Final report for GNC20-311
Project Information
Urban farmers face many unique challenges associated with the urban environment in which they produce. One of the most expensive and limited resources is access to healthy soils. There is often low organic matter and industrial contaminants present in urban soils, resulting in the need for remediation, such as capping and importing topsoil and compost. Recently, black soldier fly larvae (Hermetia illucens; BSF) have been recognized as an efficient organism used to break-down organic matter and produce a soil amendment comparable to traditional fertilizers. These fly larvae can feed on a wide range of organic waste (plant material, biosolids, food waste, etc.), can break down contaminants such as pharmaceuticals or pesticides, and impact the bioavailability of heavy metals. The resulting material is a digestate that can be applied as a soil amendment, much like the vermicomposting processes of worms. Fly pupae can be harvested and used as a nutrient dense feedstock for livestock or reared to adults to continue the cycle of composting. Knowledge gaps remain regarding the impact of feedstock on the nutritional quality of the digestate for crop production and the application and implementation of BSF composting on-farm. My research aims to fill in these gaps, increasing our understanding of BSF composting for vegetable production and implementing on-farm capabilities to generate compost with organic waste produced on-farm or within the community. Ultimately, I hope to provide a strategy for producing nutrient-dense soil amendments tailored to urban farming systems, thus increasing the sustainability of locally grown produce on limited-resource farms. Through laboratory assays and on-farm trials I will identify the optimal feed source (food waste, plant debris, spent brewing grains, manure) and stocking rates of BSF to produce viable compost for vegetable production. The digestate from this process (compost product) will be evaluated as a soil amendment for the production of vegetables. Results from this work will be disseminated through on-farm demonstrations with collaborating urban farmers, a field day at our research farm, extension publications and presentations/demonstrations at grower meetings.
Major learning outcomes from this study include: 1) optimization of feedstock for black soldier fly (BSF) compost production, 2) increased farmer knowledge and understanding of BSF composting and its application in urban farming, 3) acquisition of skills to produce and evaluate BSF digestate. Action outcomes include: 1) adoption of on-farm compost production using BSF, 2) reductions in organic waste streams moving off-farm or to landfills, 3) reduced reliance on external inputs to build organic matter on urban farms. We will achieve these outcomes through applied research, dissemination of research results with on-farm trials and learning events, the publication of extension materials and presentations/demonstrations at grower conferences. Major condition outcomes resulting from this study include improved economic well-being of urban farmers for reduced outsourcing of soil amendments, efficient use of on-farm and community organic waste streams, and increased access to healthy foods in food-insecure urban communities through sustainable urban farming. This project focuses on improving the sustainability of urban agricultural systems by reducing the need to purchase soil through an efficient way to compost on-farm.
Research
Larvae Selection
Black soldier fly larvae (BSF, BSFL) that were roughly 6 days old were ordered from Fluker’s farms, Symton, the Critter Depot, and Josh’s Frogs, in staggered purchases to establish a colony in our laboratory. Larvae arrived in a moistened peat moss substrate, which was included in the first round of composting. Larvae reared for nutritional trials were stored in 4 x 9.75 x 12.25-in, 2-gallon containers; this is also where digestate was produced. Bulk digestate was produced in 18-gallon tote BSF rearing bins using food waste as the primary input with the addition of other waste streams: plant waste, spent worts from local breweries, and sheep manure.
Larval Rearing
Young larvae were stored in an environmental growth chamber with the temperature set to 25C. From there, larvae were transferred to bins in a room within medium to large sized bug dorms to prevent escapees, underneath LED and fluorescent light. These lights are recommended for egg fertilization within indoor requirements (Nakamura et al., 2016). However, we found moving adult flies into an area with natural lighting encouraged increased mating and oviposition; this was our preferred rearing method. The adult stage of the colony was, therefore, maintained in a greenhouse.
Fluted cardboard substrates were provided to adult flies for oviposition so eggs could be collected easily, monitored, and then added to digestate production. These cardboard egg cards were placed in the environmental growth chamber at 25C until larvae reached 6 days old to ensure larval fitness. BSF adults are attracted to fluted cardboard and prefer oviposition substrates above moistened food with BSFL present to guarantee their offspring can eat after they hatch as neonates. Because of this quality we implemented ‘food traps,’ bowls with mesh lids that contain fresh food and young larvae to encourage oviposition.
