Final report for SW18-063
Project Information
Barn owls are a popular component of Integrated Pest Management (IPM) programs for the control of rodent pests across the Western United States and contribute to sustainable agriculture through integration of natural biological control, enhancing the environmental quality of agricultural regions, and sustaining the economic viability of agricultural operations. However, because farmers utilize rodenticides to control rodent pests, owls can suffer from both lethal and sublethal secondary poisoning. Despite the important role that owls can play in providing long-term, sustainable, and natural pest control services, we have little understanding of how often owls are exposed to rodenticides and what effect this exposure has on their behavior and reproductive success.
To tackle this critical gap in knowledge, our study addressed five key objectives: 1) Determine if rodenticide exposure affects growth rates in owl nestlings; 2) Understand how land-use type, rodenticide applications, and prey choice affect the frequency of rodenticide exposure; 3) Inform predictive models on the efficacy of barn owls for controlling rodent pests on farms; 4) Create stakeholder-verified recommendations for the use of rodenticides in combination with barn owls for effective IPM; and, 5) Disseminate findings to producers through publications, a field-demonstration, visits to schools, and presentations to pest-control and agricultural groups.
From 2018 to 2021, we banded 224 nestlings and 67 adults. From 2018 through 2020, 44 nest boxes were monitored revealing a 79.5% success rate of at least one chick fledging from a nest box. There was low field application of rodenticides at our study sites and blood sampling did not always align with timing or location that baiting that occurred. We screened 231 breeding barn owls (adults and nestlings between February and August 2018 to 2021) from 121 nests across three counties for circulating rodenticide exposure and found one positive result for Chlorophacinone cted at trace (<5ppb) levels.
Our morphometric monitoring of nestlings (561 measurements from the successfully fledged nestlings and 273 measurements from failed nestlings) revealed that the average fledge age for a barn owl nestling was 55 days, or 7.8 weeks.
We dissected 1,595 pellets between 2018 and 2020 to identify the diet of barn owls living in agroecosystems. Rodent pests for each habitat made up over 95% of the barn owls’ diet. In vineyard systems, the preferred prey choices were mice and vole, whereas gophers were a more opportunistic choice. Video monitoring took place in 2020 and 2021. Seven nest boxes were used with a total of 38 nights recorded. We were able to use this average to estimate the amount of prey items consumed by nestlings when in the nest box. The average biomass removed during an eight-week period averaged to approximately 30 kg per nest box.
Between the years 2019-2021, we tracked 12 breeding female barn owls using Alle-300 Ecotone GPS units. We obtained an average of 2,929 GPS data points per owl, resulting in an average of 6.5 nights of movement tracking per owl. Qualitative assessments of our owl movement data confirm that barn owls breeding within California agriculture exhibit hunting behaviors in various crop fields, adjacent grassland habitats, along roads and highways, and around crop fields they nest in.
Overall, our results indicate that barn owls in this system foraged in habitat immediately surrounding nest structures as well as nearby agricultural and grassland habitats. In these habitats, the barn owls targeted rodent pest species and removed substantial biomass of prey species from the surrounding habitats. We found limited evidence of acute anticoagulant rodenticide in blood and pellet samples collected at our study site which corresponds with information gathered from collaborating producers who reported limited baiting. Together, these results highlight the utility of barn owls as an effective tool in integrated pest management strategies at both local scale and at a regional scale.
Future research should be focused on sampling for rodenticide exposure simultaneously with field rodenticide applications and in landscapes with a larger number of buildings and facilities where second-generation anticoagulant rodenticides are regularly used.
1) Determine if rodenticide exposure affects growth rates in owl nestlings;
2) Understand how land use type, rodenticide applications, and prey choice affect
the frequency of rodenticide exposure;
3) Inform predictive models on the efficacy of barn owls for controlling rodent pests
on farms;
4) Create stakeholder-verified recommendations for the use of rodenticides in
combination with barn owls for effective IPM; and,
5) Disseminate findings to producers through publications, a field-demonstration,
visits to rural schools, and presentations to pest-control and agricultural groups.
Cooperators
- - Producer
- - Producer
- - Producer
- - Producer
Research
Our proposal named 5 specific goals within the 3-year timeframe of our proposed project:
- Determine if rodenticide exposure affects growth rates and survival in owl nestlings;
- Understand how land use type, rodenticide applications, and prey choice affect the frequency at which barn owls are exposed to rodenticides;
- Use data on prey choice, prey delivery rates, and hunting patterns to inform predictive models on the efficacy of barn owls for controlling rodent pests on farms;
- Create stakeholder-verified recommendations for the use of rodenticides in combination with barn owls for effective IPM of rodent pests; and,
- Disseminate findings to producers through publications, a field-demonstration, visits to rural schools, and presentations to pest-control and agricultural groups.
Study design and procedures are designed to collect appropriate type and amount of data for analyses surrounding barn owl survival, reproductive success, chick growth rate, nest attendance, diet, foraging ecology, and rodenticide exposure in an agricultural setting in Yolo and Solano Counties.
