prefer sites with flow rates on the stronger end of the spectrum. Thirty-five growers reported that the substrate under their farm was soft mud or mud mixed with sand or gravel with only a few farmers indicating that their farm had cobble, hard pack, shell hash or sea grass substrates.
Maine and Massachusetts. Roughly 86% of growers categorized their farms as being located in tidal rivers or bays, with 5% or less indicating their operation was located on a salt pond, marsh or tidal creek. The median depth of farms ranged from 3 to 8 feet at low and high tide, respectively, with over 70% of farms reported as being subtidal. Salinity ranged from 12-13 to 32 ppt; generally, salinity increased with increasing latitude, although farms from CT to ME had very similar and high salinities. Average minimum winter water temperatures were relative similar across the nine states (range 31 to 36°F) while there was a noticeable gradient in average maximum summer water temperatures with highest values (86°F) among farms in New Jersey and lowest values (72°F) for Maine farms. The majority of growers described their farms as having intermediate to strong tidal exchange and tidal currents (79%) while fewer than 5% indicated their farms have weak or extremely strong exchange, indicating that growers prefer sites with flow rates on the stronger end of the spectrum. Thirty-five growers reported that the substrate under their farm was soft mud or mud mixed with sand or gravel with only a few farmers indicating that their farm had cobble, hard pack, shell hash or sea grass substrates.
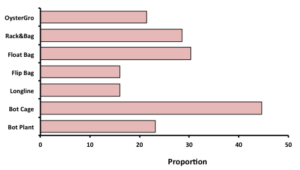 The most common gear type employed among the 56 respondents was some form of bottom cage, followed by rack and bag systems or surface floating cages. Among this latter group roughly 15% use either surface flip bags or caging systems attached to longlines. An appreciable number of growers have adopted Oyster-Gro* (or similar sink/flip cage systems) while a full 22% deploy oysters directly on the bottom (no cage). Twenty-six farmers indicated that they use multiple types of grow-out systems on their farm while the remaining 30 reported only a single grow-out system.
The most common gear type employed among the 56 respondents was some form of bottom cage, followed by rack and bag systems or surface floating cages. Among this latter group roughly 15% use either surface flip bags or caging systems attached to longlines. An appreciable number of growers have adopted Oyster-Gro* (or similar sink/flip cage systems) while a full 22% deploy oysters directly on the bottom (no cage). Twenty-six farmers indicated that they use multiple types of grow-out systems on their farm while the remaining 30 reported only a single grow-out system.
Occurrence, Prevalence, and Intensity of Blister Worm: Among responding growers, 90% indicated that they check for blister worm when they harvest their oysters and approximately 70% responded that they check more often or continuously. The majority (37) of the 56 respondents indicated they have at some time observed blister 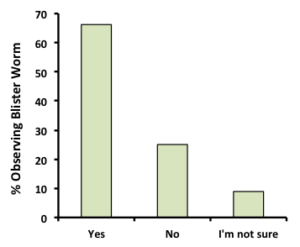 worm on their farm (see figure, at left), although we recognize that growers battling blister worm may have been more likely to respond to our survey. Among those who have observed worm, nearly 70% see it every year with 15 to 19% indicating they see blister worm often (but not every year), or rarely, respectively. The majority (55%) of respondents who observed blister worm on their farms indicate it impacts less than 25% of their crop while roughly 12-18% of respondents indicated that higher proportions of their crop are affected. The intensity of blister worm infestations (number of worms per oyster) reported by growers participating in our survey was light to intermediate. Over 70% reported light infections (1-2 worms per oyster), 13% reported intermediate infections (4-5 worms per oyster) while roughly 5% indicated that the intensity of blister worm infestations were heavy (up to 10 per oyster) or at panic stage (>10 worms per oyster). Overall, these results suggest that blister worm is relatively common on northeastern oyster farms, but the frequency of occurrence, number of oysters impacted (prevalence), and number of worms per oyster (intensity) are relatively low.