Experiment larvae were fed 163 mg (about the weight of five grains of rice) of food per larva per day (mg/larva/day) (Parra Paz et al., 2015). We chose to go on the higher end of the 95 – 163 mg of food based of the heat production of BSF larvae along with feed refusal going through an aerated composting process of its own (McEachern, 2018). Due to human error, the first feeding trial for the larvae resulted in feeding rates of 23-28 mg/larvae/day. The second and third trials were closely monitored to prevent this from occurring again and were fed at the ideal 163 mg/larva/day rate.
Measurements taken from larvae include length (mm) and weight (g). Due to the small size off larvae, 10 were selected and weighed together to get an average. For length, larvae were placed in a freezer and removed 15 minutes later so there would be limited movement. These measurements were taken as proxy of how nutritional each food stream was for larvae and if any effects in development could be monitored.
Food stream acquisition
Food waste/processed food waste was obtained from produce harvested at Meig’s Purdue Agricultural Center (Throckmorton, IN) and/or from table scraps from lab members. Plant waste/organic food waste was gathered at Meig’s from weeding plots, from scrapped flats of seedlings, or from local grocery stores with bad produce. Sheep manure was collected from the Animal Sciences Research and Education Center (ASREC; West Lafayette, IN), a local Purdue associated farm that raises multiple herds of livestock. Spent worts were obtained from local breweries.
Digestate
Following BSF pupation, vacant digestate was harvested and sent to A&L Laboratories (Fort Wayne, IN) for compost analyses. These analyses looked at micro and macro nutrients, organic matter, pH, and C:N ratios. We analyzed all four BSF treatments (food waste, plant waste, spent worts, and biosolids) in addition to a common manure compost (Black Kow), a low nutrient potting mix, and a pooled sample of BSF digestate.
Field location
The field application experiment was conducted at the Meigs Horticultural Research Farm (40.28783289649686, -86.88420847116431), part of the Throckmorton Purdue Agricultural Center located in Lafayette, IN. All three focal crops were grown in a field that was planted with soybean in the previous year and had lime applied in the spring of 2020. All crops were grown in raised beds on three-foot centers with 6.5-foot spacing between the rows. Plastic drip irrigation was installed, and plastic mulch covered the beds for the tomato and cucumber crops. Beds were oriented in a north-south direction. Each crop was planted into two rows and blocked by crop type.
Crop Selection
Three focal crops were selected based on their popularity on small farms, representation of broad crop families (Solanaceae, Cucurbitaceae, Apiaceae), and representing fruit and root crops. Seeds were obtained from commercial seed suppliers and included: Tyria cv. cucumbers, Celebrity cv. tomato and Naval cv. carrot from Johnny’s Selected Seeds (Winslow, ME) and Short N’ Sweet cv. carrot from Burpee (Warminster Township, PA).
Source of Amendments
Digestate produced from BSF was applied to soils shortly after transplant, with one additional application for tomatoes and cucumbers after flowering. Digestate was harvested from single food streams: food waste, plant waste, spent worts, and sheep manure which we refer to as biosolids, along with a pooled sample of these food streams. These samples were sent to A&L Great Lakes Laboratories (Fort Wayne, IN) for package C6 analyses along with a commercially available composted cow manure known as Black Kow and a low nutrient potting mix from Midwest Trading (Maple Park, IL; 80/20 mix). The C6 compost package includes solids, nitrogen, phosphorus, potassium, sulfur, calcium, magnesium, sodium, iron, aluminum, manganese, copper, zinc, organic matter, carbon, C:N ratio, pH, and soluble salts. Due to BSF rearing difficulties within the lab setting we ultimately decided on one pooled waste stream to feed to the larvae; this food stream was a mixture of food waste, plant waste, manure, and spent worts. Resulting crops had stem diameter, height, and yield measurements taken biweekly, along with pest and pathogen surveys weekly.