All work was conducted under appropriate federal (# 23406) and state permits (# 1259). Field sampling protocols were approved by UC Davis Institutional Animal Care and Use Committee (IACUC # 20516).
Study Sites:
Our study was conducted on agricultural properties in Yolo, Solano, Sonoma, Napa, and Monterey counties in California. The crops grown on these farms included perennial crops such as wine grapes and fruit and nut trees; forage crops such as alfalfa and winter wheat; and annual crops such as tomatoes, sunflowers, and corn. Properties varied in the amount of natural land cover, such as grassland, oak woodland, and oak savannah, in the vicinity. This work took place annually between the months of January and August for the years 2018-2021.
Nest monitoring and banding:
We monitored occupancy and nest status using a live-streaming camera (SONY Action Cam) attached to an extension pole (Kross et al. 2016; Wendt and Johnson 2017). This is an effective way to minimize nest disturbance and the chance of flushing adults from nest boxes. We recorded nests as unoccupied, roosting individuals or pairs, incubating females, eggs and/or nestlings. Nest boxes that were at appropriate stages were included in rodenticide exposure, nestling growth, telemetry, and/or video monitoring protocols.
We banded all roosting adults and nestlings (minimum of 4 weeks old; Varland et al. 2007) using US Geological Survey federal lock on leg bands. We did not disturb owls in the incubation period due to risk of nest abandonment (Taylor 1994). We used a stockinette head covering to minimize stress once owls were removed from the nest box (Colvin and Hegdal 1986). We took morphometric measurements, including mass, hallux length, wing chord, tail length, culmen length, and facial disc width. At this time, we took a blood sample for rodenticide screening and collected pellets from the nest box (see below). Individuals were returned to their nest boxes with the entrance holes temporarily plugged (i.e., wooden plug or towel) to prevent adults from flushing, which is a standard technique when capturing birds from nest boxes (Colvin and Hegdal 1986). The entrance hole was unplugged immediately before vacating the site.
Rodenticide exposure screening:
Because we wanted to understand rodenticide exposure in a living population, we used blood samples to screen for circulating rodenticide exposure. Compounds can be detected in the blood after consuming exposed prey for a window of time that may be as short as several days or as long as 2 weeks, depending on dose and compound. We sampled adults and nestlings over 4 weeks of age found in nest boxes. If nestlings were being studied for growth rates, they were sampled up to 2 times between the 4-9 week old nestling period. Individuals that were sampled multiple times were not sampled within the same 14 day period. For nests that had many nestlings and were not part of the growth or telemetry study, we selected two random nestlings for blood draws.
Prior to blood draws, we weighed each individual to ensure the maximum of 1% of body weight allowed by IACUC guidelines was not exceeded, although due the required amount of blood for rodenticide screening we collected much less than 1%. Blood was drawn via medial metatarsal venipuncture using a 25 gauge needle and a 3cc syringe. Past experience working with owls and other raptors, as well as discussion with raptor veterinarians, indicates that this is the best location for drawing blood and minimizes the likelihood of hematoma. If we were unable to obtain blood from this site we did not attempt another location. Prior to drawing blood, the metatarsus was wetted with disinfectant and pressure was applied to visualize the vein. One researcher restrained the bird (the handler) while the second performed the blood draw. Following the blood draw, the handler applied pressure to the draw site for a minimum of 1 minute and visually inspected the draw site to ensure bleeding had stopped. Prior to release of the owl back into the nest box, we inspected the draw site again to ensure bleeding had not resumed.
Blood was stored in a cooler and frozen in cryovials in the lab. Blood draws were conducted by researchers that were trained by UC Davis avian veterinarians. Rodenticide screenings took place at Texas A&M Veterinary Medical Diagnostics Laboratory via measurement by liquid chromatography/mass spectrometry (LC/MS), which detects occurrence and measures quantity of first- and second-generation anticoagulant rodenticides (Warfarin, Bromadiolone, Brodifacoum, Diphacinone, Chlorophacinone, Coumatetralyl, Difenacoum, and Difethialone).
We collaborated with Martinico et al. (GW19-200) to compare exposure rates from breeding owls to exposure rates of owls and hawks in fall and winter on the same properties. We also collaborated with Phillips et al. (in prep) to compare rodenticide exposure detection from blood samples to detection through regurgitated pellets found in the same nest boxes.
Nestling growth rates:
We visited a subset of nests with nestlings on a weekly basis to gather data to compile growth curves, test for rodenticide exposure (see above), and gather pellets for diet analysis (see above). Due to extreme heat of the Central Valley in spring and summer, all handling and measurements took place in the early morning and was completed before temperatures exceeded 80 degrees F to prevent hyperthermia. When possible, permanent and temporary shade structures were used to minimize nestlings' exposure to direct sunlight. Before entering a box, we plugged the nest box entrance to prevent any adults that might be present from flushing. We measured mass, hallux length, and culmen length for all nestlings. Once feathers emerged from the skin we took wing chord, tail length, and facial disk width measurements. Nestlings were marked with non-toxic fabric dye on downy feathers on the head for future identification prior to being old enough for banding. We banded nestlings once they reached 4 weeks old, when tarsi have fully developed (Taylor 1994, Varland et al 2007).
