Final report for PDP20-001
Project Information
The cornerstone of a sustainable plant disease control program for any farm community hinges on well-trained agricultural professionals. Diagnosing diseases in Guam and the Northern Mariana Islands is a constant challenge because of the yearly movement of nearly 2 million tourists and the importation of large quantities of fresh produce. Compounding the problem is the islands’ remoteness from mainstream plant pathology research and teaching facilities. Project’s long-term goals are to increase economic viability and enhance environmental quality of small commercial and subsistence farming operations in the region through accurate and timely diagnoses of plant diseases. The project’s immediate goal is to provide agricultural professionals with the skills and knowledge useful in discerning leaf spots caused by fungi as opposed to those caused by bacteria or chemical sprays. Trainees will be provided with the skills necessary to differentiate among similar and often confused symptoms caused by three common and virulent fungal genera: Colletotrichum, Cercospora, and Corynespora. Using trainers from Georgia and the Philippines, will bring a national and an international perspective to the workshop. Travel cost for the trainers will be kept to a minimum by scheduling the workshop to coincide with their other travel plans. Activities include: 1) Guam assembling workshop materials; 2) Guam collecting images of field symptoms and micrographs of spores from 15 foliar fungi including Colletotrichum, Cercospora, and Corynespora; 3) Guam preparing single spore isolates; 4) Georgia identifying isolates and depositing sequences to GenBank; 5) Guam updating Guam’s Index of Plant Diseases; 6) Guam, Georgia and Philippines conducting a four-day plant disease diagnostic workshop for 12 trainees composed of field trips, lectures, hands-on examination of samples, laboratory exercises, and engagement opportunities with the local farmers and the public; and 7) Guam, Georgia and Philippines posting identification tutorial online; and 8) Georgia and Guam conducting a workshop evaluation.
Objective 1) Increase the technical capacity of the University of Guam to train agricultural professionals about foliar fungal leaf spots through the assembly and development of training materials.
Objective 2) Increase diagnostic collaboration between the Universities of Guam, Georgia, and the Philippines by identifying unknown species of Colletotrichum, Cercospora, and Corynespora and other fungal leaf spots that occur on Guam and enter such information into the Guam Index of Plant Diseases and other databases.
Objective 3) Increase the skills of agricultural professionals in Guam and the Northern Mariana Islands to diagnose fungal leaf spots and to be able to pass this information on to their constituents, through the production of a diagnostic workshop composed of field trips, lectures, and microscopic examination of plant samples.
Objective 4) Use workshop evaluations consisting of surveys and pre and post-tests to measure knowledge gained and identify opportunities for future plant pathology related activities and the means through which delivery of diagnostic skills and knowledge to trainees or stakeholders in future workshops can be improved.
Proposal’s principle assumption is the belief that improvements in the skills of agricultural professionals in discerning various fungal leaf spots will translate into more specific and targeted recommendations to their clientele.
Inputs include expert workshop trainers (Dr. Schlub, Dr. Kemerait, Dr. Brewer, and Dr. Sumabat-Dacones); a mycologist with expertise in molecular techniques (Brewer et al., 2014; Stewart et al., 2015; Sumabat et al., 2018); and a Co-PI with expertise in instructional evaluations. Direct financial contributions will come from WSARE, while salary support will come from the PI, Co-PI, and trainees’ respective institutions and governments. Farmer volunteers will host field trips and participate in a half-day clinic for growers and the public.
The workshop will follow the “Train the Trainer” model and will include classroom and field instructions. On the final day, trainees will have an opportunity to use their newly acquired knowledge during a half-day clinic for growers and the public. Dr. Shelton will use the Don Dillman’s tailored Design method (Dillman et al. 2014) to formulate survey questions and the IBM SPSS Statistics software (IBM 2017) in data analysis.
Activity 1: September 2020-February 2021: Create an online workshop manual.
Activity 2: September 2020-January 2022: Guam will collect images of field symptoms and micrographs of spores of 15 foliar fungi including species of Colletotrichum, Cercospora, and Corynespora.
Activity 3: September 2020-January 2022: Two sets of 15 single spore fungal isolates will be prepared. One will be sent to Georgia for ID the other maintained on Guam.
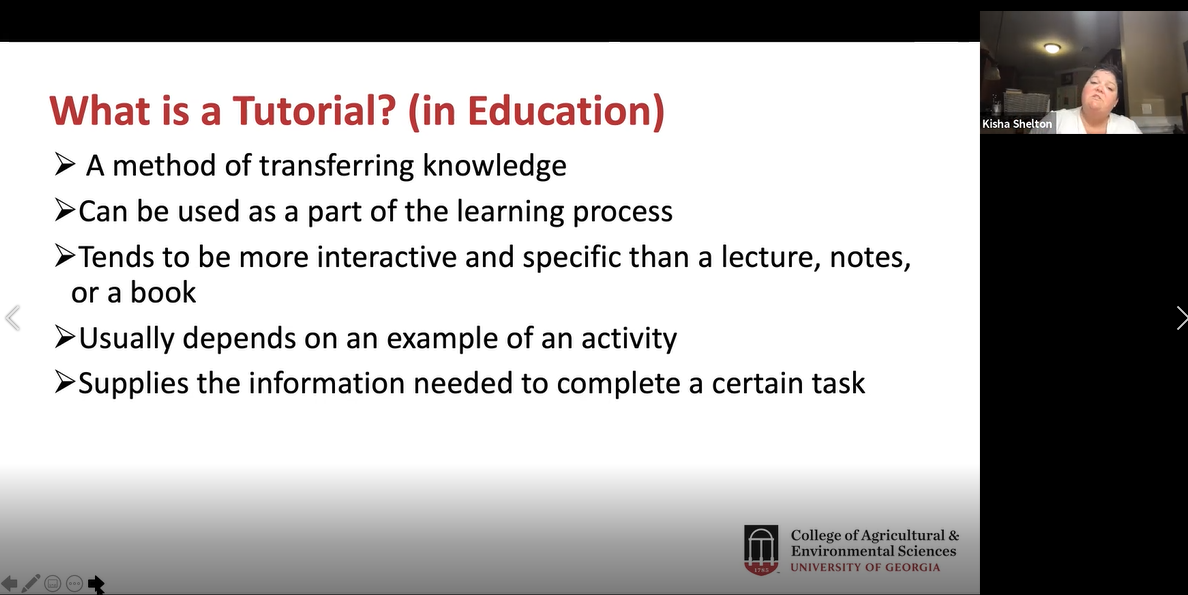
Activity 4: January 2021-August 2021: Trainers will develop a tutorial on distinguishing foliar infections caused by Colletotrichum, Cercospora, Corynespora, and other fungi. A working model will be available at the time of the workshop for trainees’ testing and feedback. When finished the tutorial will be placed online.
