Final report for LS21-357
Project Information
Many Southern farmers/ranchers are seeking ways to improve health from the ground up. One way they are doing that is by using pasture-based livestock systems to enhance the health of soils, animals, and presumably that of consumers. Beef, irrespective of rearing practices, provides many essential nutrients such as bioavailable protein, zinc, iron, and B12. Emerging data indicate that raising livestock on pasture concentrates additional health-promoting metabolites—vitamins/minerals, terpenoids, phenols, carotenoids, antioxidants, peptides, and other bioactive compounds—compared to meat from animals fed grain-based diets in conventional systems. Preliminary studies also show potential for additional anti-inflammatory effects in people after eating pasture-raised meat.
While the connections among soil-plant-beef-consumer health are often touted for why grass-fed beef has additional health benefits, no studies have systematically tested these links using a bottom-up approach. To bridge this gap, we have forged a consortium of pasture-based farmers and interdisciplinary researchers from leading agricultural (NC State University) and medical (Duke University) schools.
Using a systems approach, we will compare forage and soil metabolomes of grazed pastures in three regional grass-fed beef systems to total mixed rations and soil samples from a grain-fed beef system (Objective 1). Second, we will compare health-promoting compounds in grass-fed beef from three regional systems to grain-fed beef (Objective 2). We will then compare metabolite and inflammatory profiles of adults consuming grass-fed versus grain-fed beef to provide insight into consumer health (Objective 3). For objective 3, we propose a randomized-crossover design where participants consume each source of beef (grass-fed vs grain-fed) during separate test-days. Blood samples will be obtained before and several hours after. We hypothesize that grass-fed beef results in higher amounts of health-promoting metabolites—terpenoids, phenolics, and other antioxidants—in the metabolome of consumers and induces additional anti-inflammatory effects compared to grain-fed beef.
Metabolomics is the biological marker common to all three objectives to link shared metabolites from soil, to forage/feed, to meat, and into the body of consumers; an approach we describe as ‘from Farm to Table to Us’. The proposed study will provide a critical, and largely unstudied, link between land ecology, livestock systems, and human health—key priority areas of SARE’s Systems Research for Agriculture. Farmers will have crucial roles by assisting with sample collection, monitoring and reporting on cattle grazing habits, linking research findings back to pasture botanical composition, and disseminating findings to peers.
Findings will be shared (Objective 4) with producers, consumers, and stakeholders through NC Cooperative Extension (e.g., Amazing Grazing/NC Choices), Build-Up Dietitians, the Appalachian Sustainable Agriculture Project, Understanding Ag, and farmer cooperators (workshops/farm tours). Findings will be presented at local meetings (e.g., Carolina Meat Conference) and published in scientific journals, farm press, and social media outlets. Our work is vital to Southern pasture-based producers who work hard to nourish local communities, but lack scientific evidence on the healthfulness of their beef. Having such justification can result in tremendous “brand building” opportunities for local pasture-based producers to improve economic viability whilst enhancing human and environmental health.
Our project includes the following four objectives:
Objective 1: Compare forage and soil metabolomes of grazed pastures in three regional grass-fed beef systems to total mixed rations and soil samples from a grain-fed beef system.
Hypothesis 1a: The forage consumed by grass-fed cattle will contain higher amounts and a wider variety of phytochemicals—terpenoids, phenols, carotenoids, amines, and other organic acids— compared to total mixed rations (corn and hay) consumed by grain-fed cattle.
Hypothesis 1b: The phytochemical richness of forage and feed (TMR), as determined by metabolomics, is correlated with nutrient levels and metabolomes of the soils.
Objective 2: Compare the presence of health-promoting compounds in grass-fed beef from three regional systems to grain-fed beef.
Hypothesis 2a: Grass-fed beef contains higher amounts and a wider variety of health-promoting metabolites—terpenoids, phenols, carotenoids, phenols, peptides, unsaturated fatty acids, and other bioactive compounds—compared to grain-fed beef.
Hypothesis 2b: The metabolomes of grass-fed and grain-fed beef correlate with the metabolome profiles of consumed forage/feed by cattle.
Objective 3: Compare metabolite and inflammatory profiles of adults consuming grass-fed versus grain-fed beef to provide insight into their impact on consumer health.
Hypothesis 3a: Consuming grass-fed beef provides the body with higher amounts and a wider variety of health-promoting metabolites— terpenoids, phenols, carotenoids, phenols, peptides, unsaturated fatty acids, and other bioactive compounds—and induces anti-inflammatory effects compared to eating grain-fed beef during the postprandial period (i.e., for several hours after consumption).
Hypothesis 3b: The post-meal metabolomes of consumers correlate with the metabolomes of grass-fed and grain-fed beef.
Objective 4: Develop outreach and education efforts to translate findings to farmers, consumers, and industry through Extension programs, and regional farmer and consumer partnerships.
Expected outcomes from this project are that:
1) soil nutrient levels and metabolomes of grazed pastures and row-crop fields relate to the phytochemical richness of forage/feed consumed by grass-fed cattle and grain-fed cattle—an expectation solidly supported by work linking soil health to the phytochemical richness of plants/crops (Rajashekar et al., 2009;Reeve et al., 2016);
2) greater phytochemical richness of forage consumed by pasture-raised cattle will increase the phytochemical richness of beef compared to grain-finished animals—an expectation solidly supported by previous work from our group (Van Vliet et al., 2020a;Van Vliet et al., 2020c) and others (Larick et al., 1987;Agabriel et al., 2007);
3) these biochemicals will become available in the plasma of consumers within hours after consumption and share a resemblance to the metabolome profiles of the ingested food source (grass-fed vs grain-fed beef)—an expectation solidly supported by work from us (van Vliet et al., 2017;Van Vliet et al., 2019) and others (Pimentel et al., 2018);
4) higher post-meal circulating levels of phytochemicals/biochemicals in the plasma of grass-fed beef consumers will induce acute anti-inflammatory effects—an expectation solidly supported by previous studies on meat consumption and acute changes in inflammatory markers (Arya et al., 2010;Li et al., 2010). For example, comparable work by Pimentel et al. (2018) found that acute changes in the circulating metabolome after single meals of yogurt or milk (both from grain-fed systems) already reflected many of the changes that were observed with chronic (2-week intake) intake of yogurt or milk, respectively. While acute findings will need to be followed up with temporally longer trials, it will be critical to obtain preliminary insight into potential human health responses of grass-fed vs grain-fed beef and to establish a ‘proof of principle’ of the transfer of phytochemicals from forage into beef and the consumer before moving on to temporally longer studies.
Metabolomics has a high potential to contribute to sustainable agriculture by providing a deep understanding of linkages between soil health, agricultural production practices, the nutrient densities of foods, and their impact on human metabolism. We expect this project to result in data that can inform management practices that emphasize finishing cattle on diverse pastures to enhance the phytochemical richness of beef and consumer health outcomes. We expect that metabolomics will reveal that, despite differences in management strategies on the three pasture-based farms, high-quality forage ingestion by pasture-raised cattle with result in phytochemically-rich beef that is distinct from the feedlot finished beef, but similar to each other.
Recent research with forage-based beef production systems in LA, SC, NC, and GA showed consistency in performance and carcass quality between pasture-raised beef produced from systems provided a high level of forage management was maintained (Poore et al., 2020). Our data is expected to reinforce the notion that, as long as management practices stimulate soil health and forage quality, practices can be tailored to the individual farm (i.e., multiple pasture-based production methods can lead to nutrient-dense beef). Evidence on high-quality beef from different pasture-based management practices is important for bringing local grass-fed beef with consistent quality to wholesale markets.
The objectives and expected outcomes of this project are solidly in line with Southern SARE’s program objectives, which are based on the 1990 Farm Bill. First, we expect our results to enhance understanding of linkages between soil health, livestock production systems, and human health, which is in line with Priorities #1; “Satisfy human food and fiber needs” and #3; “Make the most efficient use of nonrenewable resources and on-farm resources and integrate, where appropriate, natural biological cycles and controls”. Second, our work will generate results directly usable by consumers and producers, which can help Southern grass-fed beef producers improve profitability in a growing market and consumers enhance health, which is in line with Priorities # 4; “Sustain the economic viability of farm operations; and 5; “Enhance the quality of life for farmers and society as a whole”. Finally, by having an evidence-based justification on the healthfulness of grass-fed beef as a result of grazing nutrient-rich pastures we expect our findings will re-enforce Priority #3: “Enhance environmental quality and the natural resource base upon which the agricultural economy depends”.
Cooperators
- - Technical Advisor - Producer
- - Technical Advisor - Producer (Researcher)
- - Technical Advisor - Producer
Research
Description of sites (Objectives 1-2):
Sample collection (soil, forage, beef) occurs on the following participating farms.
Beaver Creek Farm (Mount Airy, NC): Utilizes rotational grazing practices on botanically diverse pastures, consisting of native warm-season grasses and warm and cool-season cover crops for grazing.
Hickory Nut Gap Farm (Fairview, NC): A 400 acre-farm located in the Blue Ridge Mountains that runs a multi-species livestock operation where animals are rotationally grazed.
BDA Farms (Uniontown, AL): A mixed plant farming-livestock farm that utilizes multi-species grazing with cattle, sheep, and chickens that are moved daily amongst pastures.
The grass-fed beef from participating farmers will be compared to grain-finished beef provided by Demkota Ranch Beef (Aberdeen, SD) and Austin Ranch (Asheville, NC). Midwest grain-finished beef will provide a “real-world” grain-fed control, as Midwest feedlot-beef is widely sold in grocery stores across the South (Poore et al., 2020), while the beef from Austin Ranch is from a smaller regional Southern feedlot.
Objective 1: Compare forage and soil metabolomes of grazed pastures in three regional grass-fed beef systems to total mixed rations and soil samples from a grain-fed beef system.
1.1 Soil collection: Soil samples will be collected from participating farms during the Summer of 2021, 60-30 days prior to slaughter of cattle. Collection is led by Dr. Alan Franzluebbers and Johnny Rogers and performed in collaboration with the farmers due to their knowledge of grazed pastures. Four grazed pastures on each cooperating farm will be sampled at 0-10-, 10-30-, and 30-60-cm depths. Two sets of samples will be collected from each pasture by randomly locating a site within a pasture and collecting five cores (one at center and four at 10-m distance from center in cardinal directions) that will be composited for each set. A stainless-steel push probe (4-cm diameter) will be used to extract the 0-10-cm depth and an auger will extract other depths. A total of 24 soil samples will be collected from each participating farm (4 fields x 2 subsamples x 3 depths) (Franzluebbers et al., 2019;Franzluebbers et al., 2020). The nearest by corn-fields (within 1 mile of each of the pastures) will be sampled in an identical fashion as the pastures and will serve as a model for how growing corn for commodity markets (including animal feed in finishing rations) will impact metrics of soil health. By simply adjecent corn fields, we minimize the impact of spatial factors on soil analysis.
