Final report for GW18-156
Project Information
Beef production is at a crossroads in terms of environmental and economic sustainability. Recently, grass-fed beef has gained popularity with consumers who are concerned about the environmental impact of beef production and animal welfare. However, feedlot-finished beef has been shown to have lower rates of greenhouse gas emissions than pasture-finished beef. Here, we proposed using tannin- and saponin-containing legume forages to lower greenhouse gas (GHG) emissions and improve soil nitrogen (N) retention in pasture-finished beef systems. These forages are known for reducing methane production in cows, reducing leachable N-inputs, reducing the need for further N-additions, improving animal rate of gain, and preserving natural grassland ecosystem services. However, it was still unclear how tannins and saponins function in the soil. Previous work has shown that tannins reduce mineralization rates, although it is unclear whether this is a function of tannin structure or concentration. Saponins are another secondary plant compound which have been observed to have a similar function to tannins in the soil. To discriminate the effect of tannin or saponin source and concentration on soil N-cycling, we conducted an in vitro incubation study using varied doses of condensed tannins extracted from Lotus corniculatus (birdsfoot trefoil) and Onobrychis viciifolia (sainfoin), and saponins from Medicago sativa (alfalfa) as opposed to commonly studied commercially-available varieties, to monitor rates of N-mineralization, volatilization, and GHG production in pasture soil. Incorporating the influence of N-cycling on soil GHG emissions required a whole-farm environmental sustainability assessment. Holos is a comprehensive and user friendly GHG accounting software which uses a whole-farm approach to assess beef production environmental and economic sustainability. Holos is designed for use in Canada, restricting its adoption in the U.S. We collaborated with Agriculture and Agri-Food Canada to extend Holos’ geographic range and characteristics to include Utah for easier adaptation throughout the Intermountain West. We trained local producers and researchers to use Holos through workshops, poster presentations, talks, and a fact sheet. These activities facilitated Holos’ use, giving producers the ability to understand how management changes affect environmental and economic sustainability and giving researchers new tools to model on-farm emissions. Through our research, we found that tannins from birdsfoot trefoil were able to reduce soil soluble total N yields, N mineralization, and nitrification in a dose-dependent manner with no notable effects on C cycling. We also found that switching from grass to legume forages decreased the net GHG emissions from the finishing stage of pasture-based beef production operations by reducing fertilizer and fuel use. Based on our educational and outreach activities, all participants indicated a change in their knowledge, attitudes, and skills, as well as an intention to change their practices in the future. A majority of participants also indicated that they anticipated using knowledge from the workshop in future new and existing educational programs.
- Extract tannins from fecal samples produced by cows that have grazed on birdsfoot trefoil (BFT) and sainfoin (SFN) tannin-containing legumes to determine a baseline tannin concentration that we would expect to see in the field in April 2018. We will also extract tannins from the leaves of BFT and SFN plants, and saponins from the leaves of ALF plants in April 2018. The tannins and saponins extracted from these leaves will be added to the soils during the incubation study and used as assay standards.
- Perform a 84-day soil incubation study with varying concentrations of tannins extracted
from BFT and SFN leaves, and a single concentration of saponins extracted from ALF leaves (May-June 2018). - Determine concentrations of NH4+, NO3-, and NH3 at the start and end of the incubation, and throughout the study on the same days as headspace sampling (May-June 2018). These data will be used to calculate rates of N mineralization.
- Determine concentrations of soluble total N, total C, and organic C in soil samples at the start and end of the incubation study (May-June 2018) as an approximation of secondary compound complexation in soils. Soils will also be assayed for autoclaved citrate extractable protein (ACE protein) prior to KCl extraction at the start and end of the incubation to determine the amount of protein substrate available to be bound by tannins or saponins (May-June 2018). Soluble nutrient extractions and ACE protein assay trials will be performed prior to the incubation study.