worm on their farm (see figure, at left), although we recognize that growers battling blister worm may have been more likely to respond to our survey. Among those who have observed worm, nearly 70% see it every year with 15 to 19% indicating they see blister worm often (but not every year), or rarely, respectively. The majority (55%) of respondents who observed blister worm on their farms indicate it impacts less than 25% of their crop while roughly 12-18% of respondents indicated that higher proportions of their crop are affected. The intensity of blister worm infestations (number of worms per oyster) reported by growers participating in our survey was light to intermediate. Over 70% reported light infections (1-2 worms per oyster), 13% reported intermediate infections (4-5 worms per oyster) while roughly 5% indicated that the intensity of blister worm infestations were heavy (up to 10 per oyster) or at panic stage (>10 worms per oyster). Overall, these results suggest that blister worm is relatively common on northeastern oyster farms, but the frequency of occurrence, number of oysters impacted (prevalence), and number of worms per oyster (intensity) are relatively low.
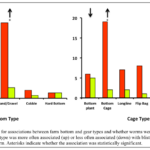 We analyzed the growers’ responses to our survey for correlations between the physical setting and gear type used by growers, with the occurrence, prevalence, and intensity of blister worms on their farms. Overall, the relatively small number of farmers responding to our survey limited our ability to statistically detect such associations. However, there were several notable strong effects associated with substrate and gear types. Of the bottom types reported by growers (soft mud, firm mud, sand/gravel, cobble, hard bottom), farms with a sand/gravel bottom were 8-fold more likely to have blister worm than farms with the other bottom types. Farms over soft mud were twice as likely to have blister worm than those with other bottom types, although this association was not statistically significant (at left, above). There were also associations between gear type and observation of blister worm on northeastern oyster farms. The use of bottom cages and rack and bag were both associated with a 7-fold higher risk of blister worm (both associations were statistically significant). In contrast, a much lower proportion of growers bottom planting their oysters or using Oyster-Gro type cage systems reported blister worm problems (55% and 45%, respectively; at right, above). While both of these latter culture approaches resulted in a decreased risk of blister worm, only the association with Oyster-Gro systems was statistically significant. Other significant associations we detected included:
We analyzed the growers’ responses to our survey for correlations between the physical setting and gear type used by growers, with the occurrence, prevalence, and intensity of blister worms on their farms. Overall, the relatively small number of farmers responding to our survey limited our ability to statistically detect such associations. However, there were several notable strong effects associated with substrate and gear types. Of the bottom types reported by growers (soft mud, firm mud, sand/gravel, cobble, hard bottom), farms with a sand/gravel bottom were 8-fold more likely to have blister worm than farms with the other bottom types. Farms over soft mud were twice as likely to have blister worm than those with other bottom types, although this association was not statistically significant (at left, above). There were also associations between gear type and observation of blister worm on northeastern oyster farms. The use of bottom cages and rack and bag were both associated with a 7-fold higher risk of blister worm (both associations were statistically significant). In contrast, a much lower proportion of growers bottom planting their oysters or using Oyster-Gro type cage systems reported blister worm problems (55% and 45%, respectively; at right, above). While both of these latter culture approaches resulted in a decreased risk of blister worm, only the association with Oyster-Gro systems was statistically significant. Other significant associations we detected included:
- Longline and Oyster Gro-like systems were associated with a decrease in worm prevalence,
- Low Salinity was associated with a decrease in prevalence and intensity,
- Maximum Temperature was associated with an increase in intensity.
Observations and Concerns: Over 70% of the growers responding to the survey indicated that they had at least some concern about blister 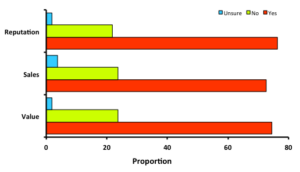 worms in their crop, or within their region. Just under 27% of respondents felt that the problem was not a concern. Though most producers felt some level of concern, a clear majority had not seen impacts to their sales numbers or perceived impacts to their reputation; only 10 of 56 replies indicated that they had received comments from customers regarding blister worms. Still, nearly 75% of respondents expressed concern for potential negative impacts to reputation, sales, or product value (at right), and over 90% of respondents said that they check their oysters for blister worms at least at harvest time, if not earlier. Only 10% of respondents indicated that they do not check for blister worms. Some comments on this issue were:
worms in their crop, or within their region. Just under 27% of respondents felt that the problem was not a concern. Though most producers felt some level of concern, a clear majority had not seen impacts to their sales numbers or perceived impacts to their reputation; only 10 of 56 replies indicated that they had received comments from customers regarding blister worms. Still, nearly 75% of respondents expressed concern for potential negative impacts to reputation, sales, or product value (at right), and over 90% of respondents said that they check their oysters for blister worms at least at harvest time, if not earlier. Only 10% of respondents indicated that they do not check for blister worms. Some comments on this issue were:
“Wholesaler asked what the blisters are but seemed unconcerned.”