Activity 5: March 2021: Trainers will conduct a four-day plant disease diagnostic workshop, composed of field trips, lectures, and microscopic examination of samples. There will be 4 trainers and 12 trainees consisting of seven from Guam and four from Northern Mariana Islands.
Activity 6: May 2021-January 2022 Guam and Georgia will provide a written evaluation of the workshop and pre and post-tests, along with solicited suggestions from trainees for future workshops.
Activity 7: January 2022-July 2022: Georgia will identify fungal cultures and deposit DNA sequences to GenBank.
Activity 8: August 2022: Guam and Georgia will add new fungal identification to Guam’s Index of Plant Diseases.
Cooperators
- - Producer
- (Researcher)
- (Educator)
- (Educator)
- (Educator)
Education
The project’s approach to education consisted of several elements: social networking, competency-based learning, real-world classroom, problem-based learning, and place-based learning. All of these elements were part of the three and a half-day plant disease diagnostic workshop, held in August 2021. Social networking consisted of fourteen professionals which attended the workshop: four from Northern Marianas College, four from Guam Department of Agriculture, one from Guam Landscape Management Systems, and five from the University of Guam. Competency-based learning consisted of participants' demonstration of learning outcomes during the lab activities. The concept of real-world classroom was embraced by focusing the workshop on foliar pathogens of Guam and the Northern Marianas Islands. Problem-based learning was incorporated into the training process by allowing small groups of individuals to work together to identify their own foliar diseases. The concept of place-based education was embraced by immersing the participants in Guam’s local culture, landscapes, and agricultural practices.
Education & Outreach Initiatives
Train Agriculture Professionals in Guam and the Northern Mariana Islands to diagnose fungal leaf spots and to be able to pass this information on to their constituents.
No educational and outreach initiatives occurred during the initial five-month reporting period, however, during this reporting period, a three and a half-day training for agricultural professionals in Guam and the Northern Mariana Islands was conducted. The diagnostic workshop was composed of field trips, lectures, and microscopic examination of plant samples. To assist trainees in passing on their newly gained knowledge, a free half-day clinic for the public was held on the last day of the workshop. The clinic covered fungal leaf diseases found on farms and home gardens in Guam and the region. Farmers and the general public were invited to attend and were encouraged to bring in their own plant specimens. Trainees were on-hand to assist the public with diagnosing plant specimens.
As a result of the workshop, trainees will be able to discern leaf spots caused by fungi as opposed to those caused by bacteria or chemical sprays.
The project resulted in an increase in the number of educational resources available for agricultural professionals to download for their own diagnostic workshops.
Educational & Outreach Activities
Participation summary:
Learning Outcomes
Project Outcomes
A training manual for identifying fungal leaf spot diseases was developed. This manual consists of fact sheets and a simplified key. The basic content of each fact sheet included a description of a pathogen including its morphological features, associated disease, and photographs of symptoms and spores. The simplified key is in a table format. It contains morphological features (size and shape of spores, hypha, fruiting bodies, etc.) and names of diseases that corresponded to 18 type specimens of foliar, filamentous pathogens known to occur in Guam. The morphological features varied in size; thereby, allowing the key to be useful even with a hand-lens.
As reported previously (Table 3) twenty-five diseased leaf samples from plants around Guam were collected and sent to the University of Georgia (UGA) to assist in morphological identification. After receiving samples, graduate student Manuela Samaco at the University of Georgia provided tentative identification of the samples (Table 4). When possible, spores were cultured and grown out. Using these cultures, the University of Georgia was able to molecularly identify 14 fungal pathogens from 9 different host plants using Fungal ITS Sequencing (Table 5).
A three and one-half day workshop/training was held August 11 to August 14, 2021 on identification of prevalent fungal leaf pathogens and their diseases. The workshop was composed of field trips, lectures, and microscopic examination of plant samples. Fourteen professionals attended: four from Northern Marianas College, four from Guam Department of Agriculture, one from Guam Landscape Management Systems, and five from the University of Guam. Topics included differentiating among similar and often confused symptoms caused by three common and virulent fungal genera: Colletotrichum, Cercospora, and Corynespora; how to collect and process fungal samples, and how to use dissecting and compound microscopes. Instructors were from the University of Guam, University of Georgia, and the University of the Philippines.