- Determine concentrations of CO2 and N2O gases using gas chromatography throughout
the incubation to determine production rates of each gas as well as cumulative production (May-June 2018). Headspace samples will be collected on days 0, 2, 7, 14, 28, 42, 56, 70, and 84. - Create Holos farm scenarios for feedlot-finished, and various pasture-finished (MBG,
BFT, SFN, ALF) beef production systems for Utah using climate and soil data from Utah sites where pasture-based beef production is or could be carried out, and quantify GHG
emissions for each scenario in units of CO2 equivalents (CO2-eq) (April-June 2018). - Create an electronic fact sheet resource explaining the benefits of using models as an exploratory tool to understand how farm management changes affect net GHG emissions (March 2019).
- Host two half-day training sessions for regional producers and outreach personnel in
partnership with USU Extension to demonstrate the use of Holos software (April 2019). - Evaluate how producers’ skills have changed with regard to Holos software abilities as
well as their understanding of how management changes influence farm sustainability
before and after the Holos training sessions (April 2019).
Cooperators
- (Researcher)
- (Researcher)
- (Researcher)
- (Educator)
- (Educator)
Research
In Vitro Incubation Experiment:
We conducted an 84-day in vitro soil incubation experiment. This experiment used various sources and doses of condensed tannins to distinguish between the effect of tannin source and concentration on N cycling dynamics. A saponin treatment with one source and concentration was added to the experiment so that the effect of tannins could be compared against the effect of other secondary plant compounds found in legume forages.
Each treatment was added to 5 g oven dry equivalent of uniform 0-15 cm soil sampled from under grass located adjacent to the pastures at the Utah State Intermountain Irrigated Pasture Project (USU IIPP) in Lewiston, Utah. Soil samples were collected on October 29, 2018 using a step-in soil corer. Soils were homogenized, sieved to 2 mm, and stored at 5 degrees Celsius until use.
The experiment consisted of 6 treatments:
- Soil control (Control)
- Birdsfoot trefoil tannins @ 3 mg tannins/g dry soil (BFT Low)
- Birdsfoot trefoil tannins @ 15 mg tannins/g dry soil (BFT High)
- Sainfoin tannins @ 3 mg tannins/g dry soil (SFN Low)
- Sainfoin tannins @ 15 mg tannins/g dry soil (SFN High)
- Alfalfa saponins @ 3 mg saponins/g dry soil (SAP Low)
Treatments were added to 5 g of the uniform soil by dissolving the dry tannins or saponins in double distilled de-ionized water. All treatments were created to deliver the correct concentration of secondary plant compounds and bring the samples to 22% moisture (approximate field capacity). Each sample was placed in a 40 mL borosilicate glass incubation vial and sealed with caps fitted with septa. Each treatment contained 39 samples: 3 samples for ammonium (NH4+) and nitrate (NO3-) extractions on days 0, 2, 7, 14, 28, 42, 56, and 70; 3 samples for NH4+, NO3- on day 84 and headspace (carbon dioxide (CO2) and nitrous oxide (N2O)) sampling throughout the experiment; 6 samples for autoclaved citrate extractable protein (ACE protein) - 3 samples for day 0, and 3 samples for day 84; and 6 samples for secondary plant compound analysis - 3 samples for day 0, and 3 samples for day 84. In addition to the 39 samples of each treatment, 3 empty jars were used as blanks and were preserved throughout the experiment (237 samples total).
Tannins were extracted from plant leaves grown at the USU IIPP according to Hagerman (2011) and purified according to Grabber et al. (2013). Tannins were also assayed from freeze-dried fecal samples collected in June 2017 from cows grazing exclusively on each forage. This was done to provide a reference value for tannin concentrations being deposited in the field. Saponins were extracted and purified according to Lee et al. (2001).
On each sampling day (0, 2, 7, 14, 28, 42, 56, 70, 84), NH4+ and NO3- concentrations were determined by performing a 2M KCl extraction on the soil. Extracts were analyzed using a Lachat Quikchem 8500 Flow Injection analyzer (Lachat Instruments, Loveland, CO, U.S.).