“Too brittle and the presence - customers don’t like the worms. They are unsightly and cause a percentage to be discarded.”
“We have had only a couple mentions of mud blisters from customers, but I as an oyster farmer CLEARLY see the mud blisters are not appealing and appear unsightly in a shucked oyster. While the meat quality of our oysters is not affected, the appearance certainly is. You know the saying, ‘you eat with your eyes first, then your mouth.’ If they do not look nice, you will likely have a less enjoyable experience eating it.”
“Weakness of the shell.”
"Questions raised by one chef regarding discoloration caused by the blister worms.”
“Appearance and [whether oysters were] healthy to eat.”
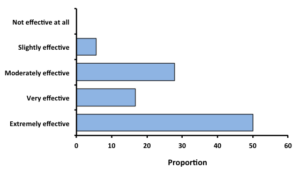 Treatments and Efficacy: When asked, 18 of 56 respondents (32%) indicated that they had tried some form of control against blister worms. The most common treatments were: freshwater or hypersaline dip, air-drying, refrigeration, bottom planting in high-flow areas, or 'working the bottom harder,' meaning increased harvesting or some sort of mechanical disturbance. In many cases, combinations of techniques were used, such as a dip followed by air-drying. Of those growers who have tried some treatment, two-thirds indicated that their treatment was either 'very effective' or 'extremely effective.' Growers were more inclined to share their treatment methods if they found them to be effective. Refrigeration and brining were generally seen as most effective, but labor costs and incidental mortality to the oysters were given as limiting factors.
Treatments and Efficacy: When asked, 18 of 56 respondents (32%) indicated that they had tried some form of control against blister worms. The most common treatments were: freshwater or hypersaline dip, air-drying, refrigeration, bottom planting in high-flow areas, or 'working the bottom harder,' meaning increased harvesting or some sort of mechanical disturbance. In many cases, combinations of techniques were used, such as a dip followed by air-drying. Of those growers who have tried some treatment, two-thirds indicated that their treatment was either 'very effective' or 'extremely effective.' Growers were more inclined to share their treatment methods if they found them to be effective. Refrigeration and brining were generally seen as most effective, but labor costs and incidental mortality to the oysters were given as limiting factors.
Information Gaps and Engagement: When asked what information was of most interest to them, producers indicated they were most interested in learning more about treatments: how to successfully treat against blister worm. Learning more about the biology of the animal was next highest (especially when applying that knowledge to inform husbandry and to reduce or avoid infestations), followed by effects of worms on the oysters themselves and lastly to learn about effects of blister worms in the marketplace. Producers listed the following methods of learning more, in order of preference: email, grower meetings, videos, web-based content, print documents, and conferences.
A formatted version of this summary along with the much longer 27 page Full Report, containing complete information on the grower responses to each question in the survey and the statistical analyses conducted on those responses, are available upon request from prawson@maine.edu.
Communication and Sharing of Results – We have worked to regularly and consistently disseminate results and findings from both our research and the grower survey with industry members, particularly those who responded to our survey and those who have had a direct role in the work under this award. These efforts include presentations at the Northeast Aquaculture Conference and Exposition (NACE) in January 2017 (Milestone 5) during which we provided updates on the field research and a preliminary analysis of the survey returns. Each of these presentations provided growers and opportunity to respond and to further shape the direction of our investigations. In addition to NACE, we presented results of our work on preventative treatments and findings of the growers survey at the World Oyster Symposium (2015) and the Annual Meeting of the National Shellfisheries Association (NSA: 2015 to 2018), and the results of our work on blister worm genetics at the Society for Ingretative and Comparative Biology (2018) and Annual Meeting of the NSA (2016 and 2017). By far, the broadest participation among industry members occurs at the NACE meetings followed by the NSA meetings.