Planting and treatment applications
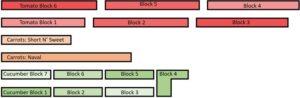
Carrots were directly seeded into beds using a Jang Clean Seeder push seeder in single rows on May-14, 2021. Seed spacing was set to 2.5 inches, however, due to the small size of the seeds they were closer. This resulted in a row length of 16.66 ft (~5.07 meters) for the variety Short N’ Sweet and 30.66 ft (~9.34 meters) for the Naval variety (see figure 1). Soil amendments were added to subplots on Jun-22; both treatments were applied to one third of the carrot rows with the last third being the control treatment. 3055 ± 100 grams of soil amendment was applied to the Naval carrots and 2516 ± 100 grams of soil amendment was applied to Short N’ Sweet carrots. Differences in amount of digestate applied can be attributed to seed size altering spacing and density.
Figure 1
Tomatoes and cucumbers were seeded on Apr-29 into 72-cell trays filled with potting mix (DETAILS FROM THE BAG OF SOIL) and germinated under mist irrigation in a greenhouse at Throckmorton Purdue Agriculture Center. Seedlings were transplanted into the field on May-24 using a rainflo model 1670 water wheel transplanter. Both crops were transplanted in parallel, adjacent rows starting on the Northwest side of the plot. Seedlings were transplanted into single rows; 24-inch spacing for cucumber plants and 48-inch spacing for tomato plants. This resulted in 35 cucumber plants and 28 tomato plants. T-posts were installed along with mesh netting to create a trellis for cucumber plants to support them, tomato plants were provided with tomato cages.
179.70 grams ± 15 grams of BSF and BK soil amendments were added to cucumber blocks C1, C2, C6, C7, and tomato blocks T1, T2, T5, and T6 on May-25. The remaining cucumber and tomato blocks (C3, C4, C5, T3, and T4) received soil amendment applications on Jun-4 (figure 1). All blocks received an additional application of soil amendment treatments applied to tomatoes and cucumbers on Jul-14 after plants had flowered and produced small fruits of roughly the same size as the first addition of soil amendments (179.7 grams). One block was made up of 5 plants within one of three treatments, only 3 plants were harvested from each treatment where available.
Data Collection
Pest and pathogen surveys were completed weekly during the summer of 2021. Subplots including 3 out 5 plants in each block were surveyed by examining the whole plant for pests and checking three leaves for aphids. Plant measurements were taken biweekly after the first three weeks. We measured stem diameter in millimeters using an automatic caliper. Plant height was measured as well in centimeters, plant height being defined as from the stem base to the apical meristem. Fruits were harvested and weighed from cucumber plants twice; Jul-14 and Jul-23. Tomato plant fruits were harvested and weighed on Aug-13, Aug-27, and Sept-11.
Cucumber plants were harvested on Jul-30 in 2021, to examine dried above and below ground biomass. Fresh weight was not taken for these plants due to bacterial wilt drying some parts of the plants out. Carrots were harvested on Aug-4, combined fresh weights of roots and shoots were collected along with dried above and below ground biomass. Tomato plants were harvested on Aug-17 and -24; fresh weight was taken in kilograms on Aug-17 and in pounds on the Aug-24 due to access of scales. Plant material was dried using a drying oven for 1 to 2 weeks at 75C. To ensure plant material was completely void of moisture, 5 bags were weighed randomly, dried for an additional 24 hours, and weighed again. When a stable weight was recorded during the 24-hr interval plants were deemed dry, removed from the oven and weighed.
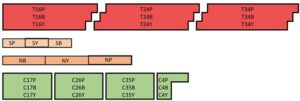
Soil samples were collected on Oct-13 in 2021, from the B1 plot at the Meig’s Horticultural Research Farm. Samples were taken using a soil corer and sent to A&L Great Lakes Laboratories (Fort Wayne, IN) for soil analysis. We ordered the analysis packages S1 and S3 which includes organic matter, available phosphorus, exchangeable potassium, magnesium, calcium, soil pH, buffer pH, cation exchange capacity, percent base saturation of cation elements along with sulfur, zinc, manganese, iron, copper, and boron. A total of 27 soil samples were sent for analyses taken from the plots outlined in figure 2.