On each sampling day, 7 mL headspace samples were taken using a syringe and analyzed for concentrations of CO2 and N2O. Concentrations of CO2 were analyzed on a HP 6890 Series Gas Chromatograph System (Hewlett-Packard, Palo Alto, CA, U.S.). Concentrations of N2O were analyzed on an Agilent Technologies 6850 Series II Network GC System (Agilent Technologies, Santa Clara, CA, U.S.). Jars were flushed to ambient atmospheric conditions after each sampling event. Headspace samples were collected from the same 3 experimental replicates of each treatment throughout the experiment.
Samples were analyzed for ACE protein on days 0 and 84 according to Hurisso et al. (2018). Protein analysis was conducted to assess the amount of soil protein available in the soil at the start and end of the incubation. This acted as a proxy for the amount of protein which was bound by secondary plant compounds throughout the experiment.
Soluble soil C and N concentrations were analyzed according to the hot water method described in Halvorson & Gonzalez (2008). Samples were subjected to one cold water, and three subsequent hot water extractions. Cold and hot water extracts were analyzed for concentrations of dissolved organic C and total dissolved N. Extracts were analyzed on a Shimadzu TOC-L Analyzer (Shimadzu Corporation, Kyoto Japan).
Concentrations of soil NH4+ and NO3- were used to calculate rates of N mineralization. Concentrations of N2O were used to calculate rates of nitrous oxide production, and concentrations of CO2 were used to monitor microbial activity.
Data was analyzed using a mixed linear model and a randomized complete block design for analysis of variance using the MIXED procedure in SAS Studio University Edition (version 9.4, SAS Institute Inc., Cary, NC, U.S.). Parameters were analyzed for the main effects of treatment, day, and their interaction at p<0.05. Parameters were transformed as necessary to attain normality. Outliers were removed by assessing residuals.
References:
Grabber, J.H., W.E. Zeller, and I. Mueller-Harvey. 2013. Acetone enhances the direct analysis of procyanidin- and prodelphinidin- based condensed tannins in Lotus species by the butanol-HCl-assay. J. Agric. Food Chem. 61: 2669–2678.
Hagerman, A.E. 2002. Tannin Purification.
Halvorson, J.J., and J.M. Gonzalez. 2008. Tannic acid reduces recovery of water-soluble carbon and nitrogen from soil and affects the composition of Bradford-reactive soil protein. Soil Biol. Biochem. 40(1): 186–197.
Hurisso, T. T., Moebius-Clune, D. J., Culman, S. W., Moebius-Clune, B. N., Thies, J. E., & van Es, H. M. 2018. Soil Protein as a Rapid Soil Health Indicator of Potentially Available Organic Nitrogen. Agricultural & Environmental Letters, 3(1).
Lee, Stephen T., Bryan L. Stegelmeier, Dale R. Gardner, and Kenneth P. Vogel. 2001. The isolation and identification of steroidal sapogenins in switchgrass. Journal of Natural Toxins. 4: 273-281.
Stark, J.M., and S.C. Hart. 1996. Diffusion Salt Solutions, Kjeldahl Digests, and Persulfate Digests for Nitrogen-15 Analysis. Soil Sci. Soc. Am. 60: 1846–1855.
Holos Greenhous Gas Emission Modeling:
We created Holos software scenarios for various pasture-finished beef production systems for the Intermountain West. Holos is a whole-farm life cycle greenhouse gas modeling software developed by Agriculture and Agri-Food Canada for animal agriculture operations. The four pasture-finished scenarios represented beef finished on a birdsfoot trefoil or sainfoin legume diet which contain condensed tannins, an alfalfa diet as a legume control, and a meadow bromegrass diet as a grass control. The scenarios were adjusted to reflect soil, climate, and yield conditions in Utah based on data taken from the USU IIPP, USU Caine Dairy Farm, and the Utah Climate Center. Greenhouse gas emissions were quantified in units of CO2 equivalents. Using a modeling approach allowed us to simulate how changes in soil N cycling dynamics as a result of the presence or absence of tannin- and saponin-containing forage legumes affected net greenhouse gas emissions at the farm scale.