We originally proposed to set up regular email or web-based communication with the growers participating in our survey (Milestone 4). We were not as successful as we had envisioned, having sent out only two email announcements to our survey respondents. A third email is planned in late January to communicate the summary report of the survey (above) and to summarize the comments from and discussions that took place at our Growers’ workshops in late 2018 (see below).
Milestone 8 of our original proposal was based on farmers attending a site visit to another farm employing preventative treatments targeting either adult or larval blister worms. We found that it was logistically difficult to organize appropriate dates for both attending and hosting growers, to participate in such site visits. During the off season the weather is not conducive and during the growing season time daylight hours are valuable for managing their farms. Thus, we opted to conduct a series of evening roundtable workshops. The first of these was held at the Freeport Community Library in Freeport, ME on October 11, 2018 and was attended by 8 participants. Joshua Reitsma and Diane Murphy of the Cape Cod Cooperative Extension hosted the second workshop, held in Barnstable, MA on October 25, 2018 and attended by 15 participants. The third workshop was hosted by Jay Baker and Michael Chambers at the UNH Judd Gregg Marine Research Complex on November 15, 2018 and was attended by 10 participants.
These workshops were organized around the results of the Grower Survey results. However, they concentrated on open discussions regarding the information attendees needed to apply the research findings to their individual circumstances and thereby develop pest management plans. At these meetings we advocated for growers creating biosecurity plans (for reducing risk) and making sure that protocols for minimizing the inadvertent movement of blister worm were a part of those biosecurity plans. In addition, growers were asked to identify the information and research gaps that we could address to help them be better informed about blister worm. A final, similarly organized workshop was held at the Northeast Aquaculture Conference and Exposition in Boston in January 2019 (Milestone 9). After the NACE meeting we sent a follow-up survey asking what information presented in the workshops was helpful and increased their knowledge of and treatments for blister worm, and whether, as a result, they had developed a management plan (Milestone 10).
Attendance at the four workshops that we offered exceeded 70 individuals of which 45 clearly indicated that they were growers. We restricted the distribution of the post-workshop survey to these 45 individuals. Of the 17 workshop participants who returned the follow-up survey, 15 indicated that they had gained new knowledge, awareness, skills or had a change in attitude about blister worm and treatments to control blister worm. Further, out of these 17 participants: 5 did not provide information about whether they have a control/treatment plan, 2 indicated they are new farmers and had not yet considered a blister worm management plan, 3 responded that the problem was not big enough to warrant a plan, 2 indicated they were developing a plan, 1 responded they did not have a plan but needed one, 4 have a plan or are trying a plan, although they did explicitly state that the plan was developed or adopted as a result of our project.
Milestones
Members of the East Coast Shellfish Growers Association receive updated online survey on the impact, prevalence and distribution of blister worm and invitation to participate in blister worm education program.
1000
56
September 30, 2014
Completed
September 30, 2016
Survey was distributed to industry members although implementation was delayed while we addressed concerns expressed by external reviewers of a draft of the survey and completed IRB clearance/certification.
Oyster growers return survey and express interesting in joining education program.
100
56
February 28, 2015
Completed
November 30, 2017
We received 56 completed surveys as of December 31, 2016. All respondents indicated an interest in blister worm education program with 37 expressing a high level of interest.
Research component of project testing preventative treatments for blister worm established at two oyster farms in Maine. Workers at these farms receive training in monitoring for blister worm larval abundance (plankton sampling), settlement, and reproduction.
2
3
April 30, 2015
Completed
October 10, 2017
Together with direct industry participation, we conducted field research on preventative treatments at three Maine farms between May and November 2015.
Participants in blister worm education program receive regular email/blog updates on project progress and efficacy of preventative treatments.
100
56
October 31, 2015
In Progress
Given the delay in implementation of our survey, we did not compile our education program participant list until December 2016. We have communicated periodically with participants by a number of means, including email and web communications, although not as regularly as we had originally planned. The final communication will be sent out after the completion of the last workshop at the NACE meeting in January of 2019.
Farmers (industry participants) attend first workshop at the Milford Aquaculture Seminar on blister worm biology, reproduction and settlement, receive update of project goals and progress and begin development of best management plans for blister worm prevention.