Figure 2
Data Analysis
Compost and soil data were both analyzed using a principal component analysis (PCA) to illuminate any latent variables that could not be measured or identified. Bartlett’s tests were run as well to confirm PCAs were an appropriate statistical analysis. Crop measurements, including above and below ground biomass, stem diameter, and growth, were measured for significant interactions using an ANOVA in addition to Tukey HSD to confirm significance. The statistical software used to analyze data generated from these methods was JMP Pro 16.0.
Digestate
Table 1 shows the results from a PCA using compost analysis data generated from A&L Great Lakes Laboratories (Fort Wayne, IN; table 2) resulting in 7 samples. Table 1 shows principal components 1-6, eigenvalues, the percentage of variation explained by latent variables (percent), the cumulative amount of variation described (cum percent), chi-square values (ChiSq), degrees of freedom (DF), and the probability of those values at a 95% confidence interval (α = 0.05). Six principal components can explain 100.0% of the variation observed in our compost samples compared to Black Know and a low nutrient potting mix. Principal component 1 and 2 can explain 70.72% of the variation observed in these data. Principal component 1 was most weighted by positive interactions from phosphorus (0.9115) and phosphate (0.91166). Principal component 2 was positively weighted by pH (0.9333) and iron (0.7987).
Table 1: PCA results for soil analyses
|
Number |
Eigenvalue |
Percent |
Cum Percent |
ChiSq |
DF |
Prob>ChiSq (α = 0.05) |
||||||
|
1 |
8.2468 |
39.270 |
39.270 |
3.579 |
206.947 |
1.000 |
||||||
|
2 |
6.6052 |
31.453 |
70.724 |
16.775 |
201.546 |
1.000 |
||||||
|
3 |
3.2172 |
15.320 |
86.044 |
47.091 |
193.019 |
1.000 |
||||||
|
4 |
1.8513 |
8.816 |
94.860 |
90.099 |
178.435 |
1.000 |
||||||
|
5 |
0.7956 |
3.788 |
98.648 |
158.820 |
162.443 |
0.5657 |
||||||
|
6 |
0.2839 |
1.352 |
100.00 |
263.536 |
145.614 |
<.0001* |
||||||
Table 2: Compost analyses results from A&L Great Lakes Laboratories
|
Treatment |
Moisture @ 70C |
Solids |
Total Nitrogen (N) |
Phosphorus (P) |
Phosphate(P2O5) |
Potassium (K) |
Potash (K2O) |
Sulfur (S) |
Magnesium (Mg) |
Calcium (Ca) |
Sodium (Na) |
Iron (Fe) |
Aluminum (Al) |
Copper (Cu) |
Manganese (Mn) |
Zinc (z) |
pH |
Soluble Salts |
Ash @ 550 C |
Organic Matter (LOI @ 550 C) |
Total Organic Carbon © |
Carbon:Nitrogen Ratio (C:N) |
|
Food Waste |
45.66 |
54.34 |
4.94 |
1.13 |
2.59 |
3.51 |
4.21 |
0.62 |
0.16 |
0.15 |
2.6 |
0.07 |
0.06 |
9 |
13 |
35 |
6.9 |
22.09 |
17.02 |
82.98 |
41.49 |
8.4:1 |
|
Plant Waste |
4.53 |
95.47 |
0.89 |
0.22 |
0.5 |
2.56 |
3.07 |
0.2 |
0.46 |
0.79 |
0.04 |
1.02 |
1.05 |
13 |
415 |
54 |
9.8 |
12.06 |
81.22 |
18.78 |
9.39 |
10.6:1 |
|
Spent Worts |
12.14 |
87.86 |
4.18 |
0.05 |
0.11 |
0.01 |
0.01 |
0.03 |
0.02 |
0.01 |
0 |
0.02 |
0.001 |
0 |
3 |
7 |
7 |
6.26 |
13.48 |
86.52 |
43.26 |