Pre-tests were given to each participant prior to the workshop, which was followed by post-tests at the conclusion of the workshop. The average gain in knowledge was 27.5 percent. A post-workshop evaluation consisting of 21 questions was also given to the participants. Questions addressed workshop planning, objectives, field trips, classroom instruction, lecturers, and overall workshop experience. Scoring was based on a scale of 1-5, with 1 being strongly disagree and 5 being strongly agree. The average overall score was 4.7. The lowest average score for a question was 4.00. Due to the increase in knowledge gained and the overall positive workshop evaluation score, the participants in the training will have a positive impact on diagnosing foliar fungal diseases for their respective agencies.
As a result of the positive workshop evaluations and the knowledge base of the participants, it is believed that the participants in the training will have a positive impact on diagnosing foliar fungal diseases for their respective agencies. Coincidentally, one of the participants, Dr. Glenn Dulla would later be hired to fill the University of Guam research plant pathology position vacated by Dr. Adrea Blas.
It is believed that there will be an increase in the economic viability and enhanced environmental quality of small commercial and subsistence farming operations in the region as a result of more accurate and timely diagnoses of foliar fungal leaf spot diseases. Since the project resulted in an increase in the number of educational resources available for agricultural professionals to download for their own diagnostic workshops, it is believed that there will be an increased awareness, knowledge, and capacity of agricultural professional to provide train-the-trainer programs.
Objective 1) Increase the technical capacity of the University of Guam to train agricultural professionals about foliar fungal leaf spots through the assembly and development of training materials.
Activity: Foliar, filamentous pathogens were identified for use in fact sheets and as type specimens for the Simplified Key. Using the Guam Plant Disease Index and other sources, 89 pathogen candidates were identified. The candidates were ranked according to their incidence on Guam (common, occasional, rare), and importance to Guam’s economy (high, moderate, low).
Product: Fact sheets were developed for 16 important pathogens (Table 1). Drafts of the fact sheets were distributed to trainees at the foliar fungal workshop held in August 2021. All 16 fact-sheets are now finalized and posted on the UOG Cooperative Extension & Outreach website here: https://www.uog.edu/extension/agriculture-and-natural-resources#Resources under the “Plant Disease Diagnostics 2021” tab. They are also attached to this report.
Table 1. Compiled fact sheets of filamentous, foliar pathogens in Guam and the diseases they cause.
| Foliar Pathogens | Disease |
| Albugo | White rust |
| Alternaria | Bull's eye spot, Early blight, Gray leaf spot, Leaf blight, Leaf spot |
| Ascochyta | Leaf spot, Gummy stem blight |
| Botrytis | Leaf spot, Gray mold |
| Cercospora | Black leaf streak, Frogeye spot, Gray leaf spot, Leaf spot |
| Cephaleuros | Algal leaf spot, Red rust |
| Colletotrichum | Anthracnose |
| Corynespora | Bull's eye spot, Leaf blight, Leaf spot, Target leaf spot |
| Erysiphe | Powdery mildew |
| Fusarium | Leaf spot |
| Helminthosporium | Leaf spot, Southern leaf blight |
| Peronospora | Downy mildew |
| Phomopsis | Leaf blight, Leaf spot |
| Phyllosticta | Leaf freckle, Leaf spot |
| Phytophthora | Late blight, Leaf spot, Leaf or canopy blight |
| Puccinia | Rust |
Product: A simplified key to asexual morphological structures to Guam’s common leaf spots including Colletotrichum, Cercospora, and Corynespora and others was developed. Based on the pathogen ranking, 18 were chosen as type specimens (Table 2). A draft key was distributed to trainees who attended the August 2021 foliar fungal workshop. After the workshop, the simplified key was finalized and posted to the UOG Cooperative Extension and Outreach website here: https://www.uog.edu/_resources/files/extension/publications/Pathogen_Simplified_Key.pdf. It is also included as an attachment on this report.
Table 2. Guam foliar pathogens chosen for inclusion in the simplified key.
| Foliar Pathogens | Disease |
| Albugo | White rust |
| Alternaria | Bull's eye spot, Early blight, Gray leaf spot, Leaf blight, Leaf spot |
| Ascochyta | Leaf spot, Gummy stem blight |
| Botrytis | Leaf spot, Gray mold |
| Cephaleuros | Algal leaf spot |
| Cercospora | Black leaf streak, Frogeye spot, Gray leaf spot, Leaf spot |
| Colletotrichum | Anthracnose |
| Corynespora | Bull's eye spot, Leaf blight, Leaf spot, Target leaf spot |
| Diplodia | Leaf spot |
| Fusarium | Leaf spot |
| Helminthosporium | Leaf spot, Southern leaf blight |
| Oidium | Powdery mildew |
| Peronospora | Downy mildew |
| Pestalotia | Gray leaf spot, Scab |
| Phomopsis | Leaf blight, Leaf spot |
| Phyllosticta | Leaf freckle, leaf spot |
| Phytophthora | Late blight |
| Puccinia | Rust |
Objective 2) Increase diagnostic collaboration between the Universities of Guam, Georgia, and the Philippines by identifying unknown species of Colletotrichum, Cercospora, and Corynespora and other fungal leaf spots that occur on Guam and enter such information into the Guam Index of Plant Diseases and other databases.
Activity: Twenty-five leaf samples were collected between the months of November and December 2020 (Table 3).