Diet monitoring:
We collected all fresh whole pellets from the inside of active nest boxes at each visit and subsequently froze pellets until further analysis. We dissected pellets to classify the diet of the barn owls living in different agroecosystems. We identified vertebrate prey items commonly found in the barn owl diet which included: deer mice (Peromyscus maniculatus), house mice (Mus musculus), California voles (Microtus californicus), pocket gophers (Thomomys spp.), Norway rats (Rattus norvegicus), and black rats (Rattus rattus). We did not measure or try to distinguish between house and deer mice and therefore used the estimated biomass of mice surveyed in a tangent study on the same vineyard (Phillips et. al., 2020). We estimated the biomass of voles and gophers using previous study methods (see Kross et. al. 2016). We chose to be conservative when dissecting the pellets and therefore jaw bones were the only identifying marker used. Any pellets that did not have jaw bones were not identified to prey species and therefore were not included in the final pellet results. We collaborated with Martinico et al. (GW19-200) to compare breeding diet to fall and winter diet on the same landscape. This collaboration assisted in if the proportions of prey shift during different times of the year, which may impact susceptibility to secondary exposure to ARs in barn owls.
Nest boxes with more than 11 intact pellets collected were used to identify diet proportion and biomass removed from the surrounding landscape (Charter et al. 2012). Jaw bone measurements from pellet dissections were used to estimate average biomass of prey items by nest box and the average biomass of prey items over the entire network of nest boxes. If a nest box had less than three gophers and/or voles found in pellets dissected, the average biomass for those nest boxes were based on the average gopher/vole mass from the entire nest box network rather than the individual nest box. Using video monitoring (see below) we were able to identify the average biomass per prey delivery (D). The average number of prey deliveries per nestling per night was calculated using prey delivery videos (4.19 deliveries per night per chick), and was multiplied by the average number of nestlings during the approximate eight week period they were in the nest box. The number was then multiplied by D to give us the average biomass delivered per night. Finally, this number was multiplied by the number of days in the nest box to give us the average biomass consumed by each nest box during the nestling period. If a nest box failed (i.e., all nestlings perished), the eight week period was decreased to the age of the nest box at failure.
Video monitoring:
Prey deliveries were recorded using a low-cost motion trigger video camera between 2019 and 2021 breeding seasons. The low-power Raspberry Pi Zero W was used to create an affordable alternative to higher-end wildlife cameras currently on the market. This micro-computer has Bluetooth and LAN technology and has an open-source operating system allowing for high customization opportunities. The computer language for coding the camera was written in Python 3 (van Rossum 1995). A tupperware container (Rubbermaid Brilliance Food Storage 3.2 cup) was used as a waterproof case. A passive infrared motion sensor was used to activate the motion capture video. Videos were saved on a microUSB (SanDisk Ultra Dual Drive m3.0 32GB) connected to the Raspberry Pi. The cameras were set up for motion capture and were set to save video that occurred three seconds prior to motion, and then ten seconds after the camera was triggered (13 seconds per recorded motion trigger).
Cameras were placed at the same time as nestling growth measurements were being taken to decrease disturbance of the nest box. The cameras were placed on the opposite side of where the nest box entrance was to capture prey deliveries brought back by adults. Batteries to operate the camera (Hiluckey 25000mAh Solar Charger, RAVPOWER 25000mAh Solar Power Charger RP-PB092; discontinued) were placed on top of the next box and were exchanged weekly during nest box visits.
Prey were identified in videos where items were clearly visible and not obstructed by the adult or nestling owls, and classified into general size categories when difficult to identify (small, medium, or large rodent, reptile, bird, invertebrate). During initial nest box monitoring, when we found an adult, we strategically placed a USGS metal band on different legs to identify male versus female later on the recorded videos. If we could not identify the adult during the video, the adult was marked as unknown.