Holos Software: http://www.agr.gc.ca/eng/science-and-innovation/agricultural-research-results/holos-software-program/?id=1349181297838
In Vitro Incubation Experiment:
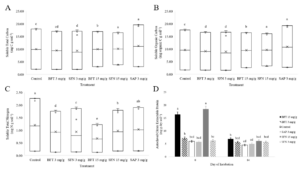
Significant differences (p<0.0001) in soluble N among the CT and saponin treatments suggested that CTs were capable of increasing N retention in a dose dependent manner (Figure 1). The control yielded significantly more N than any of the BFT or SFN treatments and yielded equal amounts of N as the 3 mg/g SAP treatment. This suggests that all CT treatments are complexing with organic N and increasing N retained in the soil. Reductions in soluble N are consistent with past studies. Like Halvorson et al. (2016), reductions in soluble N by the CTs were dose dependent when the 15 mg/g SFN treatment was excluded due to N contamination, consistent with the idea that CTs increase N retention via complexation with organic and mineral N.
Although there were significant differences in yields of soluble total (p<0.0001) and organic (p<0.0001) C among treatments, the secondary compounds appeared to have a lesser effect on C cycling than N cycling (Figure 1). The 15 mg/g SFN and 3 mg/g SAP treatments yielded significantly more soluble TC than the rest of the treatments, followed by the 15 mg/g BFT treatment which yielded significantly more soluble TC than the remaining control, 3 mg/g BFT and SFN treatments. The control yielded significantly more soluble TC than the 3 mg/g SFN treatment and the 3 mg/g BFT treatment did not differ from the control or 3 mg/g SFN treatment. Similar results were seen for total soluble organic C yields. The lack of clear trends in C cycling data suggest that secondary compounds mainly effect N cycling. This is conclusion is not surprising, as the addition of condensed tannins to soil has produced mixed results in C cycling processes in past studies (Northup et al., 1995; Kraus et al., 2003; Adamczyk et al., 2012; Halvorson et al., 2016)
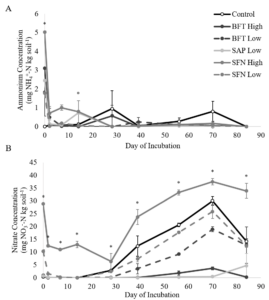
Nitrogen mineralization patterns provided evidence of increased N retention, as well as phenolic degradation and subsequent immobilization. There were significant treatment x day interactions for NH4+ (p<0.0001) and NO3- concentrations (p<0.0001) (Figure 2). At the start of the incubation, the 15 mg/g SFN treatment had significantly higher NH4+ concentrations than the control, and the control treatment had significantly lower NH4+ concentrations than the remaining treatments. When NO3- concentrations were compared among treatments by day, there was evidence of immobilization and subsequent mineralization in all treatments.
By the end of the incubation, the 3 mg/g SAP and the 15 mg/g BFT treatments had significantly lower NO3- concentrations than the 3 mg/g BFT and SFN treatments and the control. The 15 mg/g SFN treatment continued to be significantly higher than the 15 mg/g BFT and 3 mg/g SAP treatments. However, none of the treatments had NO3- concentrations that exceeded the control. This confirms that the treatments did not add significant amounts of N to the samples and suggest that low concentrations of saponins and high concentrations of CTs may decrease N mineralization and nitrification. Although the ammonium data did not directly support the idea of phenolic-driven N complexation, the nitrate data did. The lower nitrate concentrations in the 15 mg/g BFT and 3 mg/g SAP treatments did confirm that phenolics, including saponins, can inhibit N mineralization over a prolonged period (Crush, 1993; Northup et al., 1995; Crush and Keogh, 1998; Schimel et al., 1998; Kraus et al., 2003; Nierop et al., 2006a; b; Smolander et al., 2012).