1.2: Soil analysis: Soil conditions will be assessed with routine inorganic analyses and soil organic C and N fractions. Samples will be submitted to the NC Department of Agriculture and Consumer Services (NCDA&CS) for routine soil chemical analyses (P, K, Ca, Mg, S, Na, Mn, Cu, and Zn). Soil-test biological activity will be performed at the Soil Ecology and Management lab at NC State University. Briefly, a 50-g subsample will be incubated at 25 °C for 3 d after rewetting to 50% water-filled pore space and placed in a 1-L canning jar with 10 mL 1 M NaOH (Franzluebbers, 2016) after which particulate organic C and N will be determined. A separate subsample will be analyzed for C and N with dry combustion to determine total organic C and N (Franzluebbers et al., 2020). Soil properties among the farms will be tested for differences using t-Tests using all observations within a farm (Franzluebbers et al., 2020). Another soil subsample will be stored at -80°C for untargeted metabolomics as described in the Metabolomics subsection.
1.3 Forage collection: The top five plants consumed by cattle on the cooperating farms will be collected every 30 days for the last 100 days prior to slaughter; led by Dr. Matt Poore and Johnny Rogers, and performed in close collaboration with the farmers as they will know which fields have been grazed and need sampling. The forage consumed during the last 100 days prior to slaughter will mostly dictate the phytochemical composition of meat (Larick et al., 1987;Dian et al., 2007). Plant samples will be collected in conical tubes, freeze-dried, powdered, and stored at -80°C for metabolomics analysis. Feedlot TMR will be provided by Demkota, which sources their cattle and corn-based feed locally (within 50 miles). All samples (rations and forages) will be submitted for nutritional composition analysis by the NCDA&CS, while phytochemical composition will be determined using metabolomics described in the Metabolomics subsection.
1.4 Fecal collection: At the same time as plant samples are being collected, the farmers and research team will collect twelve fecal samples from cattle for DNA (fDNA) metabarcoding to quantify plant-species composition (%) of cattle’s diets (Scasta et al., 2019). Briefly, DNA will be extracted from fecal samples and amplified into amplicons using pairs of primers. Amplicons are sequenced and data will be bioinformatically processed to identify Operational Taxonomic Units (OTUs). OTUs will be referenced against a database to quantify plant species composition of cattle diets. Analyses will be performed by Jonah Ventures (https://jonahventures.com/plants/).
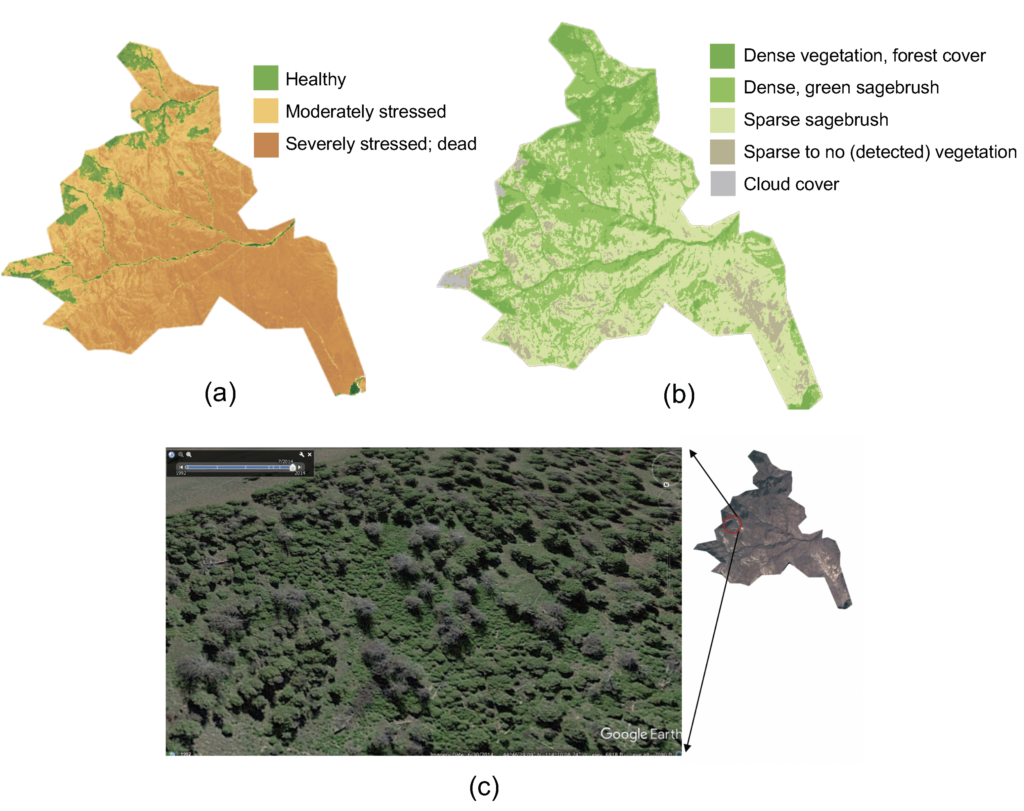
1.5 Forage classification: Farmers are asked to keep detailed records of the plants that the pasture-raised cattle had access to during the last 100 days before slaughter to complement fDNA data (Scasta et al., 2019). To assist farmer observations, an unpiloted aerial vehicle (UAV) will collect remotely sensed vegetation data (led by Dr. Sierra Young, certified Remote Pilot). A DJI Matrice 100 with a MicaSense Altum (5-band multispectral sensor with RGB, near-infrared (NIR), and red edge) will be deployed over selected grazed pastures. Flights will be conducted at each cooperating farm in coordination with plant and soil sampling. Both a calibration tarp and a minimum of 5 ground control points (four corners and center) will be captured each flight. Raw UAV images will be processed in Pix4dMapper to generate orthomosaic maps. NDVI will be calculated to determine total ground cover and provide an overall indicator of forage health using established methods (Ryan et al., 2012;Borowik et al., 2013). We will also use results from plant and soil sampling to develop a preliminary model for characterizing forage nutrient density, type, and health using reflectance data. To generate data for the model, annotated UAV images will be sent to farmers for ground-truthing the model inputs (Figure 3). Remote sensing will provide valuable information about forage availability and health, and kickstart the development of a forage assessment tool that can be further developed in the future and used by farmers.
Objective 2: Compare the presence of health-promoting compounds in grass-fed beef from three regional systems to grain-fed beef.
2.1. Beef collection: Ribeyes will be collected from 10 cattle, slaughtered during the summer of 2021, from all three cooperative farmers and the feedlot. Based on previous work it is expected that 46% of probed metabolites (167/353) are different (P≤ 0.05) between grass-fed and grain-fed beef (Carrillo et al., 2016); when we assume a P-value ≤ 0.05, Q-value ≤ 0.3, and N=10 cattle per farm is sufficient to provide true discovery rates ranging from 96-99 % assuming a fold difference of 1.1 or 1.25, respectively. The pasture-based farmers and the feedlot all raise/finish Black Angus breeds and ribeyes will be aged similarly for 14 days. Lean and fat portions of the ribeyes will be manipulated to ensure the fat content is similar among groups and confirmed using proximate analysis.
2.2. Beef analysis: A ribeye of each cattle (40 total; 10 from each farmer/feedlot) will be ground using a meat grinder and mixed thoroughly to obtain a homogenous sample mixture. 10-gram samples will be obtained from the sample mixture, frozen in liquid nitrogen, and stored at -80 °C until analysis.
Objective 3: Human intervention trials
3.1. Subjects: Thirty (N=30) middle-aged adults (30-60 years) of all races and ethnicities who are overweight (Body Mass Index [BMI] ≥25 and <35 kg/m2) without chronic disease will be recruited. The selected age and BMI range represent increased risk for low-grade systemic inflammation and future development of chronic disease. All volunteers will provide written informed consent to an IRB approved protocol (Duke University Health Sciences Pro#00102667) and screened carefully to determine eligibility using the following inclusion and exclusion criteria.
Inclusion criteria:
- Age ≥30 and ≤60 years;
- BMI ≥25 and ≤35 kg/m2;
- Weight stable in last 3 months (Loss or gain <4%);
- Hemoglobin A1C (HbA1C) ≤6.4%;
- Fasting plasma glucose concentration <126 mg/dl;
Exclusion criteria:
- Use of medications that are known to affect the study outcome measures (e.g., NSAIDs, corticosteroids) or increase the risk of study procedures (e.g., anticoagulants) that cannot be temporarily discontinued for this study;
- Consuming >14 drinks per week;
- Use of cigarettes (or other tobacco products) in last 3 months;
- COVID vaccine within the last two weeks;
- Engaged in high-level competitive exercise (e.g., triathlon, marathon, powerlifting);
- Any inflammatory diseases (e.g. autoimmune diseases, coeliac disease, glomerulonephritis, hepatitis, inflammatory bowel disease, arthritis);
- Diagnoses of active malignancy, congestive heart failure, diabetes mellitus and/or chronic obstructive pulmonary disease;
- Use of antibiotics in last 60 days;
- Pregnant or lactating women;
- Soy allergy
- Persons who are unable or unwilling to follow the study protocol or who, for any reason, the research team considers not an appropriate candidate for this study, including non-compliance with screening appointments or study visits.
3.2. Study trials: Using a double-blind, randomized cross-over design, participants will ingest 9 oz of beef (250 g) from each source (grass-fed vs grain-fed) during separate visits (7 days apart) at the Duke Stedman Nutrition and Metabolism Center. The grass-fed beef will be produced by Hickory Nut Gap Farms, as they have the scalability to provide enough beef for the study, while the grain-fed beef will be obtained from Demkota. During test-days, subjects will arrive at Stedman at 8 AM after an overnight fast, and blood will be sampled in the postabsorptive state. Participants will ingest cooked, ground beef. No spices or condiments, other than salt, are allowed. Additional blood samples will be obtained at 1h, 3h and 5h of the postprandial (post-meal) phase to determine circulating inflammatory and metabolome responses to meal ingestion.
3.3. Dietary and Activity Control: In accordance with established protocols (Porter Starr et al., 2016), we will ask participants to maintain their habitual diet, physical activity, and sleep levels. We will ask participants to fill in 3-day dietary and sleep logs for the three days prior to their metabolic visit (see attachments). We will also ask them to refrain from alcohol and strenuous physical activity (running, weightlifting etc.) for 2 days prior to each metabolic visit. The dietary and sleep logs will give us insight if lifestyle factors leading up to each visit were similar and are likely to promote compliance to ensure robust biomarker results (inflammatory cytokines and metabolomics). Participants are asked to replicate their 3-day foods logs and sleep schedule as much as possible prior to each visit. Participants are also asked to not consume red meat the day before each visit. This is done to minimize any prior presence of common red meat and soy-derived metabolites in the urine. No blood draws will occur when participants report changes in health on any of the test-days. Instead, participants will maintain the diet several days longer to ensure testing conditions are similar.