40
25
February 29, 2016
Completed
January 31, 2017
The Milford Aquaculture Seminar has been combined with the Northeastern Aquaculture Conference and Exposition (NACE). We presented the results of our field research and our online survey on blister worm at the NACE meeting in January 2017. We also presented our work at the Annual Meeting of the National Shellfisheries Association in March 2017.
Second year of activities testing preventative treatments for blister worm established at two oyster farms in Maine.
2
1
April 30, 2016
Completed
October 10, 2017
We initiated an additional experiment at one farm in Maine in May 2016. This work was conducted at only one of the three farms because one of the three original farms adopted the preventative treatments while the third could not obtain unaffected oysters for an additional trial. This work included overwintering trials and continued monitoring of blister worm through the 2017 growing season at the request of the participating farmer.
Fact Sheet on blister worm biology, reproduction, and population dynamics, and preventative treatments for blister worm distributed to members of the East Coast Shellfish Growers Association.
100
100
May 31, 2016
Completed
September 30, 2015
Fact Sheet posted September 2015. PolydoraFactSheet.
Farmers attend site visit at either of our two grower-participant farms to receive hands on training in blister worm prevention.
60
33
July 31, 2016
Completed
November 15, 2018
Given survey responses we felt that farmers were more likely to attend and participate in local workshops. It was also logistically difficult for us to organize appropriate dates for both attending and hosting growers, to participate in field site visits. Thus, instead of site visits we conducted three local workshops, one in Freeport, ME, one in Barnstable, MA and one in New Castle, NH.
Additional farmers will attend second workshop at the Northeastern Aquaculture Conference and Exposition on blister worm biology, reproduction and settlement, receive update of project goals and progress and begin development of best management plans for blister worm prevention.
40
37
December 31, 2016
Completed
January 09, 2019
We presented a second workshop at the NACE in January 2019 that was attended by 37 participants including 19 growers. There was lively discussion among the participants about the efficacy of blister worm treatments and support for the development of management plans for blister worm control.
Farmers utilize educational opportunities, outreach materials or site visits to develop, adapt and adopt best management protocols resulting in reduction of blister worm impacts on market viability of oysters.
20
17
October 31, 2017
Completed
January 31, 2019
Of the 17 workshop participants who responded to post-workshop questionnaires, 5 did not provide information about whether they have a control/treatment plan, 2 indicated they are new farmers, 3 responded that the problem was not big enough to warrant a plan, 2 indicated they were developing a plan, 1 responded they did not have a plan but needed one, 4 have a plan or are trying a plan, although it is not clear their plan was adopted as a result of our project.
Milestone Activities and Participation Summary
Educational activities:
Participation Summary:
Learning Outcomes
Three growers reported increased knowledge or awareness of blister worm biology as a result of direct participation in our research program. Out of 45 growers who attended the blister worm workshops that we presented from October 2018 to January 2019, 17 responded to our post-workshop questionnaire. Of these 15 indicated that they had gained new knowledge, awareness, skills or had a change in attitude about blister worm and treatments to control blister worm.
Performance Target Outcomes
Target #1
20
Develop, adapt and adopt best management protocols resulting in reduction of blister worm impacts on market viability of oysters
Up to $4 million in aggregate sales could be impacted by lost sales.
Reduction in pest prevalence and incidence will result in improved crop quality.
6
One grower began using Oyster-Gro cage systems for regular drying of oysters to reduce impact of not only blister worm but other shell-fouling species as a result of participating in our research program. Among the 17 growers attending one of our workshops AND responding to our post-workshop questionnaire, 5 did not provide information about whether they have a control/treatment plan, 2 indicated they are new farmers, 3 responded that the problem was not big enough to warrant a plan, 2 indicated they were developing a plan, 1 responded they did not have a plan but needed one, and 4 have a plan or are trying a plan.
Growers did not indicate whether production or sales had been lost to blister worm and thus, it is impossible to determine whether the adoption of blister worm management plans protected current production or restored lost production. However, from both our larger scale survey of growers, discussions during the workshops, and responses to our post-workshop questionnaire, it is evident that growers remain concerned about the impact of blister worm on their sales and farm reputation. This concern is likely to increase as oyster production and competition for sales increases.
Single farmer adopting system to date has reported highly reduced level of blister worm infestation.
See above.