Table 3: Sample information for fungal id
|
UOG plant clinic ID# |
Sample |
Plant |
Location |
GPS |
|
2020-023 |
1 |
Banana |
UOG, GU |
13.43016, 144.80016 |
|
2020-024 |
2 |
Papaya |
UOG, GU |
13.43015, 144.80013 |
|
2020-025 |
3 |
Wing Bean |
UOG, GU |
13.43017, 144.79982 |
|
2020-026 |
4 |
Breadfruit |
Yigo Experiment Station, GU |
13.53266, 144.87315 |
|
2020-027 |
5 |
Casssava |
Yigo Experiment Station, GU |
13.53239, 144.87305 |
|
2020-028 |
6 |
Hot pepper |
Yigo Experiment Station, GU |
13.53235, 144.87346 |
|
2020-029 |
7 |
Okra |
Yigo Experiment Station, GU |
13.53237, 144.87331 |
|
2020-030 |
8 |
Mango |
Chalan Pago, GU |
13.42799, 144.78479 |
|
2020-031 |
9 |
Mango |
Chalan Pago, GU |
13.42799, 144.78479 |
|
2020-032 |
10 |
Hibiscus tiliaceaus |
Yigo, Ming Chun farm, GU |
13.33745, 144.53522 |
|
2020-033 |
11 |
Guava |
Yigo, Ming Chun farm, GU |
13.33824, 144.53568 |
|
2020-034 |
12 |
Cucumber |
Yigo, Ming Chun farm, GU |
13.33865, 144.54568 |
|
2020-035 |
13 |
Plumeria |
UOG, GU |
13.25822, 144.48069 |
|
2020-036 |
14 |
Fig |
Chalan Pago, GU |
13.42799, 144.78479 |
|
2020-037 |
15 |
Corn |
Dededo, Ernie Wusstig farm, GU |
13.25822, 144.48069 |
|
2020-038 |
16 |
Avocado |
Dededo, John Mesa farm, GU |
13.32311, 144.51585 |
|
2020-039 |
17 |
Avocado |
Dededo, John Mesa farm, GU |
13.32311, 144.51585 |
|
2020-040 |
18 |
Calamansi |
Dededo, John Mesa farm, GU |
13.32318, 144.51623 |
|
2020-041 |
19 |
Calamansi |
Dededo, John Mesa farm, GU |
13.32318, 144.51623 |
|
2020-042 |
20 |
Ipomea pes-caprae |
Tumon, Ypao Beach, GU |
13.25880, 144.48029 |
|
2020-043 |
21 |
Heliotropium foertherianum |
Tumon, Ypao Beach, GU |
13.30160, 144.4730 |
|
2020-044 |
22 |
Panicum maximum |
Tumon, Hyatt Hotel, GU |
13.30430, 144.48160 |
|
2020-045 |
23 |
Wedelia sp. |
Tumon, Ypao Beach, GU |
13.3018, 144.47340 |
|
2020-046 |
24 |
Wedelia sp. |
Tumon, Ypao Beach, GU |
13.3018, 144.47340 |
|
2020-047 |
25 |
Banana |
UOG, GU |
13.43016, 144.80016 |
Activity: Field symptoms and microscopic images were photographed (Figure 1, 2, 3). Leica MZ95 dissecting scope was used for the photomicrographs. If spores were found they were scraped off the leaf sample and wet mounted on a glass microscope slide and examined under a Leica DM1000 compound light microscope.
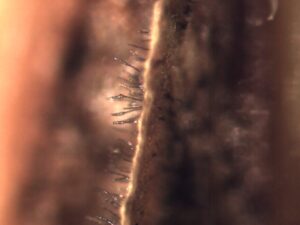
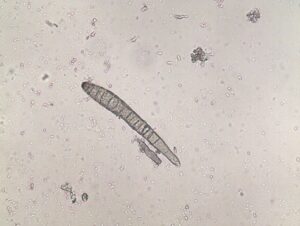

Duplicates of each leaf sample were dried over two-day periods and pressed between cardboard sheets for later use in PDP training (Figure 4).
Figure 4: Examples of duplicate samples dried on herbarium sheets for future use in diagnostic educational workshops. Credit: B. Deloso
Activity: To assist in morphological identification of leaf fungal pathogens, help from the lab of Dr. Marin Brewer at the University of Georgia (UGA) was utilized. Ultimately, the University of Georgia identified these samples molecularly. Leaf samples were sent on the same day of field collection to UGA from Guam. After receiving samples, graduate student Manuela Samaco assessed the condition upon arrival, took photographs, and attempted to identify presence or absence of spores. If spores were found photographs were taken using a light and dissecting scope. Samaco attempted to get subsets of each sample into tissue culture, some were successful. Some samples had spores observed while others did not. Spores were later observed on 11 out of 25 samples by UGA after arrival. Possible identities were listed by mycologists at UGA based on leaf spot symptoms and spore morphology (Table 4).
Table 4: Sample information and tentative identification.