3.4. Circulating immune function markers: Concentrations of various immune function, inflammatory, and oxidative stress biomarkers—CRP, IL-6, TNF-a, VCAM-1, —will be measured in plasma samples before and 5 hours after beef consumption by multiplex electro-chemiluminescence according to manufacturer’s instructions (V-PLEX Human Biomarker Kits, Meso Scale Discovery) (Bartlett et al., 2018). Change-scores (baseline – post-meal) will be created for all inflammatory biomarkers, and differences between the grass-fed and grain-fed beef group will be determined using paired t-Tests at 5% significance (P<0.05).
3.5 Power calculations: Using untargeted metabolomics for our power calculations of the number of participants we need to recruit, we assume a P-value ≤ 0.05, Q-value ≤ 0.3, and standard deviation of 0.3 based on previous work (Haro et al., 2016;Pimentel et al., 2018). Since there is not a previous study available to draw from, the effect size and number of truly different metabolites are meant to give general estimates. 600 metabolites are typically identified (Nieman et al., 2015) and conservative estimation is that 80-120 metabolites will be significantly different with a mean of 0.2624 (30% difference). An n=24 per group is expected to provide true discovery rates ranging from 89-93 % assuming differences in 80-120 metabolites. Assuming a drop-out of 20%, the final number of participants we will recruit is N=30.
Objectives 1-3: Metabolomics
1-3.1. Sample processing: The Metabolomics Laboratory at the Duke Molecular Physiology Institute (Drs. van Vliet and Bain), in collaboration with Metabolon Inc (Cary, NC), will perform untargeted metabolomics analysis of soil, plant, beef, and plasma samples (mView+ global metabolomics). Samples will be processed and analyzed as described previously (Evans et al., 2009;Van Vliet et al., 2020a). Briefly, lyophilized samples will be powdered under liquid N2 and homogenized in 50% aqueous acetonitrile containing 0.3% formic acid. To remove proteins and to recover chemically diverse metabolites, proteins will be precipitated with methanol under vigorous shaking for 2 min (Glen Mills Genogrinder 2000) followed by centrifugation. The resulting extract will be divided into four fractions: two for analysis by ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS; positive ionization), one for analysis by UPLC-MS/MS (negative ionization), and one for the UPLC-MS/MS polar platform (negative ionization). The MS analysis alternates between MS and data-dependent MS2 scans using dynamic exclusion, and the scan range is from 80-1000 m/z. Based on differences in metabolomes of forage, beef, and plasma, up to 15 discriminating phytochemicals will be probed with targeted metabolomics analysis at Duke’s Metabolomics Core (Baugh et al., 2019).
1-3.2. Metabolite Identification. Peaks will be quantified as area-under-the-curve; raw area counts for each metabolite in each sample will be normalized to correct for variation resulting from instrument inter-day tuning differences by setting the median value for each run to 1.0. Metabolites will be identified by automated comparison of the ion features in the experimental samples to a reference library of chemical standards that include retention time, molecular weight (m/z), preferred adducts, and in-source fragments as well as associated MS spectra (Evans et al., 2009). Currently, more than 5,200 purified standards of metabolites are registered and will provide a comprehensive analysis of metabolites in all samples.
1-3.3. Data Curation. Untargeted metabolomics will provide information on various metabolites—terpenoids, phenolics, carotenoids, tocopherols, nucleotides, xenobiotics, vitamins, minerals, lipids, amino acids, sugars, peptides, and organic acids—in the soil (soil metabolome), feed/forage (feed metabolome), beef (food metabolome), and consumer plasma (human metabolome). Two types of statistical analyses will be conducted: (1) significance tests and (2) classification analysis. Following log transformation, Benjamini-Hochberg adjusted p-values at 5%, to account for false discovery rates, will identify significantly different metabolites between sample profiles. Classification analyses will include principal component analysis (PCA) and hierarchical clustering (heatmap analysis) to determine the primary metabolites contributing to discrimination between groups. Bioactivities and potential health effects of annotated metabolites from the metabolomes will be explored by using the FooDB and/or PubChem databases, while metabolic pathway identification will use the Kyoto Encyclopedia of Genes and Genomes (KEGG). Metabolites will be clustered by chemical class to identify differences using freely-available ChemRICH software (Barupal and Fiehn, 2017). The most discriminatory metabolites will be kept for analysis based on variable importance in projection (VIP) scores, with a VIP > 1. Statistical analyses will be performed in SAS 9.4 or R (cran.r‐project.org/).
1-3.4. Data Analysis. Using results from the significance and classification approaches, we will perform two comparative analyses: (1) within objectives 1-3 we will compare amongst each other to determine differences between each grass-fed beef system and the feedlot system, and relate findings back to farm management practices; (2) we will compare metabolomes of each objective within the grass-fed and feedlot to study shared metabolites along the soil-plant-beef-consumer continuum (Figure 4).
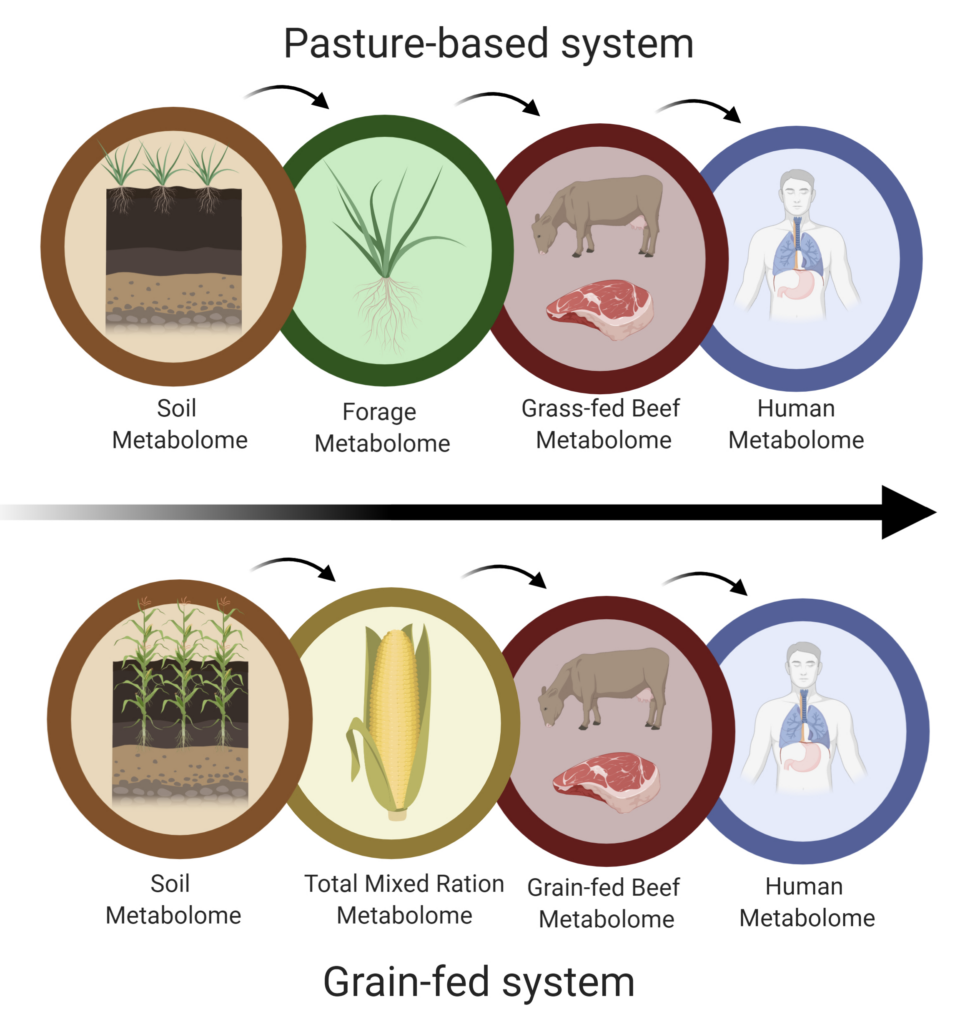
(1) Within-sample type comparison: For soils of pasture-based systems, individual metabolites with VIP scores > 1 and/or ChemRICH derived metabolite clusters with P-values < 0.05 will be correlated with extracted nutrients to understand relationships between metabolite profiles and soil characteristics. For pasture forage, pasture composition and health from UAV imagery will be correlated with metabolite classes and individual metabolites present across the sampled pastures to determine how differences in pasture composition affect the forage metabolome. The phytochemical richness of forage samples from each farm and the feedlot TMR will be compared to determine differences in phytochemical richness of forage/feed consumed by animals. For the beef, we will directly compare the differences in metabolites between beef obtained from all pasture-based farms and the feedlot. For the human nutrition study, we will compare change-scores of plasma metabolomics data (postmeal – baseline) after beef consumption from the grass-fed and grain-fed systems.
(2) Across-sample type comparison: Within Hickory Nut Gap (grass-fed) and Demkota (grain-fed); soil, plant, beef, and plasma metabolomes will be analyzed using PCA analysis and overlaid to correlate shared metabolites (Pimentel et al., 2018). It is within these farms that we will carry the metabolomics analysis forward from soil, to forage/feed, to beef, to consumers. Within the two other pasture-based farms (BDA and Beaver Creek), analysis will be carried forward from soil, to forage, to beef. This is justified based on the expectation that the beef from the three grass-fed systems will be comparable in phytochemical richness due to high-quality forage (Poore et al., 2020), and that feeding three types of beef to consumers is beyond the resources and scope of this project.
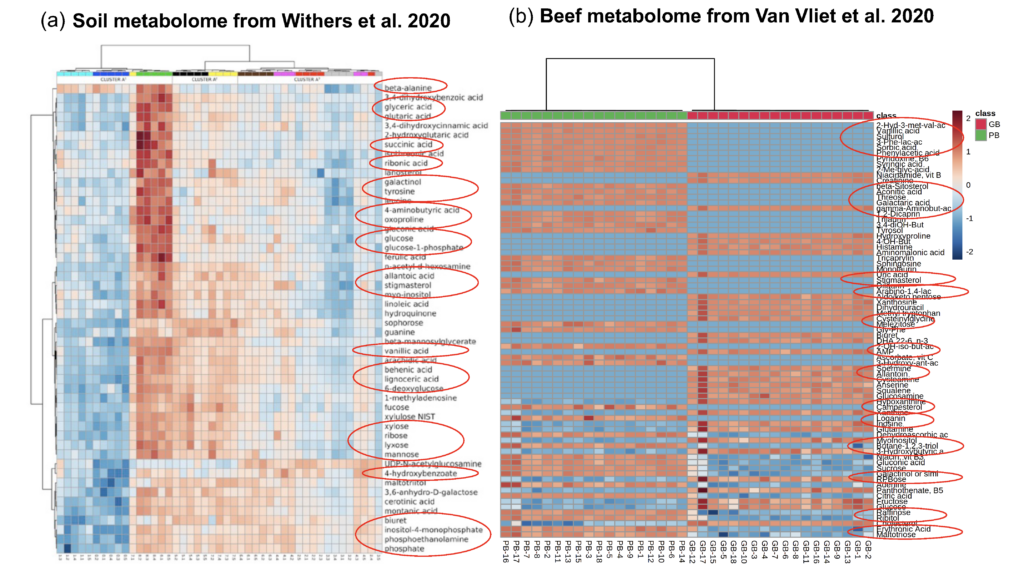
Preliminary data strongly support the hypothesis that we will observe shared metabolites along the soil-plant-beef-consumer continuum. For example, Withers et al. (2020) used untargeted metabolomics for assessing soil quality and found many similar metabolites in soil samples as we found in our beef samples (Figure 5). Moreover, Pimentel et al. (2018) found similar metabolites in both the food metabolome and acute post-meal plasma metabolome of consumers.