Telemetry:
To document spatial hunting patterns and foraging in proximity to rodenticide bait stations, we used GPS transmitters (Alle-300 models, Ecotone Telemetry, Poland). Transmitters were programmed to record 1 GPS point every minute each night from between 20:00—06:00 for the duration of battery life of the unit (expected to last 5-7 days). Females were selected for transmitter deployment if they had greater than 2 week old nestlings and were heavy enough so that the total mass of the unit and harness did not exceed 3% of body mass (IACUC guidelines; USGS Bird Banding Laboratory; Kenward 2001; Boal 2014). Transmitters were placed on the bird's back and attached with teflon ribbons that crossed at the breast at the top of the keel (this ensures the crop is not obstructed) and under each wing to connect to the two loops at the bottom of the transmitter. The teflon ribbons were carefully adjusted for fit by working them underneath the feathers and leaving one finger width of space (this ensures the transmitter is not too loose or too tight). Once the ribbons were adjusted, they were clamped in place and glued with a small amount of super glue to prevent fraying. Transmitter fit was rechecked before release by ensuring normal movement of the owl and that there is one finger width between the transmitter and the bird. Our processing time for attaching and detaching GPS transmitters of each adult did not exceed 20 minutes and was only conducted by trained and permitted individuals. We downloaded data from the transmitter’s base station, installed beneath the nest box, to infer the duration of the battery and when we should return to retrieve the transmitter. We made every attempt to recapture owls for transmitter removal before nestlings fledged.
Nest monitoring and banding:
From 2018 to 2021, we banded 224 nestlings and 67 adults. From 2018 through 2020, 44 nest boxes were studied with a 79.5% success rate of at least one chick fledging from a nest box (Table 1). Only 48% of the chicks that hatched survived to fledging. Nest boxes in the study were checked once a week to take morphological measurements of the nestlings to observe growth rates. Nestlings were sampled for rodenticide exposure via blood 0-2 times during weeks 4-9 of their nestling phase. Pellets were collected for diet analyses and were tested for rodenticide exposure in collaboration with Phillips et al. (unpublished data).
|
Year |
Habitat |
Monitored Boxes |
Failed Boxes |
Nestlings Hatched |
Nestlings Fledged |
Fledge Rate |
Success Rate |
|
2018 |
Vineyard |
14 |
1 |
51 |
31 |
66% |
93% |
|
|
Mosaic |
3 |
0 |
14 |
13 |
93% |
100% |
|
2019 |
Vineyard |
10 |
3 |
37 |
9 |
24% |
70% |
|
2020 |
Vineyard |
17 |
5 |
72 |
30 |
42% |
71% |
|
Total |
|
44 |
9 |
174 |
83 |
48% |
80% |
Table 1: Number of barn owl nest boxes monitored between 2018 and 2020 in the Central Valley of California. The success rate indicated the percentage of failed nest boxes to monitored nest boxes. Failed boxes indicate nests that did not fledge any nestlings, but does not include unhatched nests. The fledge rate is the proportion of nestlings hatched to nestlings that fledged.
Rodenticide exposure screening:
We screened 231 breeding barn owls (adults and nestlings between February and August 2018 to 2021) from 121 nests across three counties for circulating rodenticide exposure and found one positive result (Table 2). While pellets were screened for rodenticide exposure from many of these nest boxes (Phillips et al. unpublished data), there were no whole pellets available to collect from the nest box that had a positive result, so no comparison was made in this case. The positive blood test was in a 6 week old nestling in a nest box with 5 nestlings total, all which survived to fledge. The compound identified was Chlorophacinone and it was detected at trace (<5ppb) levels.
We found that a second generation anticoagulant rodenticide, Bromadiolone, was administered year-round by professional pest control companies in and around offices and processing facilities on our sites. A cluster of several buildings and facilities were present at each site, although buildings were generally scarce on the landscape. The first generation compound Diphacinone had infrequent field-use on an as needed basis, typically in spring and/or summer. There was likely unknown use of first and second generation anticoagulant rodenticides on neighboring properties because we detected two second generation compounds samples from winter months (Martinico et al., unpublished data; GW19-200) and Chlorophacinone that was not applied by land managers at our study sites.
There was low field application of rodenticides at our study sites and blood sampling did not always align with timing or location that baiting that occurred. Because we intended to study the interaction between owls and the typical pest management schedule, we did not influence the applications of rodenticides. Future research should be focused on sampling simultaneously with field rodenticide applications and in landscapes with a larger number of buildings and facilities where second-generation anticoagulant rodenticides are regularly used.
|
Location (county) |
Individuals |
Nest boxes |
Positive tests |
|
Yolo |
153 |
71 |
1 |
|
Napa |
40 |
25 |
0 |
|
Sonoma |
38 |
25 |
0 |
|
Monterey |
3 |
2 |
0 |
|
Total |
231 |
121 |
1 |
Table 2. Number of blood samples submitted for rodenticide screening for individuals across four counties and from 121 nest boxes. One positive blood test was detected in Yolo County.
Nestling growth rates:
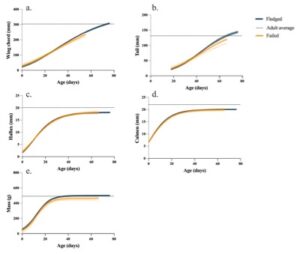
The average fledge age for a barn owl nestling was 55 days, or 7.8 weeks. We collected 561 measurements from the successfully fledged nestlings and 273 measurements from failed nestlings. Nestling growth curves were made separately for the successful and failed nestlings’ growth of the hallux, culmen, wing chord, tail, and mass (Figure 1).