|
Date specimen collected |
Specimen collected by |
Suspected disease or pathogen |
Date received at UGA |
Specimen cultured? |
Spores observed? |
Tentative ID (UGA) |
|
11/5/2020 |
B. Deloso |
Fusarium |
11/10/2020 |
yes |
yes |
Curvularia, Colletotrichum |
|
11/5/2020 |
B. Deloso |
Corynespora |
11/10/2020 |
yes |
yes |
Colletotrichum |
|
11/5/2020 |
B. Deloso |
powdery mildew |
11/10/2020 |
no |
n/a | n/a |
|
11/13/2020 |
B. Deloso & J. Hudson |
anthracnose |
11/17/2020 |
yes |
yes |
Colletotrichum |
|
11/13/2020 |
B. Deloso & J. Hudson |
unknown |
11/17/2020 |
yes |
yes |
Colletotrichum |
|
11/13/2020 |
B. Deloso & J. Hudson |
unknown |
11/17/2020 |
yes |
yes |
Colletotrichum, Pestalotiopsis |
|
11/13/2020 |
B. Deloso & J. Hudson |
unknown |
11/17/2020 |
yes |
yes |
Fusarium, Curvularia |
|
11/13/2020 |
R. Schlub |
orange algae |
11/17/2020 |
no |
n/a | n/a |
|
11/13/2020 |
R. Schlub |
anthracnose |
11/17/2020 |
yes |
yes |
Colletotrichum, Fusarium |
|
11/27/2020 |
B. Deloso & J. Hudson |
unknown |
11/30/2020 |
yes |
yes |
unidentified |
|
11/27/2020 |
B. Deloso & J. Hudson |
unknown |
11/30/2020 |
yes |
yes |
Pestalotiopsis |
|
11/27/2020 |
B. Deloso & J. Hudson |
Colletotrichum |
11/30/2020 |
yes |
no |
n/a |
|
11/27/2020 |
B. Deloso & J. Hudson |
unknown |
11/30/2020 |
yes |
yes |
Colletotrichum |
|
11/27/2020 |
R. Schlub |
unknown |
11/30/2020 |
yes |
yes |
Colletotrichum, Curvularia |
|
12/4/2020 |
B. Deloso & J. Hudson |
Bipolaris |
12/7/2020 |
yes |
no | n/a |
|
12/4/2020 |
B. Deloso & J. Hudson |
unknown |
12/7/2020 |
yes |
no | n/a |
|
12/4/2020 |
B. Deloso & J. Hudson |
unknown |
12/7/2020 |
yes |
no | n/a |
|
12/4/2020 |
B. Deloso & J. Hudson |
citrus scab |
12/7/2020 |
yes |
no | n/a |
|
12/4/2020 |
B. Deloso & J. Hudson |
unknown |
12/7/2020 |
yes |
no | n/a |
|
12/10/20 |
B. Deloso & J. Hudson |
unknown |
12/14/2020 |
no | n/a | n/a |
|
12/10/20 |
B. Deloso & J. Hudson |
unknown |
12/14/2020 |
no | n/a | n/a |
|
12/10/20 |
B. Deloso & J. Hudson |
A &B, two suspects |
12/14/2020 |
yes |
yes |
Bipolaris |
|
12/10/20 |
B. Deloso & J. Hudson |
Cercospora |
12/14/2020 |
no | n/a | n/a |
|
12/10/20 |
B. Deloso & J. Hudson |
downy mildew |
12/14/2020 |
no | n/a | n/a |
|
12/10/20 |
B. Deloso |
Mycosphaerella |
12/14/2020 |
no | n/a | n/a |
Activity: Specimens were cultured if spores were present on the tissue sample and the pathogen was one that was known to be culturable. Using spores from the culture grow-outs, the University of Georgia was able to molecularly identify 14 fungal pathogens from 9 different host plants using Fungal ITS Sequencing (Table 5).
Table 5. Fungal leaf pathogens from different host plants collected from Guam (2021) identified by the University of Georgia using ITS Sequencing. (*unidentified: based on culture and spore morphology in PDA)
| Sample | Host | Fungal identity (from culture) | BLAST search results | % identity | Unite results | % identity | Remarks |
|
1 |
Avocado |
unidentified* (sample 1) |
Diaporthe sp. |
99.12% |
Diaporthe pseudomangiferae |
99% |
fungus is Diaporthe pseudomangiferae |
| Diaporthe pseudomangiferae | 98.96% | Diaporthe sp. | 99% | ||||
|
2 |
Avocado |
unidentified (sample 2) |
Lasiodiplodia theobromae |
99.64% |
Lasodiplodia theobromae |
99% |
fungus is Lasodiplodia theobromae |
| Lasodiplodia sp. | 99% | ||||||
|
3 |
Calamansi spot |
unidentified |
Nigrospora sp. |
99.66% |
Nigrospora sp. |
99% |
fungus is Nigrospora sp. |
| Nigrospora sphaerica | 99.65% | Nigrospora oryzae | 99% | ||||
|
4 |
Calamansi scab |
unidentified |
Diaporthe phoenicicola |
98.77% |
Diaporthe |
98% |
fungus is Diaporthe sp. |
|
Diaporthe pseudomangiferae |
98.47% |
Diaporthe pseudomangiferae |
98% |
||||
|
Phomopsis sp. |
98.21& |
Diaporthe phaseolorum |
97% |
||||
|
5 |
Corn |
Bipolaris maydis |
Bipolaris maydis |
99.66% |
Bipolaris maydis |
99% |
fungus is Bipolaris maydis (100%) |
|
6 |
Guava |
Pestalotiopsis sp. |
Pestalotiopsis sp. |
99.64% |
Pestalotiopsis |
99% |
fungus is Pestalotiopsis sp. |
|
Pestalotiopsis mangiferae |
99.28% |
Pestalotiopsis mangiferae |
99% |
||||
|
7 |
Hibiscus |
unidentified (sample 1) |
Lasodiplodia theobromae |
99.46% |
Lasodiplodia theobromae |
99% |
fungus is Lasodiplodia sp. |
|
Macrophoma theicola |
99.46% |
Lasodiplodia pseudotheobromae |
99% |
||||
|
Botryosphaeria rhodina |
99.46% |
||||||
|
8 |
Hibiscus |
unidentified (sample 2) |
Nectria pseudotrichia |
99.50% |
Nectria pseudotrichia |
99% |
fungus is Nectria sp. |
|
9 |
Mango |
Fusarium sp. |
Fusarium sp. |
99.13% |
Fusarium |
99% |
fungus is Fusarium sp. |
|
Fusarium napiforme |
98.96% |
Fusarium napiforme |
99% |
||||
|
10 |
Okra |
Culvularia sp. |
Cochliobolus lunatus |
99.81% |
Culvularia kusanoi |
99% |
fungus is Culvularia sp. |
|
Curvularia kusanoi |
99.62% |
||||||
|
11 |
Pepper |
Colletotrichum |
Colletotrichum siamense |
99.83% |
Colletotrichum siamense |
99% |
fungus is Colletotrichum, species level to be determined |
|
Colletotrichum boninense |
99.83% |
Colletotrichum boninense |
99% |
||||
|
Colletotrichum endophyticum |
99% |
||||||
|