Objective 4: Outreach
Findings will be shared with producers, consumers, and aggregators via NC Cooperative Extension (NCCE) programming, and consumer and producer organizations. Our systems research reaches beyond the farm gate to include outreach on broader societal impacts of local food systems to the consumers (NCCE Local Foods Flagship Program), producers (NC Choices/Amazing Grazing), and third-party organizations (Build Up Dietitians, ASAP, and Understanding Ag). Efforts are described in detail in our Outreach Plan.
Soil, Forage, and Fecal Collection (objectives 1-2):
Soil, forage, and fecal samples were successfully collected from each cooperating farm during the grazing season (every 60 days). The research team sampled soil + forage from soon-to-be-grazed pastures (30-60 day rest) and from nearby corn fields (typically within 1 mile of the pasture), the latter representing a model for growing corn for feedlot finished animals. In contrast to the originally proposed slaughter times near the end of Summer 2021, the producers were not able to harvest cattle prior to November-December 2022. This was not an issue given the long grazing season in the South, but meant we collected samples every 60-90 days instead of every 30-60 days. Collections took place on days without precipitation and were as follows:
- Beaver Creek Farm (Mount Airy, NC):
- Collection day 1: 5/6/2021; Low Temp: 45 F; High Temp; 66 F.
- Collection day 2: 9/7/2021; Low Temp: 61 F; High Temp; 84 F.
- Collection day 3: 11/17/2021; Low Temp: 42 F; High Temp; 71 F.
- Hickory Nut Gap (Fairview, NC):
- Collection day 1: 5/18/2021; Low Temp: 58 F; High Temp; 67 F.
- Collection day 2: 7/28/2021; Low Temp: 67 F; High Temp; 90 F.
- Collection day 3: 10/27/2021; Low Temp: 42 F; High Temp; 66 F.
- BDA Farms (Uniontown, AL):
- Collection day 1: 5/14/2021; Low Temp: 47 F; High Temp; 76 F.
- Collection day 2: 9/7/2021; Low Temp: 73 F; High Temp; 91 F.
- Collection day 3: 10/18/2021; Low Temp: 42 F; High Temp; 74 F.
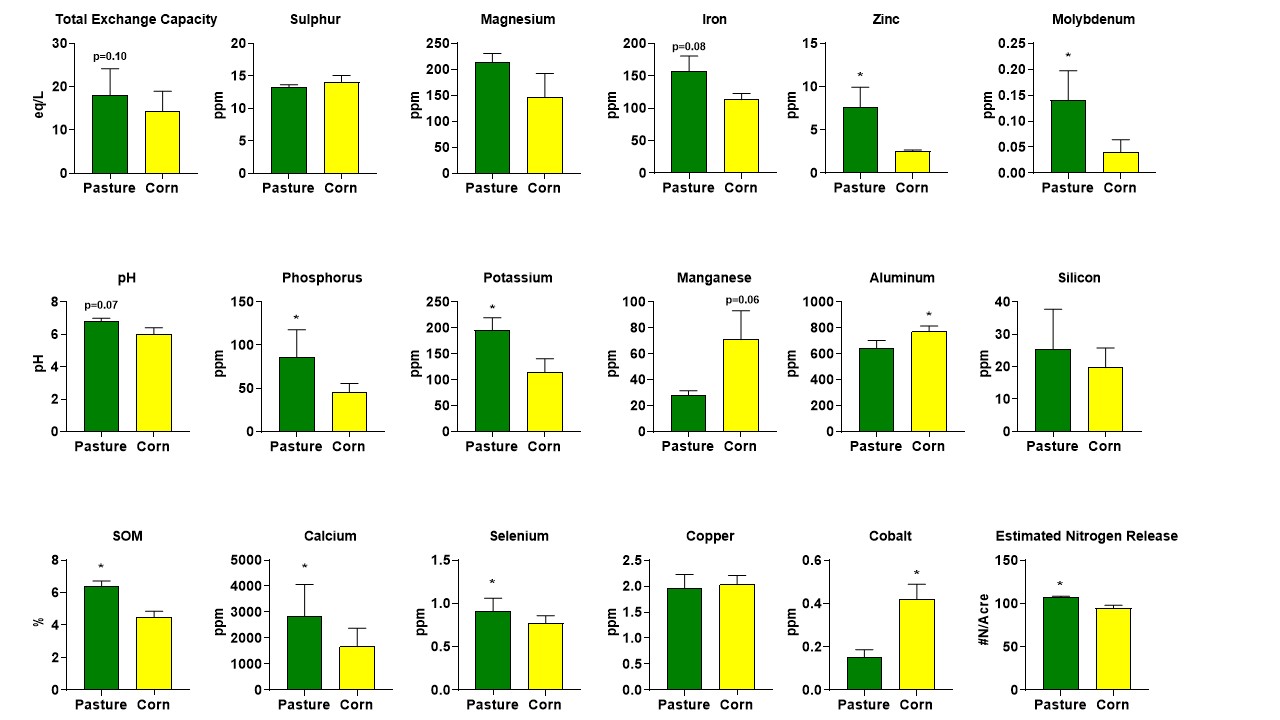
Soil Outcomes: Soils were sampled at 0-10 CM depth and analyzed at Logan Labs using standard soil testing, while a seperate subsample was immediately frozen in liquid nitrogen and kept for metabolomics analysis. The presented data (figure 1) represents the average and SD of all pasture and corn soil samples, which were sampled within 1-2 miles from each other to minimize the impact of geographical factors. We found generally healthier soils in the grazed pastures compared to the monoculture (corn) crop fields, which was in line with our hypothesis. Soil organic matter was higher in the pasture soil samples (6.36%) vs the corn soil samples (4.49%). We also found a trend for higher Total Exchange Capacity in the pasture (17.97) vs corn (14.4) soils. Soils can be thought of as storehouses for plant nutrients and a higher Total Exchange Capacity indicates more nutrients are available in the soil for plants in the pasture vs corn system. We also found higher levels of various minerals including Potassium, Phosphorus, Calcium, Selenium, Zinc, and Molybdenum in pasture soils. In contracts, corn soils contained higher amounts of Manganese, Cobalt, and Aluminum.
Figure 1: Soil analysis of pasture samples (from grass-fed farms) and nearby corn fields (as a model of corn-based total mixed rations for feedlot animals).
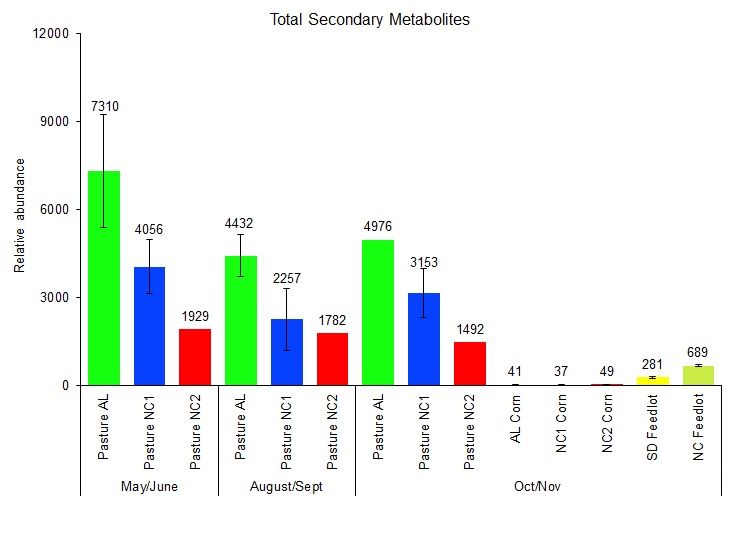
Forage Outcomes: Forage samples from the pasture-finished farms (NC1, NC2, AL1) were collected by pasture walk in near proximity to soil sample collection during the. We were also able to obtain corn from all corn fields that were sampled for soil (see Soil Outcomes). Furthermore, total mixed ration samples were collected from a Midwest feedlot as a comparator. All forage/feed samples were analyzed for phytochemical richness using ultra-high performance liquid chromatography tandem-mass spectrometry (LC/MS-MS) as described (1). A total of 136 phytochemicals were identified. Phytochemicals are plant secondary compounds, that have been studied for their potential anti-inflammatory and anti-oxidant effects. We found that the phytochemical richness of forages in May/June 2021 (4432 AU) was highest in May/June and decreased by 36% and 28% in August/September and October/November 2021, respectively (Figure 2). We also found that the phytochemical richness of forages grazed by the grass-fed animals is, on average, 680% higher than the total mixed ration (TMR) samples fed to grain-finished animals. We also studied the contribution of corn to the phytochemical richness of TMR samples and found that, despite making up 50-80% of the weight of the TMR, corn contributed only 9% of phytochemicals to the TMR. Thus, most phytochemicals in TMR samples are contributed by the hay portion of the TMR (Figure 2). We also found that vitamins known to occur in substantial amounts in fresh forages, such as Vitamins C, E (alpha-tocopherol), and B3 were 9455%, 5433%, and 458% higher in the forage samples compared to the TMR samples. In contrast, Vitamins B1, B5, and B6 were reduced by 53%, 25% and 65% in fresh forages compared to the TMR samples, which was likely the result of vitamin supplementation in the TMR as these compounds remained reduced in the corn itself.
Fecal outcomes: Fecal samples were collected by sampling 8 cow pies per field day. Stool samples have been submitted to Jonah Ventures in January 2022 for DNA Metabarcoding to complement forage questionnaires from farmers. 16s rRNA samples have been processed and submitted for analysis in February to provide insight into gut microbial health of the cattle. Once results are known they will be posted here.
Beef Collection (objective 2): All cattle from the various farms were harvested in November-December of 2022 and ribeye samples from individual animals were received by the research team and subsequently stored in a -40 ° C. We obtained 3 ribeyes from each pastured farmer totaling 9 ribeyes (biological replicates). We also obtained 8 grain-fed ribeyes from animals that were finished in a Midwest feedlot. The meat samples were analyzed for phytochemical richness using ultra-high performance liquid chromatography tandem-mass spectrometry (LC/MS-MS) similar to the plants. In the data and statistical analysis of the beef samples, the pasture-finished beef samples (Bf_Pasture) from were grouped together and the Midwest feedlot samples were grouped together (Bf_Feedlot). Eight ribeye steaks from individual animals were tested for each group. Differences in metabolites were tested using the Wilcoxon rank-sum test with false-discovery-adjusted p-values with statistical significance considered at P<0.05.