To our knowledge, these are the first comprehensive nestling growth measurements of the American barn owl (Tyto furcata). This is especially important because of interest in using barn owls as natural pest control. Understanding more of their life history also helps researchers and growers discover best practices using barn owls in integrated pest management systems. Finally, this information can further studies researching life history traits including environmental effects on nestling growth that could impact future pest control benefits provided by the birds.
Figure 1 : American barn owl growth rate curves of the wing chord (a.), tail (b.), hallux (c.), culmen (d.), and mass (e.). The dark blue line represents data from the fledged dataset and the gold line represents data from the failed dataset. Dashed lines of their respective line color represent the standard error. The adult average is the black dashed line to show nestlings have not completed their growth during their time in the nest box.
Diet monitoring:
We dissected 1,595 pellets between 2018 and 2020 to identify the diet of barn owls living in agroecosystems. The prey proportions in the diets between mosaic agriculture and vineyards differed (Table 3). In a mosaic agricultural system, barn owls consumed more voles than any other rodent pests, while in vineyards, the majority of the diet were mice. Rodent pests for each habitat made up over 95% of the barn owls’ diet.
|
Habitat |
Total Pellets |
Gopher |
Mouse |
Vole |
Rat |
Invert |
Bird |
Other |
|
Diet Proportion in Mosaic |
277 |
24% |
31% |
43% |
1.1% |
1.1% |
0% |
0% |
|
Diet Proportion in Vineyard |
1318 |
16% |
60% |
20% |
1.1% |
1.2% |
1% |
1% |
Table 3: The total number of barn owl pellets dissected from monitored nest boxes. Diet was separated between mosaic and vineyard habitat because of assumed differences in rodent species abundances. The mosaic nests relied more on voles in their diet while vineyard habitat nests had a higher proportion of mice in their diet. The other category includes fence lizards and rabbits we saw cached in nest boxes or seen on video.
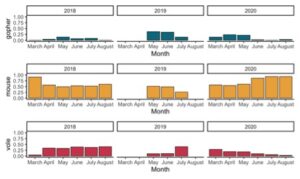
In vineyard systems, the preferred prey choices were mice and vole, whereas gophers were a more opportunistic choice (Figure 2). Because rodent pests make up the majority of the diet, we are able to see comparable changes between these prey choices. As the number of mice in the owls’ diet changes, there is an inverse relationship with the number of voles in the diet. Based on the differences in diet between the mosaic and vineyard systems, we can assume that barn owls will hunt for any rodent pest available, but may change focus to a better prey choice if one increases in abundance. Future research may be beneficial in looking at this change in hunting behavior to identify barn owl practicality if growers have specific rodent pest problems.
Figure 2: Proportion of rodent pests found in the diet of barn owls nesting in vineyards. A relationship between mice and voles in the barn owls’ diet can be seen. Gophers in the diet do not show any pattern.
Video monitoring:
Video monitoring took place in 2020 and 2021. Seven nest boxes were used with a total of 38 nights recorded (Table 4). Based on the number of hours recorded and the number of nestlings at the time, the adults brought on average four prey items per nestling per night. We were able to use this average in our biomass estimation formula to estimate the amount of prey items consumed by nestlings when in the nest box. The average biomass removed during an eight week period averaged to approximately 30 kg per nest box. We estimated the total biomass of rodent pests removed from barn owl nests to be approximately 1,030 kg. This biomass estimation accounts for only the nest boxes we monitored (44) and does not take into account any other nest boxes, nor the prey items that adults consumed themselves while out hunting. Thus, this is a conservative number and we expect future research to better identify biomass estimation removed from an entire nest box network.
Our analysis shows the number of prey deliveries differed significantly between the adults, where the male averaged 10.7 deliveries per night to a females’ 5.5 deliveries per night. It is important to note that one of our boxes had a single female attending to the nestlings due to either male abandonment or perishing. Despite being a single female, she averaged the most deliveries per night out of the seven nest boxes at over five deliveries per nestling per night. While the parental attendance behavior appears to be a tradeoff between the female incubating and the male hunting, this nest showed the female could make up for the loss of her mate.
While we do not have data on rodent populations prior to these nest box networks being erected a decade ago, it is still obvious that barn owls are effectively decreasing the amount of rodent pests on the landscape. By gathering this data from a single system (see Kross and Baldwin 2016 using combined studies for biomass estimation removal) we have a better understanding of what a nest box network can accomplish on a specific landscape.
|
Box |
Nights Recorded |
Number of Nestlings |
Deliveries |
Delivery Rate/hour |
Average deliveries per nestling per night |
|
20 (2020) |
1 |
5 |
19 |
2.1 |
3.8 |
|
22 |
2 |
4 |
30 |
1.7 |
3.8 |
|
33 |
3 |
4 |
52 |
1.9 |
4.3 |
|
29 |
5 |
5 |
79 |
1.8 |
3.2 |
|
10 |
5 |
5 |
111 |
2.5 |
4.4 |
|
14 |
11 |
3 |
186 |
1.9 |
5.6 |
|
20 (2021) |
11 |
5 |
152 |
1.3 |
2.8 |
|
Total |
38 |
31 |
629 |
1.9 |
4.0 |
Table 4: American barn owl nest box recordings during 2020 and 2021.