10.3:1 |
|
Biosolids |
37.15 |
62.85 |
3.16 |
1.09 |
2.5 |
2.46 |
2.95 |
0.42 |
0.71 |
3.36 |
0.33 |
0.08 |
0.02 |
22 |
97 |
317 |
8 |
8.21 |
15.44 |
84.56 |
42.28 |
13.4:1 |
|
Black Kow |
59.83 |
40.17 |
1.63 |
0.01 |
0.02 |
0.08 |
0.1 |
0.1 |
0.12 |
0.33 |
0.01 |
0.06 |
0.04 |
5 |
33 |
4 |
6.7 |
0.81 |
50.88 |
49.12 |
24.56 |
15.1:1 |
|
LNPM |
73.03 |
26.97 |
0.92 |
0.03 |
0.07 |
0.1 |
0.12 |
0.16 |
1.42 |
4.24 |
0.15 |
0.21 |
0.21 |
4 |
130 |
19 |
6.6 |
0.45 |
25.30 |
74.70 |
37.35 |
40.6:1 |
|
Pooled |
8.71 |
91.29 |
4.54 |
0.88 |
2.02 |
3.13 |
3.76 |
0.46 |
0.39 |
1.35 |
1.27 |
0.09 |
0.08 |
19 |
63 |
80 |
7.7 |
7.22 |
7.06 |
92.94 |
46.47 |
10.2:1 |
We observed high variability in the levels of micro and macronutrients measured, along with salts and organic matter, when comparing the single source waste streams. When the waste streams were pooled, which is what we applied in our experiments, there were increased levels of some of the nutrients that are identified as important contributors to soil conditioning and plant growth (NPK, Organic Matter, Ca, Zn) but others that may raise concerns and need to be monitored (pH, soluble salts) when compared to the use of commercial composted cow manure.
Soil
Table 3 shows the results from a PCA using soil analysis data generated from A&L Great Lakes Laboratories (Fort Wayne, IN; table 4), resulting in 27 samples. Table 3 shows principal components 1-13, eigenvalues, the percentage of variation explained by latent variables (percent), the cumulative amount of variation described (cum percent), chi-square values (ChiSq), degrees of freedom (DF), and the probability of those values at a 95% confidence interval (α = 0.05). 13 principal components can explain 100.00% of the variation observed between soil core samples. Principal components 1 and 2 are latent variables that can explain 58.25% of the variation seen between soil core samples. Iron (Fe) was the most weighted positive interaction in principal component 1 (0.8665) and soil pH was the most weighted negative interaction (-0.8614). Principal component 2 had only heavily weighted positive interactions such as calcium (0.8506).
Table 3: PCA results for soil analyses
|
Principal Component |
Eigenvalue |
Percent |
Cum Percent |
ChiSq |
DF |
Prob>ChiSq (α = 0.05) |
|
1 |
4.7035 |
36.181 |
36.181 |
260.591 |
76.493 |
<.0001* |
|
2 |
2.8686 |
22.066 |
58.247 |
191.661 |
71.840 |
<.0001* |
|
3 |
1.6862 |
12.971 |
71.218 |
138.739 |
64.236 |
<.0001* |
|
4 |
1.0171 |
7.824 |
79.042 |
103.718 |
55.271 |
<.0001* |
|
5 |
0.9016 |
6.935 |
85.978 |
82.851 |
46.063 |
0.0007* |
|
6 |
0.6515 |
5.012 |
90.898 |
58.117 |
37.298 |
0.0161* |
|
7 |
0.3502 |
2.694 |
93.683 |
36.222 |
29.299 |
0.1769 |
|
8 |
0.2624 |
2.018 |
95.701 |
26.840 |
22.014 |
0.2180 |
|
9 |
0.1917 |
1.475 |
97.176 |
19.824 |
15.756 |
0.2156 |
|
10 |
0.1682 |
1.294 |
98.469 |
15.090 |
10.434 |
0.1493 |
|
11 |
0.1039 |
0.799 |
99.269 |
8.856 |
6.047 |
