Final report for ONE19-353
Project Information
American chestnut was once the dominant tree species in much of the eastern United States. Breeding efforts following the devastating chestnut blight in the early 1900s have made growing chestnuts an increasingly viable option for producers. Pest pressures still limit production, however. Lesser chestnut weevil larvae feed inside of chestnuts rendering them unmarketable and causing substantial losses. This project evaluates the viability of two biological control agents, entomopathogenic nematodes and entomopathogenic fungi, for the control of chestnut weevil. The entomopathogens have proved effective in controlling other, related, weevils. We seek to establish their effectiveness as an attractive, environmentally friendly, control option for organic chestnut production. Success in this project would remove a primary barrier to productive chestnut production and facilitate economic development in this nascent industry.
This project seeks to demonstrate that biological control using entomopathogenic fungi and entomopathogenic nematodes is a viable method for control of chestnut weevil in the Northeast. Success in this project will provide producers with an environmentally friendly, effective method at increasing organic and conventional chestnut production.
This project evaluates the viability of two biological control agents, entomopathogenic nematodes and entomopathogenic fungi, for the control of chestnut weevil. The entomopathogens have proved effective in controlling other, related, weevils. We seek to establish their effectiveness as an attractive, environmentally friendly, control option for organic chestnut production. Success in this project would remove a primary barrier to productive chestnut production and facilitate economic development in this nascent industry.
Cooperators
- (Researcher)
Research
Project Period: April 2019 until now
Methods:
- Field Map:
- Soil samples:
-
- Before treatments: Collected soil samples from the field to evaluate the presence of native fungi and native EPN.
- Soil samples: April, 22th (5 samples/treatment)
- Add gallerias: April,23th( 10 unit/soil sample); Dried dish: May, 1st; Fungi evaluation May, 9th (a. Metarhizium; b. Beauveria) EPN evaluation May, 19th (c. H. bacteriophora; d. S. feltiae).
- After season: Collected soil samples from the field to evaluate the presence of native fungi and native EPN.
- Soil samples: Nov, 14th (5 samples/treatment)
- Add gallerias: Nov, 15th( 10 unit/soil sample); Dried dish: Nov, 25th; EPF evaluation Dec, 2nd (a. Metarhizium; b. Beauveria) and EPN evaluation Dec, 10th (c. H. bacteriophora; d. S. feltiae)
- Before treatments: Collected soil samples from the field to evaluate the presence of native fungi and native EPN.
-
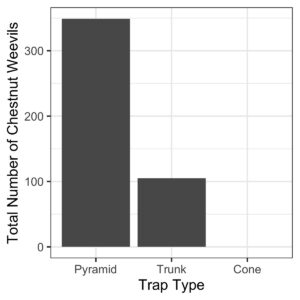
- Traps evaluation: Evaluate different traps to monitor Chestnut weevil. To evaluate we collected all the insect samples present in each trap, including Chestnut Weevil. The evaluation happened roughly every 15 days.
- Cone trap:
- Pyramid trap:
- Trunk trap:
- Treatments: Four treatments were tested: EPF (entomopathogenic fungi); EPN (entomopathogenic nematode); EPN+EPF (combination of entomopathogenic nematode and entomopathogenic fungi) and control (water)
- Control: Applied 500mL water application around each trunk tree.
- EPF: Mycotrol ESO from Bioworks (1.5quarts per 50 gallons evenly distributed among all EPF treated trees). EPF was applied on June 17.
- EPN: Applied 500mL EPN solution to each trunk tree (50:50 S. feltiae: H. bacteriophora). EPNs were applied on May 23, June 6th, and July 1.
- EPN+EPF: Applied 500mL EPN solution to each trunk tree (50:50 S. feltiae: H. bacteriophora) + EPF solution as described above.
- Microcosm Assay: Plastic buckets (37cm high and 30cm diameter) with ten screened rolls (10 cm diameter) were added in the field with soil inside and 25 Chestnut Weevil grubs placed in each one. The treatment application was the same as we used in the field.
- Add buckets and grubs: October 30th, 2019.
-
- Soil sample before treatments: October 30th, 2019. One sample for each bucket - 20 samples)
-
-
- Galleria added (November, 12th, 2019): 10 Galleria larvae were added in each soil sample to evaluate the presence of EPF (a. Metarhizium; b. Beauveria) and EPN (c. H. bacteriophora; d. S. feltiae). EPF evaluation Nov, 22nd (a. Metarhizium; b. Beauveria) and EPN evaluation Dec, 2nd (c. H. bacteriophora; d. S. feltiae).
-
- b.
- d.
- Soil sample the end of the season:
- Treatments: Four treatments were tested: EPF (entomopathogenic fungi); EPN (entomopathogenic nematode); EPN+EPF (combination between entomopathogenic nematode and entomopathogenic fungi) and control (water). Application on Nov 13th, 2019.