Telemetry:
Between the years 2019-2021, we tracked 12 breeding female barn owls using Alle-300 Ecotone GPS units. GPS units were programmed to record 1 GPS point every minute from between 20:00—06:00 each night. Nine of the owls tracked were breeding in nest boxes installed in vineyard and olive orchard habitats. Three owls tracked were breeding in nest boxes surrounded by various crop types, including forage and row crops, fruit and nut orchards, and fallow fields. We affixed GPS units to adult females when nestlings were 2-4 weeks old to capture movement of when the female’s hunting resumes.
For vineyard and olive orchard breeding owls, we obtained an average of 2,929 GPS data points per owl, resulting in an average of 6.5 nights of movement tracking per owl. For owls breeding in mosaic habitats, we obtained an average of 2,489 GPS data points per owl, resulting in an average of 7 nights of movement tracking per owl. Qualitative assessments of our owl movement data confirm that barn owls breeding within California agriculture exhibit hunting behaviors in various crop fields, adjacent grassland habitats, along roads and highways, and around crop fields they nest in. We also observed movement patterns and habits where females would commute up to ~4km away from nest box to forage. We are currently conducting statistical analyses to quantify home range sizes, resource selection patterns, habitat use, and hunting behaviors in relation to agricultural land-use and areas of rodenticide application.
|
Habitat |
Individuals tracked |
Ave. nights tracked per individual |
Ave. GPS data points per individual |
|
Vineyard/Olive orchard |
9 |
6.5 |
2,929 |
|
Mosaic |
3 |
7 |
2,489 |
From 2018 to 2021, we banded 224 nestlings and 67 adults. From 2018 through 2020, 44 nest boxes were monitored revealing a 79.5% success rate of at least one chick fledging from a nest box. There was low field application of rodenticides at our study sites and blood sampling did not always align with timing or location that baiting that occurred. We screened 231 breeding barn owls (adults and nestlings between February and August 2018 to 2021) from 121 nests across three counties for circulating rodenticide exposure and found one positive result for Chlorophacinone cted at trace (<5ppb) levels.
We dissected 1,595 pellets between 2018 and 2020 to identify the diet of barn owls living in agroecosystems. Rodent pests for each habitat made up over 95% of the barn owls’ diet. In vineyard systems, the preferred prey choices were mice and vole, whereas gophers were a more opportunistic choice. Video monitoring took place in 2020 and 2021. Seven nest boxes were used with a total of 38 nights recorded. We were able to use this average to estimate the amount of prey items consumed by nestlings when in the nest box. The average biomass removed during an eight-week period averaged to approximately 30 kg per nest box.
Between the years 2019-2021, we tracked 12 breeding female barn owls using Alle-300 Ecotone GPS units. We obtained an average of 2,929 GPS data points per owl, resulting in an average of 6.5 nights of movement tracking per owl. Qualitative assessments of our owl movement data confirm that barn owls breeding within California agriculture exhibit hunting behaviors in various crop fields, adjacent grassland habitats, along roads and highways, and around crop fields they nest in.
Overall, our results indicate that barn owls in this system foraged in habitat immediately surrounding nest structures as well as nearby agricultural and grassland habitats. In these habitats, the barn owls targeted rodent pest species and removed substantial biomass of prey species from the surrounding habitats. We found limited evidence of acute anticoagulant rodenticide in blood and pellet samples collected at our study site which corresponds with information gathered from collaborating producers who reported limited baiting. Together, these results highlight the utility of barn owls as an effective tool in integrated pest management strategies at both local scale and at a regional scale.
Future research should be focused on sampling for rodenticide exposure simultaneously with field rodenticide applications and in landscapes with a larger number of buildings and facilities where second-generation anticoagulant rodenticides are regularly used.
Research Outcomes
Education and Outreach
● Project Facebook page: https://www.facebook.com/BarnOwlProject/?ref=br_rs
● Project Instagram page: https://www.instagram.com/barnowlproject/
● Project twitter feed (@barn_owlproject): https://twitter.com/barn_owlproject
● Ryan Bourbour twitter: https://twitter.com/talonDNA
In addition to the work we did with farmers, agricultural professionals, students, and the general public Emily Phillips, Breanna Martinico, and Ryan Bourbour mentors seven student interns who participated in aspects of the project-- including field work, labwork, and outreach. Interns gained valuable experience that allied them to obtain related jobs and apply to graduate school and vet school.
Participation Summary:
Consultations (5)
- Emily Phillips, Ryan Bourbour, and Breanna Martinico worked with a landowner in Yolo County on placement of 11 barn owl boxes and 3 kestrel boxes at an almond orchard.