Untargeted metabolomics analysis of the beef samples identified a total of 781 compounds. We found that 193 compounds (24%) differed in abundance (false discovery rate-adjusted P<0.05) between grass- and grain-fed beef samples. Sparse Partial Least-Squares Discriminant Analysis (sPLS-DA) with 20 compounds per components found pasture-finished- and feedlot-finished samples differed in composition with component 1 explaining 22.9% of the variation (Figure 3).
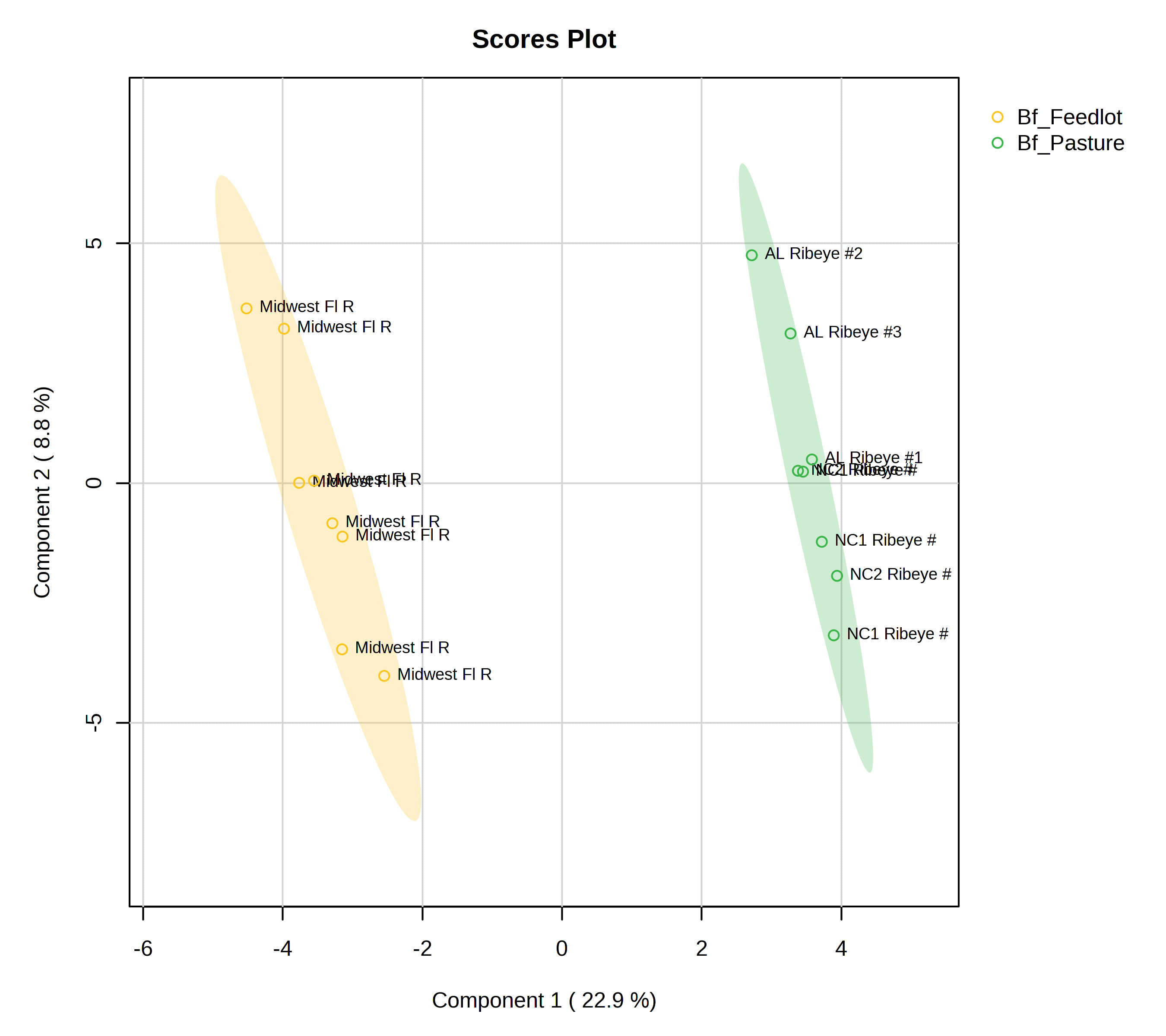
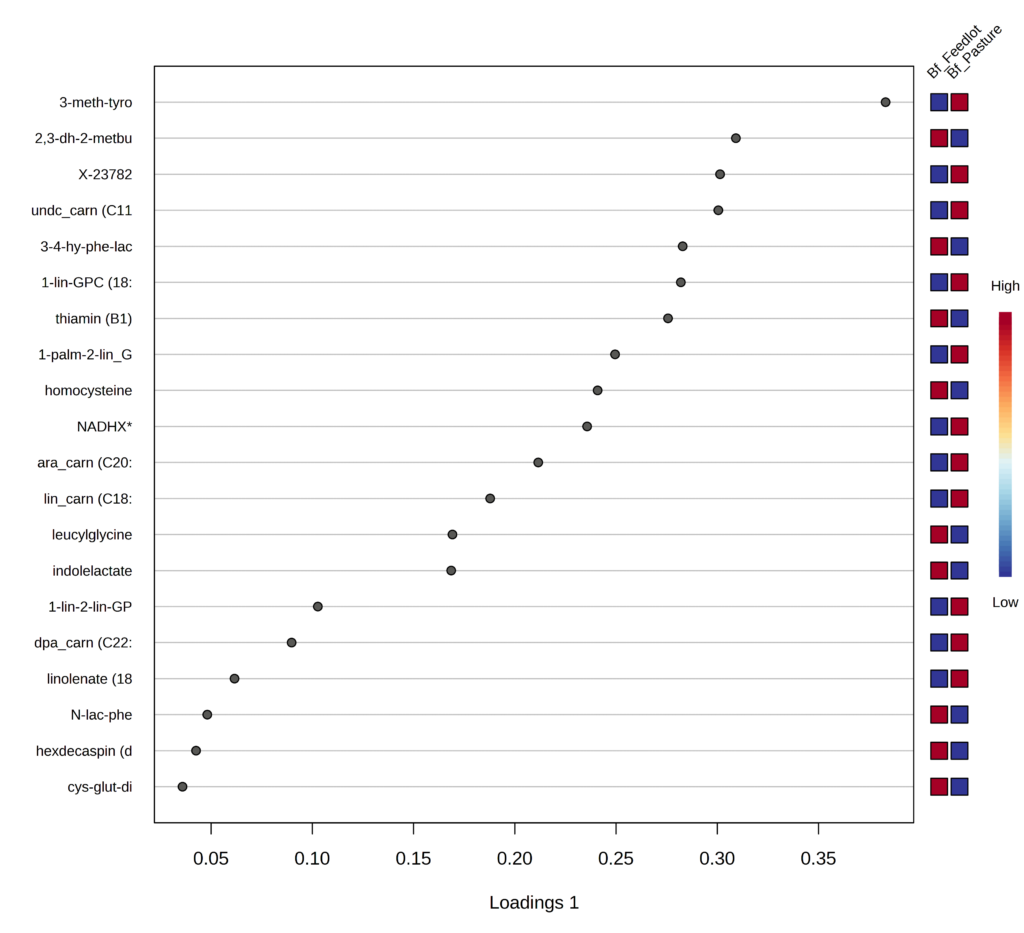
Highlighting the top 20 metabolites important for the separation between grass- and grain-fed, the biochemical importance plot ranks compounds in order of increasing importance from bottom to top on the y-axis (Figure 4).
The higher phytochemical richness in forages vs. TMR subsequently led to a 107% higher phytochemical richness in the grass-fed meat vs the grain-finished meat samples. For example, two major biomarkers of phenolic intake, namely cinnamoyl glycine and catechol sulfate, were 321% and 507% elevated in the grass-fed vs grain-fed beef samples (Figure 5).

We also studied two anti-oxidant metabolites that are produced by soil fungi and cyanobacteria, namely ergothioneine and its metabolite histidine betaine, which have been becoming of increasing interest with regards to their relationship to soil health as they are upcycled from soil bacteria to our food (2). We found ergothioneine and histidine betaine to be 179% and 234% higher in the pasture-finished beef samples when compared to the feedlot finished samples. We consider these data to tie in with our soil health biomarkers (Figure 1), which found higher SOM and TEC (an indicator of plants and soil to exchange nutrients) in the pasture samples compared to nearby corn field samples (which were used as a model for the TMR samples).


We also found 173% and 392% higher concentrations of Vitamins A (carotene) and E (alpha-tocopherol), respectively in the grass-fed vs grain-fed beef samples, similarly reflecting the greater amounts of these in the forage vs. TMR samples (Figure 7). On the other hand, Vitamins B1 and B2 were 45% and 40% reduced in the grass-fed vs grain-fed beef samples (Figure 8), reflecting the findings we made in the forages. In other words, we found strong suggestions that the cattle “are what they eat”. More phytochemicals in the forages resulted in higher amounts of these in the grass-fed beef, while the higher content of various b-vitamins in the TMR samples were also reflected in the meat.

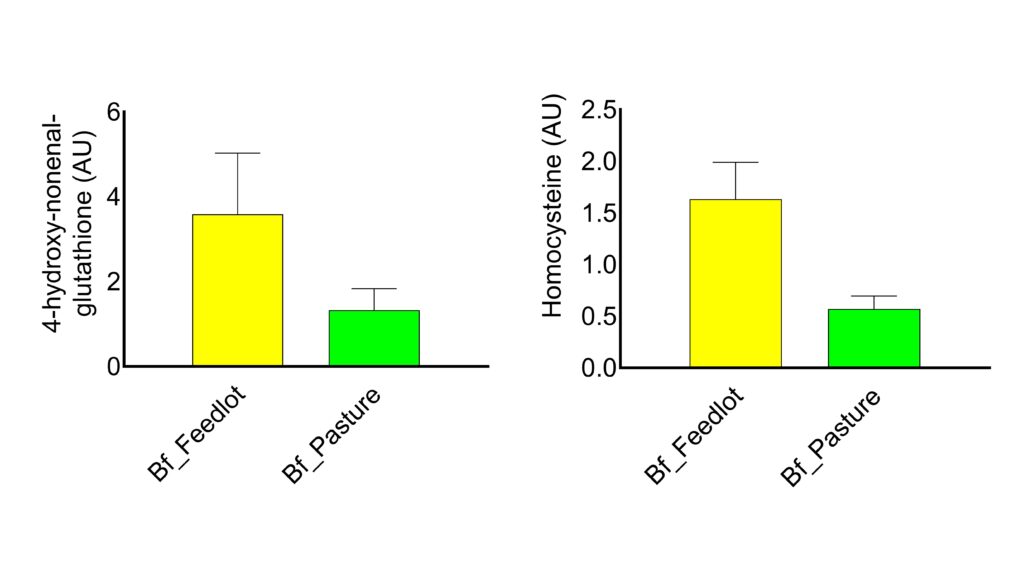
We also found that 4-hydroxynonenal glutathione, an advanced lipoxidation end product associated with worsened metabolic health, was 129% elevated in grain-fed samples. Similarly, homocysteine, a common inflammation marker was 165% higher in the grain-fed samples. Whether this has an appreciable effect on human health remains unknown.