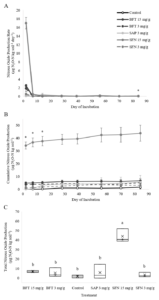
The addition of the phenolic treatments generally did not increase total N2O emissions over the course of the incubation. There was a significant treatment x day interaction for N2O production rate (p=0.0064) and cumulative N2O production (p<0.0001) (Figure 3). However, with the exclusion of the SFN High treatment due to N contamination, there was a no treatment effect for total N2O production (Figure 3). This suggested that none of the secondary compound additions stimulated significant N2O production over a prolonged period of time. The reduction of mineral N pools without an increase in total N2O production suggests that tannins complex with soil N to increase overall soil N retention.
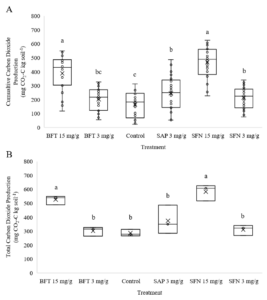
The secondary compound treatments appeared to provide a C source and increased C mineralization. There was no treatment effect for CO2 production rate, but there were significant treatment effects for cumulative (p<0.0001) and total CO2 production (p<0.0001) (Figure 4). The 15 mg/g SFN and BFT treatments had significantly higher cumulative CO2 production than all other treatments, indicating a stimulatory effect from high doses of CTs. The 3 mg/g SAP and SFN treatments had significantly greater cumulative production than the control indicating a lesser stimulatory effect. The 3 mg/g BFT treatment’s cumulative production was intermediate to the control, 3 mg/g SAP, and 3 mg/g SFN treatments. By the end of the incubation the only significant differences in total CO2 production were observed for the 15 mg/g SFN and BFT treatments which had significantly higher total CO2 production than the rest of the treatments. This suggests a stimulatory effect of CTs on C mineralization at high doses. The effect of tannins on CO2 production in the literature has been mixed. The dose-dependent effect on C mineralization observed in our study would suggest that C from the CT treatments was used as a labile C source, as several past studies have indicated (Schimel et al., 1998; Nierop et al., 2006a; b).
There was a significant treatment x day interaction (p<0.0001) for ACEP (Figure 1). On day 0, the 15 mg/g SFN and BFT treatments yielded significantly more ACEP than all treatments. By day 84, the 15 mg/g BFT treatment only yielded more ACEP than the 3 mg/g SAP and control treatments. This indicates that protein generally decreased through time, but decreased significantly more for the 15 mg/g BFT and SFN treatments. Past studies have used now outdated measures of soil protein such as Bradford reactive soil protein, and our data will need to be compared against future studies. The apparent dose-dependent increase in ACEP would suggest that the assay is either extracting proteins contained in the treatments, or that high doses of CTs make soil protein more available since reduction in soluble C was not dose dependent.
Soil N cycling results for ammonium, nitrate, and N2O production were unexpectedly high for the 15 mg/g SFN treatment and caused concerns of possible N contamination in that treatment. Upon further analysis, the 15 mg/g SFN treatment was the only treatment with detectable amounts of total N. Since the basic catechin and epicatechin building blocks of condensed tannins do not contain N, it is suspicious that the SFN, but not the BFT treatments, would add such a considerable amount of N. Statistical analysis was run with and without the 15 mg/g SFN treatment included. The removal of the 15 mg/g SFN treatment did not generally alter the results of the experiment or our conclusions. Despite the results of the 15 mg/g SFN treatment, low doses of saponins and high doses of BFT-derived condensed tannins did appear to increase soil N retention in a pasture soil.
References
Adamczyk, B., J.P. Salminen, A. Smolander, and V. Kitunen. 2012. Precipitation of proteins by tannins: Effects of concentration, protein/tannin ratio and pH. Int. J. Food Sci. Technol. 47(4): 875–878. doi: 10.1111/j.1365-2621.2011.02911.x.
Crush, J.R. 1993. Effect of tannin in animal diet on nitrification rate of pasture soil under dung patches. AgResearch Session 8(ID No. 576): 1991–1992.
Crush, J.R., and R.G. Keogh. 1998. A comparison of the effects of Lotus and white clover on some nutrient cycling factors. Proc. New Zeal. Grassl. Assoc. 60: 83–87. https://www.grassland.org.nz/publications/nzgrassland_publication_220.pdf (accessed 25 July 2017).