12 |
Plumeria |
Colletotrichum |
Colletotrichum fructicola |
99.83% |
Colletotrichum fructicola |
99% |
fungus belongs to Colletotrichum gloeosporioides species complex |
|
Colletotrichum queenslandicum |
99.83% |
Colletotrichum queenslandicum |
99% |
||||
|
13 |
Plumeria |
unidentified (sample 1) |
Neofusicoccum occulatum |
99.66% |
Neofusicoccum occulatum |
99% |
fungus belongs in Neofusicoccum parvum-Neofusicoccum ribis species complex |
|
Neofusicoccum kwambonambiense |
99% |
||||||
|
14 |
Plumeria |
unidentified (sample 2) |
Colletotrichum siamense |
99.35% |
Colletotrichum siamense |
99% |
fungus belongs to Colletotrichum gloeosporioides species complex |
|
Colletotrichum gloeosporioides |
99.35% |
Colletotrichum gloeosporioides |
99% |
Product: The University of Georgia tissue sample analyses were added to the Index of Plant Diseases in Guam. The updated Index can be found on the University of Guam Cooperative Extension and Outreach website here: https://www.uog.edu/_resources/files/extension/publications/Plant_Diseases_Guam_Index_10_25_2022.pdf
Objective 3) Increase the skills of agricultural professionals in Guam and the Northern Mariana Islands to diagnose fungal leaf spots and to be able to pass this information on to their constituents, through the production of a diagnostic workshop composed of field trips, lectures, and microscopic examination of plant samples.
Activity: A three and one-half day workshop/training was held August 11-14, 2021 on identification of prevalent fungal leaf pathogens and their diseases. Fourteen professionals attended: four from Northern Marianas College, four from Guam Department of Agriculture, one from Guam Landscape Management Systems, and five from the University of Guam. Topics included differentiating among similar and often confused symptoms caused by three common and virulent fungal genera: Colletotrichum, Cercospora, and Corynespora, how to collect and process fungal samples, and how to use dissecting and compound microscopes. The workshop was hosted by the University of Guam PDP Project Coordinator Dr. Robert Schlub, University of Georgia Professor of Plant Pathology Dr. Robert Kemerait, University of Georgia Associate Professor of Mycology Dr. Marin Brewer, University of Georgia Plant Pathology Academic Professional Associate Dr. Kisha Shelton, and University of the Philippines Assistant Professor Dr. Leilani Sumabat-Dacones.
Prior to the start of the workshop, trainees were given hard copies of all training materials and print-outs of PowerPoint presentations. Training materials included final drafts of 14 foliar fungal factsheets, final draft of the simplified table key, a copy of the Index of Plant Diseases in Guam, excerpts from The Diseases of Cultivated Crops in Pacific Island Countries, and numerous other excerpts from publications, PowerPoints, and online materials which contained pictures and descriptions of foliar fungal diseases. Trainees were also given a tote bag which contained sample collecting and processing supplies, including a clipboard, a notepad, pen, sharpie, Ziploc bags, flagging tape, a hand lens, and a basic dissection kit. All training materials were posted on the University of Guam website, and can be found here: https://www.uog.edu/extension/agriculture-and-natural-resources#Resources under the “Plant Disease Diagnostics 2021” tab.
The following was the agenda for the workshop: [WSARE PDP Workshop Final Agenda]
During the first two days of the workshop, classroom training was held in the morning hours and lab/field training was held in the afternoon. On the third day, the field training was held in the morning to accommodate the farmer’s schedule, and classroom training was held in the afternoon. Trainees were given instruction on identifying foliar pathogens and how to collect samples. During the fourth and final day, the Guam public was invited to bring in suspected plant disease samples for a trainee-hosted half-day diagnostic clinic at the University of Guam Agriculture and Life Science building laboratory. Over the course of the workshop, trainees were instructed on how to create their own YouTube tutorial which could be used teach others how to diagnose foliar fungal pathogens and specifically how to differentiate between Colletotrichum, Cercospora, and Corynespora.
During the first day of training, trainees received an introduction to foliar pathogens, how to ID foliar pathogens, and how to collect and process foliar fungal samples (Figure 5). Instructors were Drs. Schlub, Kemerait, and Brewer. In the afternoon, trainees moved into the lab where Extension Associate Ben Deloso gave a quick review of how to use the dissecting and compound microscopes which were set up for trainees to use (Figure 6). This was followed by a quick walking tour of the campus demonstration garden, where trainees were encouraged to flag and collect samples. Trainees then headed back to the lab where they examined and processed any samples collected (Figure 7).
Figure 5. Dr. Marin Brewer gives a presentation on foliar pathogens.