Human Nutrition Trials (objective 3): The trial completed with n=36 participants having finished the study.
Introduction
This project aimed to evaluate the acute inflammatory responses to three different types of burger patties: grass-fed beef, grain-fed beef, and plant-based meat alternatives (PBMA). Inflammation was measured by tracking three key biomarkers—TNF-α, IL-6, and C-reactive protein (CRP)—over a 5-hour post-meal period. This study provides information into how different types of food products may affect short-term inflammation. Persistently elevated inflammatory markers, when inadequately regulated, are associated with an increased risk of developing chronic diseases.
Study Design
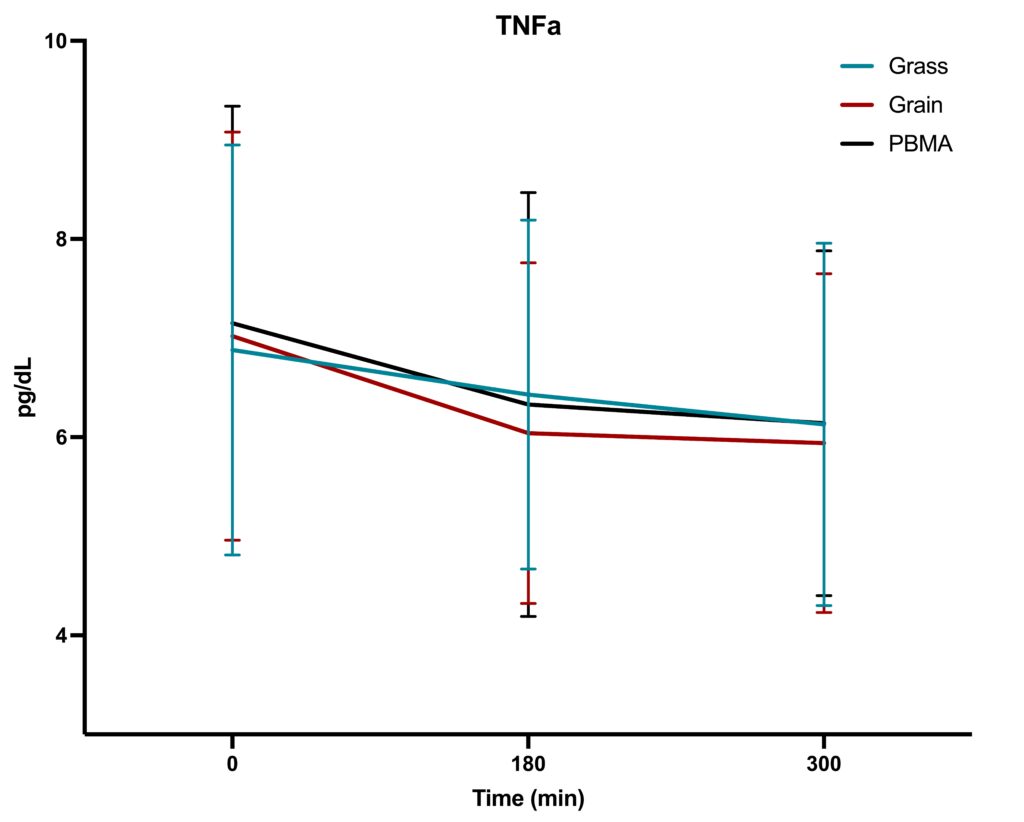
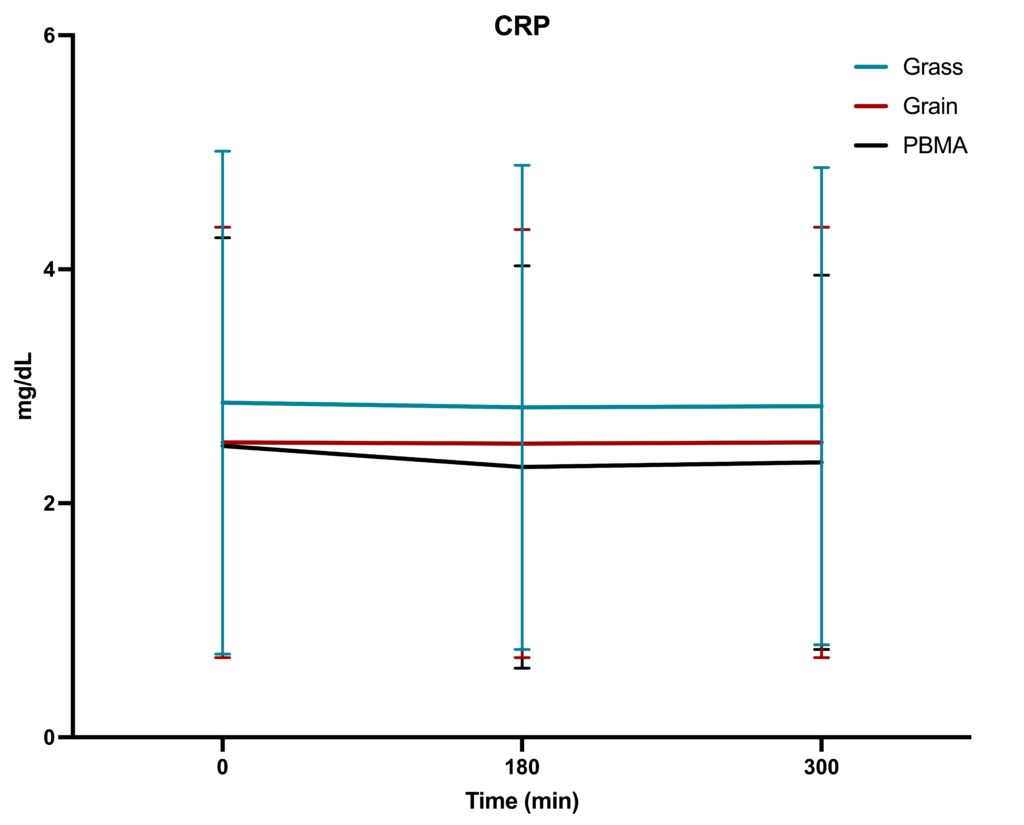
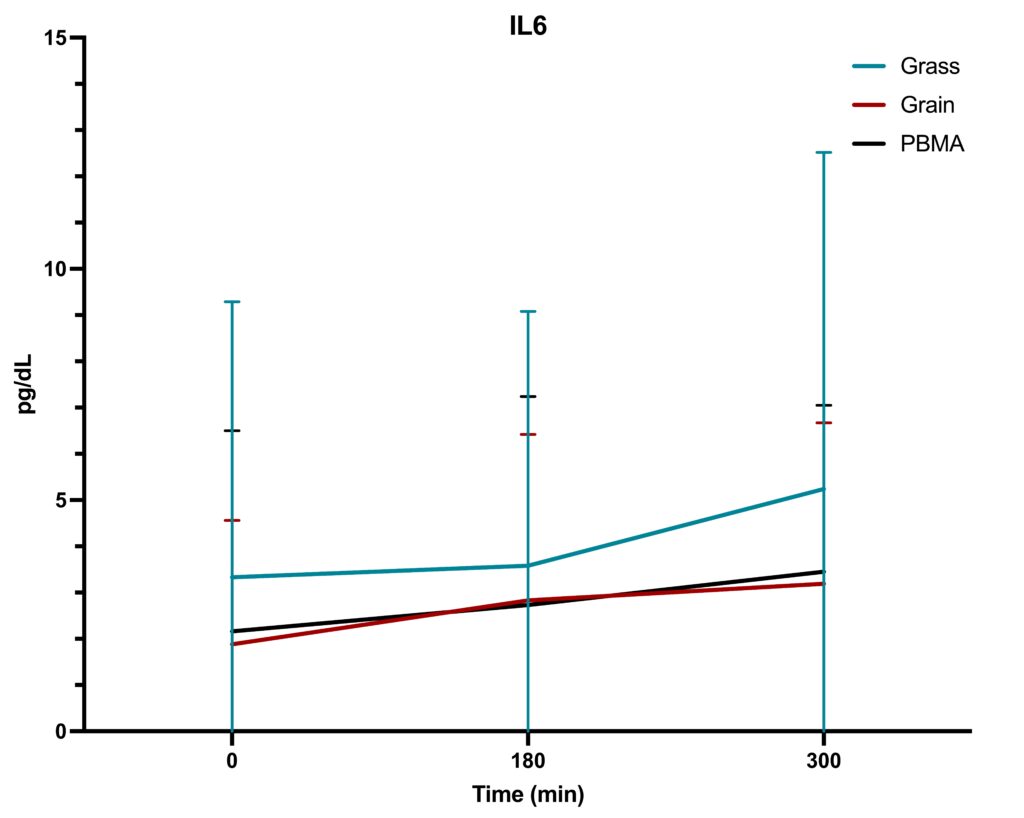
The study followed a randomized, acute, crossover design, with n=36 participants consuming three types of burgers at separate visits, followed by a 5-day washout period between meals. The primary objective was to assess whether the type of burger consumed (grass-fed, grain-fed, or PBMA) resulted in different acute inflammatory responses within-subject. Blood samples were collected at baseline (0 minutes), 180 minutes, and 300 minutes after each meal to measure changes in TNF-α, IL-6, and CRP levels.
Key Findings
The statistical analysis revealed that none of the three inflammatory markers—TNF-α, IL-6, or CRP—differed significantly between the burger types. Below are more detailed observations for each marker:
- TNF-α: As shown in the corresponding graph, the levels of TNF-α steadily declined over the 5-hour period across all three interventions (grass-fed, grain-fed, and PBMA). This pattern reflects the typical postprandial response, where TNF-α levels decrease after an initial food-induced rise. However, no significant differences were observed between the burger types.
- CRP: The CRP response was relatively stable across the three burger types, as reflected in the graph. Despite slight fluctuations, CRP levels remained within similar ranges across the 5-hour period, with no notable differences among grass-fed, grain-fed, or PBMA consumption.
- IL-6: The IL-6 data demonstrated a slight upward trend in levels over the course of 5 hours, particularly for the grass-fed burger, which showed a more pronounced increase compared to the other two options. Nevertheless, these differences did not reach statistical significance. As with TNF-α and CRP, the IL-6 levels were primarily influenced by time rather than the type of burger consumed.