0.1853 |
|
12 |
0.0739 |
0.568 |
99.837 |
5.315 |
2.514 |
0.1075 |
|
13 |
0.0212 |
0.163 |
100.00 |
0.000 |
0.417 |
0.9973 |
Table 4: Soil analyses results from A&L Great Lakes Laboratories
|
Sample ID |
Organic Matter % |
Phosphorus bray-1 equiv ppm-P |
Potassium K ppm |
Magnesium Mg ppm |
Calcium Ca ppm |
Soil pH |
CEC meq/100g |
Sulfur S ppm |
Zinc Z ppm |
Manganese Mn ppm |
Iron Fe ppm |
Copper Cu ppm |
boron B ppm |
|
C4P |
3.1 |
19 |
123 |
385 |
2300 |
6.5 |
17.4 |
7 |
1.7 |
30 |
20 |
2.1 |
0.5 |
|
C4B |
3.2 |
28 |
146 |
440 |
2700 |
6.6 |
18.7 |
8 |
1.9 |
28 |
27 |
2.4 |
0.6 |
|
NB |
3.1 |
17 |
115 |
365 |
2300 |
6.5 |
17.2 |
9 |
1.7 |
24 |
23 |
2.2 |
0.5 |
|
NY |
3.1 |
27 |
126 |
415 |
2450 |
6.9 |
16.3 |
8 |
1.8 |
30 |
20 |
2.3 |
0.5 |
|
NP |
3.3 |
30 |
136 |
360 |
2300 |
6.6 |
16 |
8 |
1.8 |
29 |
24 |
2.1 |
0.5 |
|
SP |
3.1 |
23 |
125 |
395 |
2350 |
6.8 |
15.8 |
8 |
1.8 |
28 |
26 |
2.3 |
0.5 |
|
SY |
2.9 |
35 |
133 |
405 |
2400 |
6.9 |
16 |
9 |
3 |
30 |
25 |
2.2 |
0.7 |
|
SB |
3 |
26 |
129 |
445 |
2400 |
7.2 |
16 |
7 |
1.7 |
30 |
15 |
1.9 |
0.6 |
|
T16Y |
3.1 |
25 |
117 |
405 |
2250 |
6.7 |
16.1 |
8 |
2.2 |
29 |
25 |
2.3 |
0.5 |
|
T16P |
2.9 |
19 |
122 |
415 |
2400 |
6.5 |
18.2 |
7 |
1.8 |
26 |
28 |
2.1 |
0.5 |
|
C17Y |
3.2 |
14 |
128 |
435 |
2500 |
6.8 |
17 |
8 |
1.7 |
28 |
25 |
2.1 |
0.5 |
|
C17P |
3 |
14 |
121 |
390 |
2300 |
6.8 |
15.5 |
9 |
1.7 |
25 |
19 |
1.8 |
0.5 |
|
C17B |
3 |
20 |
132 |
390 |
2250 |
6.6 |
16 |
8 |
1.6 |
21 |
17 |
1.7 |
0.5 |
|
C26Y |
2.8 |
24 |
130 |
445 |
2400 |
7 |
16 |
7 |
1.6 |
30 |
17 |
1.8 |
0.5 |
|
C26P |
2.8 |
19 |
146 |
480 |
2600 |
6.9 |
17.6 |
7 |
1.6 |
31 |
20 |
2 |
0.5 |
|
C26B |
2.9 |
25 |
138 |
435 |
2500 |
6.8 |
17 |
9 |
1.7 |
27 |
20 |
2 |
0.6 |
|
C35Y |
2.9 |
17 |
134 |
410 |
2400 |
6.8 |
16.2 |
8 |
1.8 |
29 |
21 |
2.2 |
0.6 |
|
C35P |
2.8 |
19 |
141 |
410 |
2500 |
6.7 |
17.5 |
9 |
1.8 |
30 |
21 |
2.3 |
0.5 |
|
C35B |
3.3 |
33 |
149 |
400 |
2400 |
6.7 |
17.2 |
8 |
2.1 |
28 |
23 |
2.6 |
0.5 |
|
C4Y |
3.3 |
29 |
142 |
430 |
2500 |
6.4 |
18.8 |
9 |
1.9 |
27 |
24 |
2.3 |
0.6 |
|
T16B |
2.6 |
26 |
117 |
455 |
2450 |
7 |
16.3 |
8 |
1.8 |
30 |
20 |
2 |
0.5 |
|
T25Y |
2.7 |
32 |
133 |
415 |
2450 |
6.9 |
16.3 |
8 |
2 |
30 |
26 |
2.6 |
0.5 |
|
T25P |
2.9 |
24 |
114 |
350 |
2200 |
6.4 |
16.6 |
9 |
2.1 |
20 |
27 |
2.3 |
0.5 |
|
T25B |
2.7 |
25 |
124 |
380 |
2250 |
6.5 |
17.1 |
9 |
2.1 |
26 |
29 |
2.6 |
0.5 |
|
T34Y |
3.1 |
24 |
134 |
400 |
2450 |
6.3 |
19.5 |
10 |
2.3 |
24 |
31 |
2.4 |
0.6 |
|
T34P |
3.6 |
25 |
122 |
375 |
2450 |
6.1 |
19.3 |
8 |
2.1 |
18 |
29 |
2.4 |
0.6 |
|
T34B |
3.4 |
35 |
125 |
385 |
2350 |
6.2 |
18.9 |
9 |
2.5 |
24 |
34 |
2.5 |
0.6 |
We are still working through the soil sample results from the field trial.
Crop Performance