- Emily Phillips, Ryan Bourbour, and Breanna Martinico worked with a second landowner in Yolo County on placement of 8 barn owl boxes and 2 kestrel boxes at a vineyard.
- Emily Phillips worked with a university student in placing a barn owl box in their backyard in a suburban environment.
- Breanna Martinico spoke with a grower relations representative for Napa Valley winegrowers about best practices to integrate barn owl boxes on the properties he represents. They discussed nest box number, placement, monitoring, and natural pest control.
- Sara Kross provided technical advice to the UC Extension farm on construction plans for a demonstration barn owl box.
Curricula, factsheets or educational tools
- Breanna Martinico, Emily Phillips and Ryan Bourbour created a poster outlining the goals and objectives of our work.
- Breanna Martinico and Ryan Bourbour were interviewed in short educational videos about barn owls and raptors on farms. https://www.youtube.com/watch?v=Xn5noyhFr9E&t=1s https://www.youtube.com/watch?v=cnZgdg0AzuM&t=1s
- Ryan Bourbour directed and filmed a video for World Migratory Bird Day showcasing the barn owl research done for this project. https://www.youtube.com/watch?v=ztzP52XagAc&t=232s
- Breanna Martinico, Emily Phillips, and Ryan Bourbour, helped to develop a fact/coloring sheet on barn owls that was distributed to winery customers to educate their customers on the importance of barn owls on their property.
- Breanna Martinico, Emily Phillips, and Ryan Bournour helped develop the Western SARE Quick Guide: Welcome in Barn Owls to Provide Rodent Control which summarizes information for farmers and land managers that want to encourage barn owls to nest on their property. https://western.sare.org/resources/welcome-in-barn-owls-to-provide-rodent-control/
Journal articles (3)
- Smith O, Bourbour RP, Cornell KA, Groendyk S, Hannay M, Martinico BL, Utley, O, Snyder W, and Lindell C. (2020) Promoting beneficial raptors: Identification, pest control, and management. EOrganic. Contributed by co-authoring sections on barn owls and red-tailed hawks. https://eorganic.org/node/34052
- Bourbour RP, Martinico BL, Phillips EM, Schlarbaum, JN, Hawkins MG, Hull JM, and Kross SM. (2022). Banding records of nesting barn owls reveals optimal timing for nest box management in California. Journal of Wildlife Management. (Accepted, in press)
- Phillips EM, Martinico BL, Bourbour RP, Baldwin RA, Kross SM, and Hull JM. (2022) A proposed framework to investigate the interactions between barn owls and anticoagulant rodenticides in an integrated pest management program. Proceedings of the Vertebrate Pest Conference. (in review)
On-farm demonstrations
- A group of twelve 6th graders from Woodland, CA met with Breanna Martinico, Emily Phillips, and Josh Hull at one of our vineyard study sites to learn about barn owl natural history and their role in natural pest control in agriculture. We were accompanied by the vineyard manager who also talked about pest control and growing vines from his perspective.
- A visiting international pest management research professional from the Julius Kühn-Institut in Germany met with Emily Phillps and Breanna Martinico to learn about barn owl boxes, placement, and role of barn owls and other raptors as natural pest control in agriculture.
- A group of seven grade school aged children from Sacramento, CA met with Breanna Martinico, Emily Phillips, and Ryan Bourbour at our study site and learned about barn owl natural history and their role as natural pest control agents in agriculture.
- Local landowner/farmer from Dixon, CA came to our study site to discuss barn owl box management, placement, and the role of barn owls and other raptors as natural pest control agents in agriculture with Breanna Martinico, Emily Phillips, and Ryan Bourbour.
Online trainings (0)
Published press articles, newsletters
- Our project was highlighted by the Western IPM center in a newsletter and video post. http://westernipm.org/index.cfm/ipm-in-the-west/agriculture/helping-barn-owls-help-farmers/
- Our project was featured in Western Farmer-Stockman magazine: https://www.westernfarmerstockman.com/crops/your-pest-control-killing-beneficials
- Our project was highlighted by The College of Agricultural and Environmental Sciences at UC Davis through a video on their YouTube channel- https://www.youtube.com/watch?v=N0mjvyZhgnQ&feature=youtu.be&fbclid=IwAR2yDzrgyhdx-EdASA0IgoKjmBNrPkbJYLFupPyE66DK7BZ8aYOyFd8DPHY
- We provided background information and relevant papers for an article published online by Environmental Health News called “Protecting Crops with predators instead of poisons” which covered multiple types of natural pest control services including by owls: https://www.ehn.org/back-to-basics-tackling-farm-pests-with-predator-birds-2546940909.html
- Sara Kross wrote a longform article about the role of beneficial birds in agriculture: https://www.yumpu.com/en/document/fullscreen/62280511/organic-farmer-dec-jan-2019
- Breanna Martinico was interviewed for an article in Bay Nature regarding barn owls and rodenticide exposure: https://baynature.org/article/raptors-rather-than-rodenticide/
Tours (0)
Webinars, talks and presentations
Talks to growers:
- Sara Kross gave a lecture on pest control services from owls and how to control pest birds in vineyards for a workshop hosted by the Napa County Agricultural Commissioner’s Office.