Halvorson, J.J., M.A. Schmidt, A.E. Hagerman, J.M. Gonzalez, and M.A. Liebig. 2016. Reduction of soluble nitrogen and mobilization of plant nutrients in soils from U . S northern Great Plains agroecosystems by phenolic compounds. Soil Biol. Biochem. 94: 211–221. doi: 10.1016/j.soilbio.2015.11.022.
Kraus, T.E.C., R.A. Dahlgren, and R.J. Zasoski. 2003. Tannins in nutrient dynamics of forest ecosystems-a review. Plant Soil 256: 41–66. doi: Doi 10.1023/A:1026206511084.
Nierop, K.G.J., C.M. Preston, and J.M. Verstraten. 2006a. Linking the B ring hydroxylation pattern of condensed tannins to C, N and P mineralization. A case study using four tannins. Soil Biol. Biochem. 38: 2794–2802. doi: 10.1016/j.soilbio.2006.04.049.
Nierop, K.G.J., J.M. Verstraten, A. Tietema, J.W. Westerveld, and P.E. Wartenbergh. 2006b. Short- and long-term tannin induced carbon, nitrogen and phosphorus dynamics in Corsican pine litter. Biogeochemistry 79(3): 275–296. doi: 10.1007/s10533-005-5274-0.
Northup, R.R., Z. Yu, R.A. Dahlgren, and K.A. Vogt. 1995. Polyphenol control of nitrogen release from pine litter. Nature 377: 227–229.
Schmidt, E.L., and L.W. Belser. 1994. Autotrophic nitrifying bacteria. p. 159–177. In Methods of Soil Analysis, Part 2. Microbiological and Biochemical Properties - SSSA Book Series, no. 5. Soil Science Society of America, Madison, WI.
Smolander, A., S. Kanerva, B. Adamczyk, and V. Kitunen. 2012. Nitrogen transformations in boreal forest soils — does composition of plant secondary compounds give any explanations ? Plant Soil 350: 1–26. doi: 10.1007/s11104-011-0895-7.
Holos Greenhouse Gas Emission Modeling:
Holos software scenarios for all beef-finishing scenarios are currently in the final stages of preparation. Preliminary results suggest that legumes may decrease total greenhouse gas emissions when expressed per kg of live weight gain. In a direct comparison of birdsfoot trefoil, meadow bromegrass, and cicer milkvetch finishing systems, the grass-finished system had the highest net greenhouse gas emissions due to N fertilizer use and the energy required to produce it. Grass forages also decreased feed quality, meaning that average daily gains are less. As a consequence, cattle are required to graze for a longer period of time during which they are producing enteric methane and manure. The use of both tannin- and non-tannin-containing legumes were able to decrease net GHG emissions compared to the grass system. Based on field data, the use of legumes lowered enteric methane production and eliminated the need for N fertilizer due to N fixation. In a separate comparison of alfalfa, birdsfoot trefoil, and sainfoin finishing systems, all three systems minimized external N inputs due to the use of legumes and their effect on enteric methane production and fertilizer needs. Although further adjustments are being made to these scenarios, forage yield and amount of residue left on the field appear to be important determinants of total greenhouse gas production in legume finishing systems. At this time, the Holos greenhouse gas models may not be sensitive enough to distinguish the effects of tannin- vs. non-tannin-containing legume finishing systems on whole-farm greenhouse gas emissions. These results confirmed the benefits of using legumes as an alternative forage that have been previously described in the literature, such as increased feed quality and average daily gains, reduced fertilization, and decreased enteric methane production (Phelan et al. 2015, Soussana & Lemaire, 2014; Waghorn, 2008).