Cucumber height (F2,18 = 4.6768, p = 0.0252*) and stem diameter (F2,18 = 4.1080, p =0.0363*) at the end of the growing season had significant differences between treatments. After material was dried, dry weight was taken of roots (below ground biomass, BGB) and shoots (above ground biomass excluding fruits, AGB). These showed significant differences between treatments applied to crops for BGB (F14,32 = 9.3648, p = <.0001*) and AGB (F18, 39 = 2.1442, p = 0.0479*). Differences in degrees of freedom can be attributed to loss of samples in drying ovens.
Tomato height (F2,17 = 1.1175, p = 0.3529) and stem diameter (F2,17 = 2.9636, p = 0.0823) had no observable effects from soil amendment treatments. However, after the material was dried there were observable, significant effects for BGB (F17,53 = 2.5953, p = 0.0080*) and AGB (F17,53 = 1.9352, p = 0.0474*).
Carrot growth was measured through shoot height (F5,100 = 5.4831, p = 0.0002*) and number of leaves present (F5,100 = 1.3776, p = 0.2396). BGB and AGB were recorded from 20 randomly selected carrots from each treatment and variety, resulting in 120 samples. AGB (F5,119 = 2.1845, p = 0.0607) had no significant differences between treatments, however, BGB (F5,119 = 6.9068, p = <.0001*) did.
Educational & Outreach Activities
Participation summary:
In the fall semester of 2020 I gave talk in SFS 312, Urban Agriculture taught by Dr. Lori Hoagland at Purdue University, expressing the importance of insects in sustainable agriculture with a focus on black soldier flies and the compost they are able to generate.
In March 2021, I was able to share my research in the form of a poster through the Virtual Indiana Small Farms Conference.
I attended and informed others of my research and the potential of black soldier flies at the Small Farm Education Field Day in July 2021.
In November 2021, I attended the annual meeting for the Entomological Society of America, where I was able to interact with hundreds of people. During my poster presentation, 10 individuals directly asked me questions on my research. Due to the exhibit hall being open for a few hours it is hard to estimate how many individuals interacted with just the poster.
These are instructions that were created and given to participants in the event that they would want to build their own rearing bins in the future:
Project Outcomes
This project has highlighted some of the true potential that black soldier fly larvae composting can have on small farms. They are an avenue to create circular economies and increase sustainability through waste recapture on resource limited farms in urban environments and beyond. BSFL composting, from a variety of food waste streams, was shown to generate a soil conditioner/amendment that is comparable to traditional composting methods and in our case providing higher levels of nutrition and organic matter, compared to composted manure. Farmer participants highlighted some added benefits including an avenue to dispose of culled livestock, manure reduction on-farm as a result of colonization by this insect and an added source of protein for livestock feed on their operations.