- Ryan Bourbour and Emily Phillips gave a talk on the role of barn owls and diurnal (day-hunting) raptors at a ‘Ground Squirrel and Gopher Management Workshop’ hosted by the Santa Clara County Division of Agriculture & UCCE Santa Clara County.
- Breanna Martinico gave a similar talk at a UCCE ‘Ground Squirrel and Gopher Management Workshop’ due to the overwhelming positive response from the first workshop participants in San Martin, CA.
- Breanna Martinico spoke at a UCCE workshop ‘The use of barn owls and other raptors in rodent control’ in Merced, CA
- Sara Kross gave a talk about barn owls at the UC Davis Alfalfa/Forages Field Day.
- Breanna Martinico spoke at a UCCE workshop ‘Managing burrowing rodents on the organic farm’ about barn owls and raptors as natural pest control in agriculture.
- Breanna Martinico spoke at the Wild Farm Alliance Virtual Field Day about raptors and non-toxic vertebrate pest control in agriculture.
Scientific conferences:
- Martinico, Bourbour, Phillips, Baldwin, Hull, Kross. Raptors and vertebrate pest control in agriculture. Agroecosystems Symposium, American Ornithological Society Annual Conference. Anchorage, AK. June 2019.
- Kross, Martinico, Bourbour, Phillips, Baldwin, Hull. Is it a trap? Raptors, rodenticides, and rain in California’s agroecosystems. North American Congress for Conservation Biology. July 2020.
- Kross, Martinico, Bourbour, Phillips, Baldwin, Hull. Is it a trap? Raptors, rodenticides, and rain in California’s agroecosystems. North American Ornithological Conference. August 2020.
- Martinico, Bourbour, Phillips, Baldwin, Hull, Kross. Anticoagulant rodenticide exposure in raptors in California’s agroecosystems. Raptor Research Foundation Annual Conference. October 2021.
- Phillips, Martinico, Bourbour, Baldwin, Hull, Kross. Measuring biomass removed from landscapes by breedings pairs of barn owls. Raptor Research Foundation Annual Conference. October 2021.
- Phillips, Martinico, Bourbour, Baldwin, Kross, Hull. Are barn owls impacted by rodenticide use in agriculture? A long-term study design and suggestions. Vertebrate Pest Conference. March 2022
General public:
- Breanna Martinico, Emily Phillips, and Ryan Bourbour presented the project at the annual California Raptor Center open house on two occasions.
- Emily Phillips spoke at Peregrine Elementary School to 3rd and 4th grade students about raptor research at their Women in Science event.
- Breanna Martinico spoke at the California Raptor Center Virtual Open House about raptors and non-toxic vertebrate pest control
Workshop / field days
- Breanna Martinico participated in an agroecosystems workshop at the American Ornithologists Society Annual Conference in Anchorage, AK and discussed net benefits of ecosystem services provided by raptors in agriculture and future research directions and possibilities.
- Breanna Martinico presented at Wild Farm Alliance field day in Cupertino, CA to winegrape growers about managing barn owls and raptors for natural pest control.
- Ryan Bourbour and Breanna Martinico met in the field with Sonoma County Wildlife Rescue, Sonoma, CA, to discuss and teach field research techniques and protocols for rodenticide screening samples from wild barn owls for collaboration on breeding work.
- Ryan Bourbour and Breanna Martinico hosted researchers from Golden Gate Raptor Observatory in the field to teach GPS harnessing techniques on owls.
- Breanna Martinico, Ryan Bourbour, and Emily Phillips hosted a researcher studying barn owls in regenerative agriculture in Oklahoma to teach field methods.
Other Educational Activities
We started social media accounts to share photos and insights from our work with a wide audience.
- Project Facebook page: https://www.facebook.com/BarnOwlProject/?ref=br_rs
- Project Instagram page: https://www.instagram.com/barnowlproject/
- Project twitter feed (@barn_owlproject): https://twitter.com/barn_owlproject
- Ryan Bourbour twitter: https://twitter.com/talonDNA
In addition to the work we did with farmers, agricultural professionals, students, and the general public Emily Phillips, Breanna Martinico, and Ryan Bourbour mentors seven student interns who participated in aspects of the project-- including field work, lab work, and outreach. Interns gained valuable experience that allied them to obtain related jobs and apply to graduate school and vet school.Quick-Guide-Barn-Owls
Education and Outreach Outcomes
Future research should be focused on sampling for rodenticide exposure simultaneously with field rodenticide applications and in landscapes with a larger number of buildings and facilities where second-generation anticoagulant rodenticides are regularly used.
- Use of barn owl box networks as component of IPM strategies in vineyards