Phelan, P., Moloney, A. P., McGeough, E. J., Humphreys, J., Bertilsson, J., O’Riordan, E. G., & O’Kiely, P. (2015). Forage Legumes for Grazing and Conserving in Ruminant Production Systems. Critical Reviews in Plant Sciences, 34, 281–326. https://doi.org/10.1080/07352689.2014.898455
Soussana, J. F., & Lemaire, G. (2014). Coupling carbon and nitrogen cycles for environmentally sustainable intensification of grasslands and crop-livestock systems. Agriculture, Ecosystems and Environment, 190, 9–17. https://doi.org/10.1016/j.agee.2013.10.012
Waghorn, G. (2008). Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production—Progress and challenges. Animal Feed Science and Technology 147, 116–139. https://doi.org/10.1016/j.anifeedsci.2007.09.013
Research outcomes
Education and Outreach
Participation summary:
1) A fact sheet co-authored by student Kathryn Slebodnik, Jennifer Reeve, and collaborators at Utah State University and Agriculture and Agri-Food Canada is currently in review for publication with Utah State University Extension.
2) Final data from the in vitro incubation experiment has been included in graduate student Kathryn Slebodnik's masters thesis. Kathryn's thesis was successfully defended on December 3, 2019 and has been approved by the academic committee and Utah State University's office of graduate studies. Data from the in vitro incubation experiment will be submitted to peer review journals such as Soil Biology and Biochemistry, or Agriculture, Ecosystems and Environment in 2020.
3) Final data from the in vitro incubation experiment was presented as a poster at the American Society of Agronomy Annual Meeting in San Antonio, TX on November 13, 2019 by principal investigator Dr. Jennifer Reeve.
Slebodnik Agronomy Society Poster
4) Preliminary data from the in vitro incubation experiment was presented as a poster at the Utah State University Student Research Symposium in Logan, UT on April 10-11, 2019 by graduate student Kathryn Slebodnik. Poster from event #5 was presented.
5) Preliminary data from the in vitro incubation experiment was presented as a poster at the Utah State University Department of Plants, Soils and Climate Department Showcase in Logan, UT on March 25, 2019 by graduate student Kathryn Slebodnik.
Slebodnik Showcase Poster 2019
6) Preliminary data from the in vitro incubation experiment was presented during an oral presentation at the Utah State University Dept. Plants, Soils and Climate Seminar Series in Logan, UT in March 18, 2019 by graduate student Kathryn Slebodnik.
7) Preliminary data from the in vitro incubation experiment was presented as a poster at the Intermountain Sustainability Summit at Weber State University in Ogden, UT on March 21, 2019 by graduate student Kathryn Slebodnik. Poster from event #11 was presented.
8) A second Holos training workshop, hosted by the same presenters as above, was held at Utah State University on February 22, 2019. The workshop was attended by 7 participants, including 2 research faculty, 4 graduate students, and 1 producer from a local dairy operation. The workshop trained attendees to use Holos software by walking them through a hands-on demonstration where they modeled a typical beef production operation. Participants practiced modeling alternative management strategies and analyzing their impact on the GHG intensity of the beef produced. All participants completed a pre- and post-workshop survey. This survey assessed how participants' thoughts and attitudes towards Holos software and on-farm environmental and economic planning had changed over the course of the presentations and workshops. Attendees were provided with an instructional handout to follow and take home for future reference.
9) A Holos training workshop was presented by Dr. Roland Kroebel, Dr. Sarah Pogue, and Aaron McPherson from Agriculture and Agri-Food Canada with assistance from graduate students Kathryn Slebodnik and Anthony Whaley immediately after the Urban and Small Farms Conference at the Utah Cultural Celebration Center in West Valley City, UT on February 21, 2019. Unfortunately, there were no attendees.
10) A Holos informational presentation was presented at the 2019 Urban and Small Farms Conference in West Valley City, UT on February 21st, 2019. Co-presenters included graduate student Kathryn Slebodnik and collaborator Dr. Roland Kroebel from Agriculture and Agri-Food Canada. The presentation was attended by ~30 participants which included local producers as well as Utah State research and extension faculty.
Urban and Small Farms Presentation
11) Preliminary data from the in vitro incubation experiment was presented as a poster at the Soil Science Society of America 2018-2019 International Soils Conference in San Diego, CA on January 7th, 2019 by graduate student Kathryn Slebodnik.