The economic benefits include reduced costs to import/purchase soil amendments and conditions and reduced costs of waste disposal. These can be very steep costs for a farmer and through our investigation we have identified that many of the common waste streams on or near urban farms (food waste (household or restaurant), plant waste, manure, and other streams) can be recycled quickly by these insects, and in contained environments such as the bins we used in our research, providing multiple benefits for the community and avoiding the downfalls of traditional composting related to space and time requirements.
This initial project has ignited interest from the farming and research community in the North Central Region and beyond. Dr. Ingwell will continue to build collaborations and research programs stemming from the findings of this study. Future directions and research questions that have been identified include: increasing the efficiency of separating the flies from the digestate, examining the food safety aspects and potential pathogen load in the digestate, examining the overwintering biology and capacity to compost 12 months of the year with this insect, identification of additional streams of feedstock and maximizing the composition to facilitate development and growth of the BSF.
We anticipate the generation of two peer-reviewed publications detailing the findings of this research, one Extension publication communicating the "how-to's" of BSF composting including detailed diagrams and testimonials from participants and 1-2 YouTube demonstrational videos showing how to implement this system.
During this project I learned a lot alongside my advisor, Dr. Ingwell, about sustainable agriculture (SA) and black soldier fly (BSF) possibilities. Personally, I was exposed to a lot of new methods and practices surrounding farming and insect husbandry that I had not had the opportunity to learn prior to this project. Sustainable agriculture was not something that I thought I would be able to implement in my own life, but I wasn't aware it was as easy as reducing food waste and being aware of where food comes from. Getting into the literature at the start of the project opened my eyes to the endless possibilities that SA has to offer. However, as promising as some sustainable agriculture ideas have been, I've learned it's important to be realistic about them. For example, at the start of the project, prior to having every calculation required, I pitched an idea that would end up requiring two thousand pounds of material. This project has not only made me a better researcher because of the experiences I was able to have, but also a better person by becoming more involved with the food producing world.
Dr. Ingwell increased her connection to urban farmers and learned more about the motivations driving innovative and sustainable practices on urban farms/gardens. She built relationships with new farmers and had numerous conversations, through interactions with collaborators and at Extension events, that have positively impacted her approach to sustainable agriculture and has helped refined the priorities of her research program to address the needs of urban farmers and gardeners in relation to pest management and food production. Given the interest and excitement generated around this project, she will continue to pursue funding and research related to black soldier fly composting and creating circular economies on urban farms to increase sustainability and reduce reliance on external inputs.
We both benefited from the financial support provided by SARE allowing us to work together on Sustainable Agriculture incorporating insects as waste recyclers.
Residential chicken farmer in Indianapolis, 55-gallon bin:
"My top two discoveries:
1 - We are currently raising meat chickens and ended up needing to cull two due to injuries at different times. One was about 2.3 lbs and the other 3.5 lbs when they went in the bin whole. The BSF larvae ate them, feathers, bones, and all, in about 2-3 days. There was a little "death" smell for about half a day each time, and I think in the future I would cut up the chicken into a few pieces so the larvae had more surface area to work on. Either way, it was super impressive. I was not expecting them to get through feathers, bones, and egg shells so easily and quickly.
2 - We put our bin right behind the layer coop. Inside the coop, we have a poop board that gets a layer of pine shavings on it below the roosts which collects just about any manure the hens leave in the coop. We usually clean the board twice a week, but now if I do it only once a week the manure is crawling with tiny to small BSF larvae. There is also much less chicken manure smell because the larvae are actively breaking it down. That all then goes right into my compost pile. Just an added bonus and less mucking out the coop for us!"
Backyard gardener in a residential area, 18-gallon bin:
"The bin is easy to use but getting compost (larva free) to use while keeping the bin going for multiple generations was difficult. The compost built up (looks great!), but it is not easy to separate from the colony. I did have some moisture issues when the humidity in later summer was higher, but that is probably easily solved by adding drier compost materials."
We are excited to see continued support from SARE and other funding agencies to support using insects to reduce waste streams and generate organic material for soil conditioning. This is an emerging field and the potential applications of these insects are really exciting!