Inflammatory marker levels changed over the 5-hour window, but there were no significant differences attributable to the type of burger consumed. Our work contrasts with previous work by Arya et al. (2010), who found that eating pasture-fed meat attenuated postprandial inflammatory profiles, including CRP, TNF-α, and IL-6. We conclude that eating beef from grass-fed or grain-fed sourcing has no impact on acute inflammatory markers, despite containing a wider variety and greater concentration of phytochemical antioxidants. Future longer-term trials are required to determine whether the increased phytochemical richness and improved omega-3 fatty acid profiles have an appreciable effect on biomarkers of human health, including inflammatory markers.
Conclusion
This study found that while time significantly influenced the levels of TNF-α, IL-6, and CRP, the type of burger—grass-fed beef, grain-fed beef, or a plant-based meat alternative—did not result in significant differences in acute inflammatory responses. These findings suggest that the type of protein source consumed in a single meal may not have a substantial impact on immediate postprandial inflammation. However, further research could explore the effects of long-term consumption of these foods to assess any chronic inflammatory differences that may arise.
Educational & Outreach Activities
Participation Summary:
Outreach 2023-2024
The research team has performed field days at the following farms:
- Beaver Creek Farm (second field day)
- Carolina Meat Conference (Booth at conference + Keynote by PI Dr. van Vliet)
The research team also presented this data at the following workshops/conferences for about 1000+ people total:
2024 Rutgers University, New Brunswick, NJ. Linking Plant, Animal, and Human Health in Livestock Systems: a Metabolomics Approach.
2024 Carolina Meat Conference, Boone, NC. The Impact of Soil Health and Plant Diversity on Meat Nutrient Density
2024 Bionutrient Summer Series, Webinar. Linking Plant, Animal, and Human Health in Livestock Systems: a Metabolomics Approach
2024 Soil Health in the West Conference, St George, UT. The Impact of Soil Health and Plant Diversity on Meat Nutrient Density.
2024 Northeast Grazing Conference, Amherst, MA. Nutrient Density in Meat Systems
Outreach 2022-2023
The research team has at NCSU has performed field days at the following farms:
- Beaver Creek Farm
- Hickory Nut Gap
A total of 190 farmers participated in these events. Additionally 65 ag professionals participated.
The research team also presented this data at the following workshops/conferences for about 1000+ people total:
2022 Global Conference on Sustainable Beef, Denver, CO. Balancing Production, Consumption and Nutritional Needs.
2022 Silver Fern Farms, New York, NY. Linking Plant-Animal-Human Health in Livestock Production Systems.
2022 Bionutrient Institute Webinar. Beef Nutrient Density Study Preliminary Results Webinar.
2022 Rhizoterra, LLC, Webinar. Differentiating grassfed from conventionally fed beef and their nutrition compositions.
2022 Green Cover Seed Webinar. Nutrient Density in Grass-fed Meat and Milk with Nutrition Scientist, Dr. Stephan van Vliet - YouTube
2022 Global Food & Farm Online Community Webinar. The effect of grazing practices on the nutritional composition of meat and milk.
2022 Northeastern Pasture Conference Webinar. NEPC 2022 Plant, Animal & Human Health Linkages: A Metabolomics Approach, Dr. Stephan van Vliet - YouTube
Outreach 2021-2022
The research team has presented the concept and initial findings of this project on various occasions including the following so far.
- 5 invited talks to farmer organization including Understanding Ag, NorthEast Pasture Conference, Bionutrient Institute, Southern Food and Farming, Regen-Ag Day (about 1000 farmers total combined).
- Partnership with the Bionutrient Institute (a non-profit farmer led organization), which led to the launch of the Beef Nutrient
Density Project, where we will collect samples from grass-fed and grain-fed beef systems across the US for metabolomics profiling.
- 3 invited talks at various universities (TSU, Wisconsin, Queens)
- two documentary interviews related to this project
Specific outreach during grant performance period related to this work:
Farmers:
2021 Bionutrient Institute. Invited talk. S. van Vliet. 1.5 hour roundtable webinar. Attended by 200 farmers. Defining Nutrient
Density in Beef--A Round Table Discussion.
2021 Regen Ranch Day, Marion, TX. S. Van Vliet. Invited talk. 1-hour presentation to a group of approx 80
farmers. (68) The Regen Ranch Field Day: Fred Provenza & Stephan van Vliet - YouTube
2021 Southern Family Farms and Food Systems Conference, San Marcos, TX. S. Van Vliet. Invited
talk. Animal nutrition and phytochemicals. 45 min presentation to approx. 150 farmers/consumers/academics.
2021 Understanding Ag Soil Health Academies. Invited talk. 1.5 hour webinar with 120 farmers. The links
between food production systems and human health in the context of grazing systems.
Consumers:
2021 Mensa Foundation, Houston, TX. S. Van Vliet. Invited talk with 200 consumers. Using metabolomics to
understand linkages between food production systems and human health.
Podcasts:
2022 Optimize Paleo Podcast. Interview about our work on grass-fed beef and plant-based alternatives.
2021 Sight and Life's Brain Food: Disruptive Science podcast. Plant-based meats: Getting the nutrition facts
straight.
2021 Muscle Memoirs Podcast. Interview about our metabolomics work on grass-fed beef and plant-based meat
alternatives.
2021 Peak Human Podcast. Interview about meat, protein, and human health.
2021 Ag-Emerge Podcast. Interview about our metabolomics work on grass-fed beef.
Academics: The PD, Dr. van Vliet has presented findings related to this project at the following conferences/events.
Academic Talks
2022 Utah State University. Departmental talk. 1-hour webinar to 30-40 faculty. Linking plant, animal, and human
health.
2021 University of Wisconsin, Madison, WI. Invited talk. 1-hour webinar to 30-40 faculty. Understanding linkages
between animal and human health: A metabolomics approach.
2021 Queens University, Kingston, ON, CA. S. Van Vliet. Invited talk. 1-hour webinar to 30-40 faculty?. Using
metabolomics to understand linkages between food production systems and human health
Target Audience for Project Participation
Our target audience for Project Participation primarily includes farmers, and secondarily includes consumers. The cooperating farmers participating in this project are located within the Southern region and include Beaver Creek Farm in Mount Airy, NC, Hickory Nut Gap Farm in Fairview, NC, and BDA Farms in Uniontown, AL. We will work directly with the farmers at each of these locations when selecting locations for soil, plant, and beef samples for analysis, and involve them directly in supplemental data collection by obtaining farmer observations regarding grazing patterns and plant species consumed by their cattle. We will also directly involve a small population of consumers in our project by their recruitment and participation in the human nutrition research study. Through our study, we will engage with each participant on the purpose and objectives of the trial, and the methods we will use to determine human health outcomes.
Target Audience for Information, Education, and Outreach
Our target audiences for Information, Education, and Outreach are farmers, consumers, and third-party industry stakeholders across the Southern states. To further grow the local food movement, it is important that our scientific findings on the healthfulness of pasture-raised beef and health connections across soils, plants, meat, and humans be communicated to all three groups. This will ensure that evidence-based justification is available for: i) producers to increase plant-species richness of their pasture to increase the phytochemicals richness of their meat, ii) consumers to seek out and continue to ‘demand’ locally produced pasture-raised meat, and iii) industry stakeholders to make decisions regarding beef product purchasing, sales, and marketing.
By taking a ‘whole systems approach’ to both our research and outreach activities, we aim to build community health by bridging our efforts from producers to consumers with the ultimate goal of re-connecting our food system. Our approach largely focuses on augmented and strengthening existing outreach and education efforts with data and outcomes from this project to maximize budget and personnel resources. To do this, we will leverage the strong resources available within the NC Cooperative Extension (NCCE) programs, with a focus on programming related to the Center for Environmental Farming Systems. Specific programs and approaches that will be used to target each target audience group are outlined below. Note that evaluation plans for our outreach and education efforts are described in detail in the Evaluation Plan subsection.
Producers:
Our work will compare the nutrient density of grass-fed beef from three different producers, each using production methods that are tailored towards the local ecosystem and resource base - results from which will be directly applicable for producers across Southern states. For example, BDA Farms (AL) produces grass-fed beef, and recent research with forage-based beef production systems in LA, SC, NC, and GA showed little difference between forage types or systems with a high level of forage management. We expect that the results from our study will include new information and insight on the connections between plant diversity, soil health, beef nutrient quality, and human health outcomes, which will form the basis of outreach and education programming across multiple stakeholders.
Amazing Grazing: The primary program for our producer-focused education and outreach efforts is Amazing Grazing, a pasture-based livestock education program affiliated with CEFS. The three major themes of the Amazing Grazing program are 1) Improved Profitability; 2) Improved Animal Health and Well Being; and 3) Improved Environmental Sustainability. This program includes producer workshops, interagency advisor workshops, and research and demonstration projects, and is coordinated by Johnny Rogers (Educational Coordinator for Amazing Grazing) and Dr. Matt Poore (Director and co-PI). In particular, Amazing Grazing will present twelve workshops in support of this project. These workshops will be held in conjunction with six multi-day schools in pasture ecology which will be held as part of an NRC Conservation Collaboration. All workshops and schools will provide education on links between soil health, animal health and well-being, and environmental stewardship. We will augment existing curriculum with comparative overlays of metabolomics data and the differences across pasture management systems included in this project to highlight the connectivity between soils, plants, and nutrition of beef products; this will directly target existing themes 2 and 3. As our human health findings accumulate and provided our hypothesis holds, we will add a fourth theme in the future centered around Improved Healthfulness to Consumers. The addition of this new theme and integration of human health and nutrition data into existing programming efforts will ensure our goals of this project extend beyond the life of the grant. Amazing Grazing workshops on focused topics are well attended and impactful. As part of a recent NRCS Conservation Innovation Grant (2015-2018), 44 on-farm workshops with 2204 participants improved producer understanding of Soil Health in pasture-based systems. Further, on-farm demonstrations supported by Amazing Grazing have previously resulted in 93% adoption rates (of 78% response rate) of grazing management and fencing technologies (Freeman et al., 2019).
NC Choices: NC Choices (NCC), an initiative of CEFS and NC Extension, promotes sustainable food systems through the advancement of the local pasture-based meat supply chain in North Carolina. NCC has spent almost 20 years helping to build niche meat value chains and providing technical assistance for hundreds of producers, inspected meat processors, buyers, and food professionals throughout the Southeast. Events such as the Carolina Meat Conference, the nation’s largest gathering of local meat professionals with 400+ attendees from 23 states, has brought industry needs to the forefront of Extension and research activities, and deepen collaboration with CEFS’ Amazing Grazing program. NCC also incubated Firsthand Foods, a food hub‐ modeled business that aggregates pasture‐raised beef, lamb, and pork products from a network of more than 80 livestock producers. NCC’s activities include delivering training to ~ 800 niche meat professionals/yr., a sold-out bi-annual Meat Conference, maintains 3,000+ online followers, and disseminates industry-relevant data to grow local meat in NC. Since the inception of NCC in 2002, the number of NC livestock producers selling meat directly to consumers has gone from one to over 1,000.