- Control: Applied 500mL water application to each microcosm.
- EPF: Mycotrol ESO from Bioworks (3.75ml of Mycotrol solution per 500ml of liquid applied to each treatment.)
- EPN: Applied 500mL EPN solution in each microcosm (50:50 S. feltiae: H. bacteriophora).
EPN+EPF: Applied 500mL total volume solution to each microcosm containing EPN (50:50 S. feltiae: H. bacteriophora) + EPF (3.75ml of Mycotrol ESO)
- Field Soil Sample before treatment:
-
- No EPN presence detected.
-
- EPF were present.
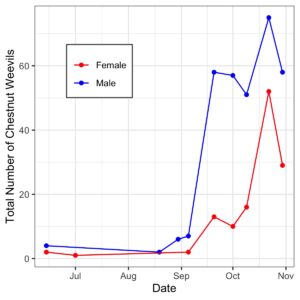
- Chestnut Weevil Populations Peaked in Late October/November
- EPF Treatment has consistently lower Chestnut Weevil compared to controls
- Addition of EPF significantly reduced Chestnut Weevil Populations by ~20%
- Pyramid traps caught more Chestnut Weevils
Chestnut Weevils have multi-year generations
Larvae burrow underground, pupate, eclose as adults then stay ~ 2in below the soil surface for at least another year before emerging again.
Chestnut Weevils are pernicious nut pests
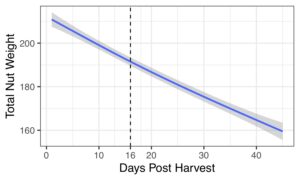
Multiple (up to 9!) chestnut weevils can live in and emerge from a single nut. Nuts lose a substantial amount of weight post-harvest. EPF treatments reduced percentages of damaged nuts. 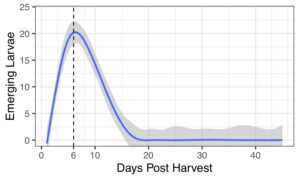
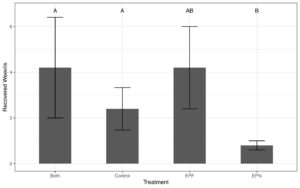
- Native EPN species effectively killed Chestnut Weevil Larvae
- Particularly H. bacteriophora and S. feltiae
- Both laboratory trials and field microcosms showed that EPN presence reduced Chestnut Weevil Populations
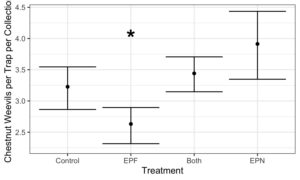
The goal of this project was to develop biological control options for the Chestnut Weevil. We have identified two biological control options that are successful in reducing populations of Chestnut Weevils. Entomopathogenic nematodes are effective at controlling belowground larval Chestnut weevils. Entomopathogenic fungi reduce populations of aboveground adult chestnut weevils and reduce nut infestations. Together, these biological control options could provide long term control of this emergent pest. Implementation of these strategies could return much of lost nut yield to the grower. Results from two field seasons suggest that reducing pest levels below economic damage thresholds could be a possibility if these techniques are used together over time.
Education & Outreach Activities and Participation Summary
Participation Summary:
Results from this field season are being prepared to disseminate in publications and upcoming workshops. Additionally, a video was prepared highlighting IPM strategies in Chestnut that is being distributed through Cornell's network.
Individual meetings with growers in NY, NJ, VA, OH, and Michigan. 2 Presentations to extension agents. 1 Workshop with 61 participants. Fact sheets and extension publications are being prepared.
Learning Outcomes
- Knowledge of chestnut weevil population dynamics
- Use of EPF for control of chestnut weevil
- Use of EPN for control of chestnut weevil
- Biological control principles and practices
Project Outcomes
This project has only been active since August. We have documented control practices that are being adopted by our grower collaborator and developed an educational video for dissemination. We will be pursuing additional grant funding and interacting with additional stakeholders throughout the upcoming year.
A number of successes from this year:
We showed that that the weevil stays underground for more than 1yr which means that growers are managing multiple populations.
We showed that EPNs can reduce chestnut weevil larvae in the field and that EPF can reduce nut damage.
We have some of the first photos of weevil pupae and adults underground. 
We also used the data from this project to support a successful grant application from the NNGA for further population studies.
Continue evaluating control strategies and expand trials over the next year. Critical need for more research into basic biology of the pest and chemical ecology control methods. Chestnut growers will need the research - the pest is becoming a substantial problem that may limit development of this burgeoning industry.
This year highlighted the need to better understand the pest life cycle - knowing that multiple generations could persist in the soil for more than a year can change pest management practices.