Figure 6. Extension Associate Ben Deloso reviews how to use a microscope.
Figure 7. Trainees returned to the lab and examined samples under the microscope.
During the second day of training, instruction began with a zoom presentation on how to create a YouTube tutorial by Dr. Shelton (Figure 8). Next, instruction focused on the life cycle and ID of Colletotrichum, Cercospora, and Corynespora. Instructors were Drs. Brewer and Sumabat-Dacones. In the afternoon, trainees and instructors made a field trip to Bernard Watson’s farm in Yigo, where a brief introduction was conducted by Dr. Robert Schlub and Mr. Frank Cruz, who has assisted Watson’s farm for many years (Figure 9). Mr. Watson was unable to be present due to health concerns. The Watson farm mainly grows banana plants and noted their two most concerning diseases are banana bunchy top virus and black sigatoka. Participants were encouraged to collect as many leaf samples as they could (Figure 10). Next, the field trip moved on to John Mesa farm in Dededo. Upon arrival, the farmer gave a brief introduction to the farm, and discussed his struggles with pests, certain pesticides, and fertilizers (Figure 11). John Mesa farm grows a wide variety of vegetable and fruit crops. Trainees were allowed to speak to the farmers and ask questions, collect samples, and take notes (Figure 12).
Figure 8. Dr. Kisha Shelton gives a presentation via Zoom.
Figure 9. A brief introduction of Bernard Watson's farm was conducted by Dr. Robert Schlub and Frank Cruz.
Figure 10. Trainees collect leaf samples from a banana tree plot at Bernard Watson's farm.
Figure 11. One of the workers at John Mesa farm presented a short history of the farm.
Figure 12. Trainees collect samples at John Mesa's farm.
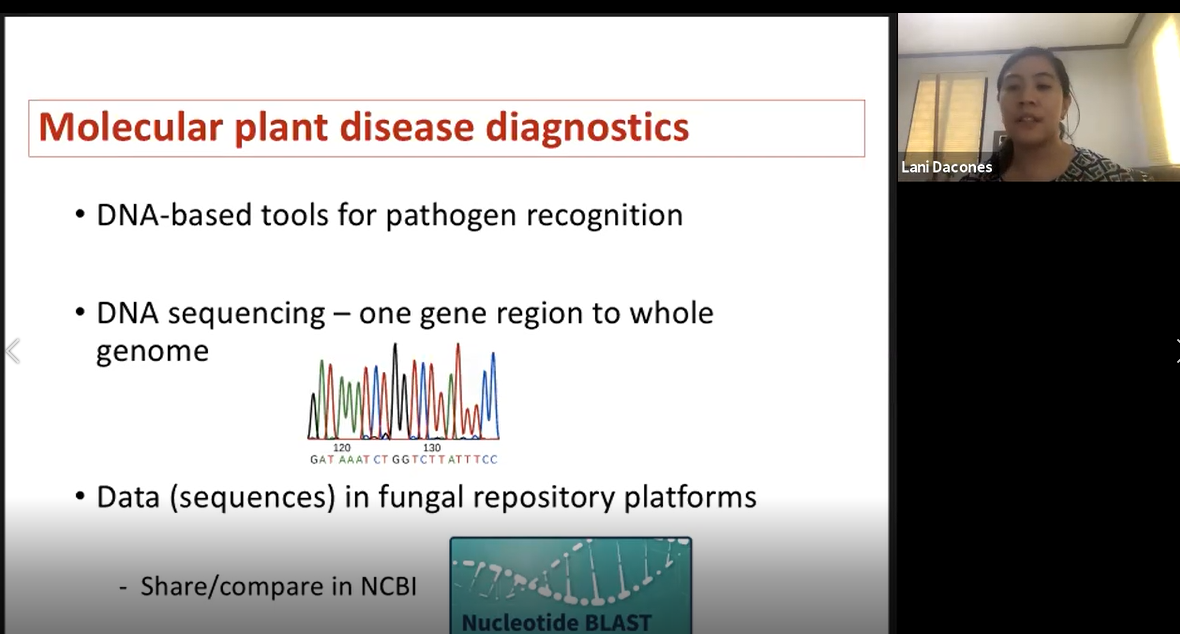
During the third day of training, trainees and instructors began with a field trip to Ernie Wusstig’s farm and the University of Guam Triton Farm. Mr. Ernie Wusstig introduced his farm (Figure 13), which manly focuses on corn and field crops. Trainees were allowed to explore the fields, examining and collecting samples (Figure 14, 15). Next, the field trip moved on to the University of Guam Triton Farm. Trainees continued collecting samples, and Dr. Robert Schlub pointed out specific diseases found at the farm (Figure 16). In the afternoon, Dr. Leilani Sumabat-Dacones gave a virtual lecture on Molecular tools in fungal detection (Figure 17). This was followed by a two-hour open lab where trainees were given the opportunity to examine any samples collected over the past two days (Figure 18).
Figure 13. Mr. Ernie Wusstig gives an introduction to his farm to trainees.
Figure 14. Trainees walk through corn fields. Mr. Wusstig is available to answer any questions.
Figure 15. A trainee uses a hand lens to examine a disease symptom on a corn leaf.
Figure 16. At the UOG Triton Farm, Dr. Robert Schlub points out damaged roots caused by a fungal pathogen.
Figure 17. Dr. Leilani Sumabat-Dacones gives a virtual presentation.
Figure 18. Dr. Robert Kemerait (red hat) gives instruction to a trainee during the open lab period.