We will utilize NC Choices to integrate our project findings into existing programming, such as workshops on profitable price strategies, marketing, and product development, in addition to developing new programming pertaining to project research assessing the correlation of meat quality, animal diet, and human health. Based on our findings and existing curriculum, we will provide a set of principles regarding grazing management and pasture diversity to increase the phytochemical richness of meat that farmers can adapt and tailor to their local operation. Further, we will introduce new sessions at key NCC training, notably the Carolina Meat Conference, focused around pasture quality and composition, beef nutrition, and human health to build awareness of and disseminate findings from our project. We will leverage CEFS’ communication channels (e.g., listservs and social media) and NC Choices’ online platforms to disseminate fact sheets and resources to meat producers, allied professionals, and consumers. Dr. Matt Poore (Co-PI) and Johnny Rogers currently serve on the NC Choices board of advisors.
Understanding Ag, LLC: Understanding Ag is a regenerative agricultural consulting company that educates and mentors farmers, ranchers, landowners, businesses, and communities in the principles and practices needed to restore, repair, rebuild, regenerate their farming and ranching ecosystems. Dr. Allen Williams and Ray Archuleta, founding partners of Understanding Ag will organize workshop curriculums in years 2 and 3 (4 total) in the Southern region and will incorporate our findings in their Soil Health Academy (50 attendees/workshop) and self-guided farm-tours at BDA Farms in Alabama (approximately 5,000 attendees per year). By incorporating our findings in presentations given by the partners of Understanding Ag; Ray Archuleta, Dr. Allen Williams, Shane New, and Gabe Brown it is expected that our findings will reach well over 5,000 people through workshops and conferences and well over 100,000 people on social media and Youtube channels.
NC Cooperative Extension: To broaden our reach across North Carolina, we will take a ‘teach the teacher’ approach and train up to twenty NC Cooperative extension agents in the Agriculture/Livestock extension program (https://beef.ces.ncsu.edu/). Training materials will include scripted presentations, infographics, and summary materials focusing on decision-making regarding the incorporation of various grasses, grass-forb, and/or grass-legume mixtures in their pasture that maximize the nutrient density of beef and soil/plant health based on our findings. We will also utilize field days and demonstrations to raise awareness about this work.
Consumers:
The local food movement has grown rapidly across the South in the last two decades (Poore et al., 2020), and there is an especially strong interest in locally produced pasture-raised meat; more recently, we have seen this trend become even further accelerated by the COVID-19 pandemic. We will continue to accelerate this growth by contributing to education and outreach efforts focused on the healthfulness and environmental benefits of consuming locally produced pastured beef. CEFS leads or partners in a variety of initiatives and programs that target consumer stakeholders; these include NC Choices, the NC 10% Campaign, and Farm to Fork NC, in addition to Extension programming in nutrition through Family & Consumer Sciences (FSC) and the Extension Local Foods Flagship Program. We will also target outreach to dieticians through the Duke University School of Medicine and the Duke Health program.
NC Cooperative Extension: We will train up to thirty Family & Consumer Sciences (FCS) agents (https://fcs.ces.ncsu.edu/) with educational programming focused on human nutrition and human health of pastured beef products, including summary materials, infographics, and presentations. We will also utilize the Local Foods Flagship Program, in which each county’s Cooperative Extension office has a designated Local Food Coordinator to support local food system efforts. Consumer education through this program will include workshops on cooking and food preparation, nutrition, and food tastings at farmers' markets and local food events. Additionally, the FSC agents write regular articles for digital distribution through NCCE. We will also utilize the NC 10% Campaign, a statewide initiative led by CEFS and NC Cooperative Extension that encourages at least 10% of food sales to be “NC-grown”, to market and raise awareness of the health and environmental benefits of consuming local pastured beef discovered through this project. Through local workshops, events, presentations, social media, and extension agents we anticipate that our findings will reach hundreds of consumers per year.
Farm to Fork NC: Farm to Fork NC is another initiative led by CEFS which conducts events focused on local food to support new farmer training programs. We will use the Farm to Fork Picnics, in the Triangle and Charlotte areas, to highlight local pastured beef producers and highlight the findings from our work related to the healthfulness of the beef products directly to consumers in attendance. These events draw about 1,500 participants every year and have cultivated a strong social media presence.
Build Up Dietitians: Founded in 2014, Build Up Dietitians is a global social media presence dedicated to promoting science-based nutrition messages. The team of dietitians that curates Buildup Dietitians content represents dietitians from different countries around the world and reaches thousands of followers on various platforms. Build Up Dietitians holds regular Q&A sessions, Facebook live events, and publishes social media posts. NC-based Leah McGrath, RD, LDN, is the founder of Build Up Dietitians and will assist the research team with outreach efforts by communicating human health and nutrition findings to the broader dietetics community. We will use this digital platform to pose questions (e.g., during Facebook Live events or Twitter polls) regarding consumer perception and demand for the healthfulness of local pastured beef products.
Industry stakeholders:
NC Choices: In addition to farmer outreach, NC Choices is uniquely positioned to directly educate other stakeholders in the meat supply chain. These include chefs, butchers, distributors, ag educators such as Cooperative Extension agents, and allied professionals. Through trainings such as the Carolina Meat Conference, we will highlight results that are relevant to these stakeholders in tailored sessions; for example, we can create a special program for chefs and butchers on highlighting beef nutrition and associated flavor profiles of beef resulting from different forages, or incorporate these results into sessions focused on making finishing recommendations to farmers. Historically, NC Choices programming has focused on feed/meat quality, including sessions on meat quality and health impacts; however, the comprehensive data has been limited, especially on relating health to animal feed and production, so results from this project that assesses all of these components will be a new addition.
Hickory Nut Gap Farms: Hickory Nut Gap farms partners with 80 grass-fed beef producers throughout North Carolina, South Carolina, Georgia, and Louisiana supporting family farms and local food economies. They sell locally-raised beef produced from their family farm network to more than 40 grocery stores across the Southeast. Hickory Nut Gap typically organizes 100 consumer farm tours per year, which are attended by consumers and school groups, and 10-20 industry tours per year, which are attended by chefs or other food industry stakeholders such as Whole Foods and Ingles Market staff. They also provide newsletters to over 50-100 restaurants across Southeast, who regularly purchase their beef. As research findings accumulate, the research team will provide Hickory Nut Gap staff with research summary materials and infographics for newsletters and presentations, which will incorporate newsletters, farm tours, and outreach activities to consumers and food industry stakeholders.
Appalachian Sustainable Agriculture Program (ASAP): Founded in the mid-1990s, ASAP runs multiple programs aimed at linking farmers to markets and consumers, and building healthy communities through local food connections. As research findings accumulate, the research team will provide ASAP with research summary materials and infographics for newsletters and presentations, which will be communicated to stakeholders where appropriate through their Local Food Campaign and Growing Minds Farm to School Program. ASAP’s Local Food Campaign is aimed at increasing public demand for local farm products and by building farmer capacity to access market opportunities and includes initiatives such as the Local Food Guide, the Asheville City Market and the Business of Farming conference. Through the Growing Minds Farm to School Program, ASAP provides training and resources to educators and health professionals to create local food and farm experiences that promote community health. ASAP engages its community through a number of avenues. ASAP communications outlets include 850,000 page views across ASAP’s websites, 24,000 e-newsletter subscribers, including monthly news, weekly farmers market reports, and farm to school news, and 24,000 social media followers. This audience includes farmers, farmers market customers and managers, educators, health care professionals, and more. ASAP will share findings that match our efforts to promote the consumption of locally grown farm products.
COVID-19 Contingency Planning
Although we hope the impacts of COVID 19 will be less significant in years 2 and 3 of this project, we have developed contingency plans for the in-person programs mentioned above. For our producer outreach and education, all in-person workshops by Amazing Grazing, NC Choices, and Understanding Ag will be delivered remotely through NCSU Zoom services (NC State’s license includes a Pro account for active university employees and students). Additionally, depending on state policies, NC State has an exemption process in-place for holding in-person events, with proper social distancing and safety protocols, which we can apply for on-farm events. If restrictions prevent any on-farm events, content will be delivered in a hybrid Zoom and pre-recorded video content format. Pre-recorded videos and recorded workshops can be made available for later viewing. NC Extension has already shifted toward both digital and remote delivery of workshops and trainings this year with great success, and it is expected that this can be used as a successful mode of delivery if needed. Further, the Carolina Meat Conference was unfortunately canceled this year, however in future years with sufficient planning, it is likely that this will be held virtual if necessary. The remote delivery is not expected to impact our evaluation plans.
For consumer education, specialized annual events, such as the Farm to Fork Picnic, may unfortunately no longer be held under COVID 19 restrictions. However, we have a diversity of avenues to reach consumers, many of which will be largely unaffected. These include our digital and social media content, marketing at farmers' markets (which are considered essential), and the NC 10% Campaign. Further, all in-person workshops and training content through NCCE FSC can be transitioned to remote delivery, as is currently being done by utilizing the Extension portal, Facebook, Zoom, and Twitter. For industry stakeholder education and outreach, programming held through NC Choices and ASAP will be held virtually as explained above, including Zoom webinars, posted recordings, and digital campaigns.
Learning Outcomes
Having scientific data on the nutrient density of their product quality. Many farmers expressed the belief that by rotationally grazing cattle on plant-diverse pastures they are improving the health and wellness of their animals, which they suspect should translate into beef with added nutritional benefits. This work was largely able to confirm that notion by finding improved markers of animal health and more antioxidants + omega 3 fatty acids in the beef.
Improvement soil health by finding more soil organic matter and minerals in pastures compared to paired feedcrop lands.
Ability to market meat to consumers
Project Outcomes
The data that has been disseminated from this project has reached considerable number of grass-fed beef farmers (1000+) through a keynote at the Carolina Meat Conference, webinars through the Bionutrient Institute and Understanding Ag, and popular farming podcasts such as the Regenerative Agriculture Podcast. We have had direct contact with 50 farmers that indicated usefulness of the data.
The economic benefits include having scientific data for farmers to indicate that the beef they are raising can be more nutrient dense in terms of antioxidants and omega-3 fatty acids. This allows them to market this to consumers and wholesalers, thus commanding a premium. We have various examples of farmers who have been successful in direct to marketing such as the partners included in this work. We also have reached a broader number of grass-fed beef farmers across the country, who our lab is now routinely testing samples for. Thus, this the work performed as part of the SSARE grant has resulted in broad reach.
The environmental benefits are demonstrated in this work, by finding improved soil health in rotationally grazed pastures. This provides further evidence to the scientific community that such grazing practices can have environmental benefits. As a result of this project, we have started to work with Regenified, who provide regenerative organic certification to farmers that have improvements in soil health.
The social benefits typically include selling products to local consumers and providing good governance of the local eco- and social system.
Future studies, I believe, should include a broader number of crops as well.