During the fourth day, the Guam public was invited to bring in suspected leaf disease samples for a free trainee-hosted diagnostic clinic at the University of Guam Agriculture and Life Sciences building laboratory. The free clinic began with a short presentation by Dr. Robert Schlub on how to identify Guam’s common fungal diseases. The group was then taken on a short walking tour of the campus demonstration garden, where Dr. Schlub pointed out some fungal pathogen symptoms on a breadfruit leaf (Figure 19). The public was allowed to collect samples as they walked. At the end of the tour, the agricultural trainees assisted the public in diagnosing foliar plant disease samples using what they had learned over the previous three-day workshop (Figure 20).
Figure 19. Dr. Robert Schlub points out fungal symptoms on a breadfruit tree leaf.
Figure 20. Trainees assisted in diagnosing disease samples from the public and used field trip samples to provide additional samples to share with island residents.
Product: A YouTube tutorial was developed. On the last day of the workshop, trainees and instructors worked together to develop ideas and a working model for a YouTube tutorial to distinguish between these foliar infections. With assistance from Dr. Kisha Shelton at the University of Georgia, Extension Associates at the University of Guam assembled the YouTube tutorial and placed it online. It can be found on the UOG Cooperative Extension and Outreach YouTube page here: https://www.youtube.com/watch?v=QQiSizXChtw&ab_channel=UOGCNAS
Objective 4) Use workshop evaluations consisting of surveys and pre and post-tests to measure knowledge gained and identify opportunities for future plant pathology related activities and the means through which delivery of diagnostic skills and knowledge to trainees or stakeholders in future workshops can be improved.
Activity: Participant change in knowledge was measured using pre and post-tests. Pre-tests were given to each participant prior to the workshop, which was followed by post-tests at the conclusion of the workshop. Three participants were not present to take the post-test. The average gain in knowledge from the 11 agricultural professionals who completed both pre- and post-tests was 27.5 percent.
A post-workshop evaluation was also given to the 14 participants and consisted of 21 questions:
1) Pre-workshop communication was well organized; 2) Was it clear that the workshop objective was to provide you with an opportunity to gain skills and knowledge useful in discerning leaf spots caused by fungi as opposed to those caused by other pathogens or pesticides?; 3) After completing the workshop, do you feel like you were well informed about the objectives of this workshop?; 4) I found the content of this workshop to be relevant to my job; 5) I found the classroom materials to be useful; 6) The workshop activities stimulated my learning; 7) The activities in this workshop gave me sufficient practice and feedback; 8) The difficulty level of this workshop was appropriate; 9) The pace of this workshop was appropriate; 10) The field trips were a good addition to the classroom instruction; 11) The lecture room was clean and well set-up; 12) The laboratory was clean and well set-up; 13) I was satisfied with the snacks, lunch, and beverages provided; 14) The instructor was well prepared; 15) I learned something from this instructor; 16) The instructor was helpful; 17) I learned how to diagnose common foliar fungal genera found on Guam; 18) My skills/ knowledge have improved by attending this workshop; 19) I learned valuable information/ skills that can be applied to my work; 20) I am confident that I can teach others what I learned; 21) Rate your overall workshop experience.
Score was based on a scale of 1 (strongly disagree) to 5 (strongly agree). The average overall score was 4.7. The lowest average score for a question was 4.00 while the highest score for a question was 5.00.
Due to the increase in knowledge gained and the overall positive workshop evaluation score, the participants in the training will have a positive impact on diagnosing foliar fungal diseases for their respective agencies.
As a result of the August 2021 foliar fungal workshop, an article was posted by Western SARE news:
The University of Guam College of Natural and Applied Sciences included an article about the Western Region SARE Professional Development Program workshop in their August 2021- Issue no. 9 of Dive Into CNAS:
https://us2.campaign-archive.com/?u=c605e34edd3522d806f45846d&id=2ac618d1e2
Dr. Robert Kemerait of the University of Georgia, who assisted with presentations and training during the August 2021 workshop, provided the following statement:
"Having been Extension for over 21 years, I can say that this workshop- to include presentations, field trips, and work in the "lab" with diseased samples, was as good or better than any I have ever attended. The obvious planning and extensive coordination that went into this training event was evident throughout; the participants remained fully engaged from the beginning to the end and clearly learned a great deal."
Dr. Leilani Sumabat-Dacones of the University of the Philippines participated in the workshop via zoom, and provided the following statement at the conclusion of the workshop:
"I would also like to express my gratitude for the opportunity to have been part of the workshop. This was my first experience of delivering presentations to non-plant pathologists who provide ag extension services. It was a meaningful exposure and I am very honored to be part of this capacity building activity."
Information Products
- Foliar Pathogens in Guam: Albugo
- Foliar Pathogens in Guam: Ascochyta
- Foliar Pathogens in Guam: Botrytis
- Foliar Pathogens in Guam: Cercospora
- Foliar Pathogens in Guam: Colletotrichum
- Foliar Pathogens in Guam: Corynespora
- Foliar Pathogens in Guam: Erysiphe (Oidium)
- Foliar Pathogens in Guam: Fusarium
- Foliar Pathogens in Guam: Helminthosporium
- Foliar Pathogens in Guam: Peronospora
- Foliar Pathogens in Guam: Phomopsis
- Foliar Pathogens in Guam: Phyllosticta
- Foliar Pathogens in Guam: Phytophthora
- Foliar Pathogens in Guam: Puccinia and Uromyces
- Foliar Pathogens in Guam: Cephaleuros
- Foliar Pathogens in Guam: Alternaria and Stemphylium
- Index of Plant Diseases in Guam